Introduction
Renal cell carcinoma (RCC) is a common type of
malignant neoplasm of the urinary system with increasing morbidity
and mortality worldwide (1).
Histopathologically, the clear-cell (cc) sub-type accounts for 80%
of all renal-cell carcinoma (2).
While surgical approaches represent a curative treatment method for
RCC, one-third of patients develop metastasis and require systemic
therapy (3). However, as end-stage
RCC is frequently resistant to radiotherapy and chemotherapy, the
affected patients have a poor prognosis. Therefore, novel molecular
targets in RCC are required to be identified in order to provide
more efficient therapeutic approaches.
Forkhead box transcription factors, class O (FOXO)
include four important components: FOXO1, −3, −4 and −6, which have
been reported not only to have crucial roles in cellular
proliferation, inflammation, stress resistance and apoptosis
(4–7), but to also exert anti-tumor effects
in certain types of malignant neoplasm (8,9).
FOXO4 is highly expressed in kidney, muscle and colorectal tissues,
and has protective effects against cardiovascular diseases
(10) and malignancies. Studies
have reported that FOXO4 exerts tumor-suppressive effects in
cholangiocarcinoma (11) and
gastric cancer (12), while its
potential effect on RCC has remained elusive and was therefore
assessed in the present study. For this, the expression of FOXO4 in
RCC tissues and cell lines as well as normal renal cells was
assessed, and the effects of vector-mediated overexpression of FOXO
on the apoptosis of RCC cell lines were investigated. Furthermore,
the role of apoptotic proteins in this process was assessed to
determine the underlying molecular mechanism.
Materials and methods
Patients and samples
Fifty-eight human RCC tissue samples and adjacent
normal tissues were obtained from RCC patients (49 males and 9
females; age, 46–70 years with mean of 58.6 years) undergoing
nephrectomy at the Department of Urology, Renmin Hospital of Wuhan
University (Wuhan, China). All of the tumor samples were of the
clear-cell sub-type as verified by pathological examination, and
the corresponding normal samples obtained from adjacent tissues did
not exhibit any pathological abnormalities. The samples were frozen
in liquid nitrogen and stored at −70°C prior to protein and RNA
extraction. The use of human tissues was approved by the
Institutional Review Board of Wuhan University (Wuhan, China) and
conformed to the Declaration of Helsinki. All of the patients
provided written informed consent.
Cell culture
The 786-0 and HK-2 cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA). The Caki-1
and Caki-2 cell lines were purchased from the China Center for Type
Culture Collection (Wuhan, China). All cell lines were maintained
in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1%
(v/v) penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere containing 5%
CO2.
Plasmid construction and purification of
cultured 786-0 cells
FOXO4 overexpression plasmids were constructed with
pcDNA3.1/Flag (Clontech Laboratories, Inc., Mountainview, CA, USA)
as the vector, FOXO4 as the expression gene and flag as a tag. The
primer sequences for FOXO4 were as follows: Sense,
5′-ATGGATCCGGGAATGAGAAT-3′ and anti-sense,
5′-TCAGGGATCTTGGCTCAAAG-3′. The constructed vector was verified
using standard DNA sequencing using an ABI PRISM 310 Genetic
Analyzer (PerkinElmer, Inc., Waltham, MA, USA). An empty expression
plasmid was used as a control. A total of 1×105 cells
were seeded into each well of a six-well plate. Upon 70%
confluency, the medium was discarded and replaced with fresh
non-supplemented, serum-free medium. A total of 1.5 µg
pcDNA3.1/Flag/FOXO4 plasmid or pcDNA3.1/Flag was mixed with 100
µl Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.).
Furthermore, 4.5 µl Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was mixed with 100 µl Opti-MEM and
incubated for 5 min at room temperature. Subsequently, the plasmid
and lipofectamine 2000 solutions were mixed and incubated for
another 20 min prior to addition to the cells. Following 24 h of
incubation, the transfection efficiency was determined by western
blot analysis and reverse-transcription quantitative polymerase
chain reaction (RT-qPCR) analyses.
Bim small interfering (si)RNA
transfection
Bim siRNA and scrambled control siRNA were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Cells were
transfected with siRNA following the manufacturer's instructions.
In brief, 2×105 786-0 cells were cultured in 2 ml
antibiotic-free medium in a six-well plate and transfected with
pcDNA3.1/Flag/FOXO4 24 h prior to treatment with Bim siRNA. First,
2–8 µl of siRNA duplex was added to 100 µl siRNA
transfection medium (solution A) and 2–8 µl of siRNA
transfection reagent was added to 100 µl siRNA transfection
medium (solution B). Subsequently, solutions A and B were mixed and
incubated at room temperate for 30 min. Following washing of each
well with 2 ml siRNA transfection medium, 0.8 ml siRNA transfection
medium was added to each well containing the siRNA transfection
reagent mixture (200 µl from solutions A and B). After
incubation for 8 h, the supernatant was removed and replaced with
normal medium, followed by incubation for another 24 h prior to
analysis. The silencing efficiency was examined using western blot
and RT-qPCR. All assays, including transfections, western blots and
PCR, were performed as at least three independent experiments.
Flow-cytometric cell sorting (FACS)
analysis of cell apoptosis
ccRCC cells were transfected with
pcDNA3.1/Flag/FOXO4 or pcDNA3.1/Flag and incubated for 24 h.
Subsequently, all cultured cells and the media were collected, and
following centrifugation at 1,000 × g for 5 min, cells were
re-suspended in 300 µl ice-cold binding buffer. Cells were
then stained with 10 µl propidium iodide (PI) and 5
µl Annexin V-fluorescein isothiocyanate (FITC) using an
Annexin V-FITC kit (Beyotime Institute of Biotechnology, Haimen,
China) and incubated for another 5 min. Ten thousand events were
analyzed to determine the proportion of apoptotic cells using a
FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ,
USA).
Western blot analysis
Tissue and cell extracts were obtained using
radioimmunoprecipitation assay lysis buffer (Biyuntian
Biotechnology Co., Shanghai, China) on ice and the lysates were
centrifuged for 15 min (12,000 × g, 4°C). Protein concentration was
determined using the QuantiPro™ BCA assay kit (Sigma-Aldrich, St.
Louis, MO, USA). Proteins (1 µg/µl; 20 µl)
were separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (Shanghai Haling Biotechnology Co., Ltd., Shanghai,
China) and subsequently electroblotted onto polyvinylidene fluoride
membranes (Millipore, Billerica, MA, USA). After blocking
non-specific binding to the membrane with 5% non-fat milk, the
membranes were incubated overnight at 4°C with the following
primary antibodies at a 1:1,000 dilution: Rabbit monoclonal
anti-FOXO4 (Abcam, Cambridge, MA, USA; cat. no. ab128908), rabbit
polyclonal anti-Bim (Abcam; cat. no. ab7888), rabbit polyclonal
anti-B-cell lymphoma 2 (Bcl-2; Abcam; cat. no. ab59348), rabbit
monoclonal anti-Bcl-2-associated X (Bax; Abcam; cat. no. ab32503),
rabbit monoclonal anti-cleaved-caspase 3 (Cell Signaling
Technology, Inc., Danvers, MA, USA; cat. no. 9664), rabbit
polyclonal anti-cytochrome c (Abcam; cat. no. ab90529) and
rabbit polyclonal anti-β-actin (Santa Cruz Biotechnology, Inc.;
cat. no. sc-130657). Membranes were washed three times with
Tris-buffered saline with Tween 20 (TBST; Wuhan Guge Biotechnology
Co., Ltd, Wuhan, China) and incubated with corresponding IRDye
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (LI-COR Biosciences, Lincoln, NE, USA; cat. no. 925-32211)
for 2 h at room temperature. The membranes were rinsed three times
with TBST and the protein bands were visualized using a two-color
infrared imaging system (Odyssey; LI-COR Biosciences).
RT-qPCR
Total RNA was isolated from the kidney tissues and
cultured cells with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized using the PrimeScript™ RT
reagent kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's instructions. RNA (1.5 µg) extracted from
tissues was used as a template to perform one-step RT-PCR, and
human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as
an internal control. The resulting cDNA was amplified using
real-time PCR in a volume of 20 µl with the SYBR Green
Master mix (Takara Bio, Inc.) method and the ABI 7500 Real-Time
RT-PCR system (Thermo Fisher Scientific, Inc.). The conditions of
the PCR were initial denaturation at 95°C for 30 sec, followed by
40 cycles of 5 sec at 95°C, 30 sec at 60°C and 1 min at 72°C. The
Cq values of each samples were calculated using the
2−ΔΔCq data analysis method (13). The primer sequences were generated
by Sangon Biotech Co., Ltd. (Shanghai, China), as follows: GAPDH
forward, 5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′; and FOXO4 forward,
5′-CTTTCTGAAGACTGGCAGGAATGTG-3′ and reverse,
5′-GATCTAGGTCTATGATCGCGGCAG-3′.
Statistical analysis
Experiments were repeated three times independently
and representative data are shown. Values are expressed as the mean
± standard error of the mean. Student's t-test was used for
comparison between two groups, and one-way analysis of variance was
used for comparisons between groups. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
FOXO4 is downregulated in RCC tissues and
cell lines
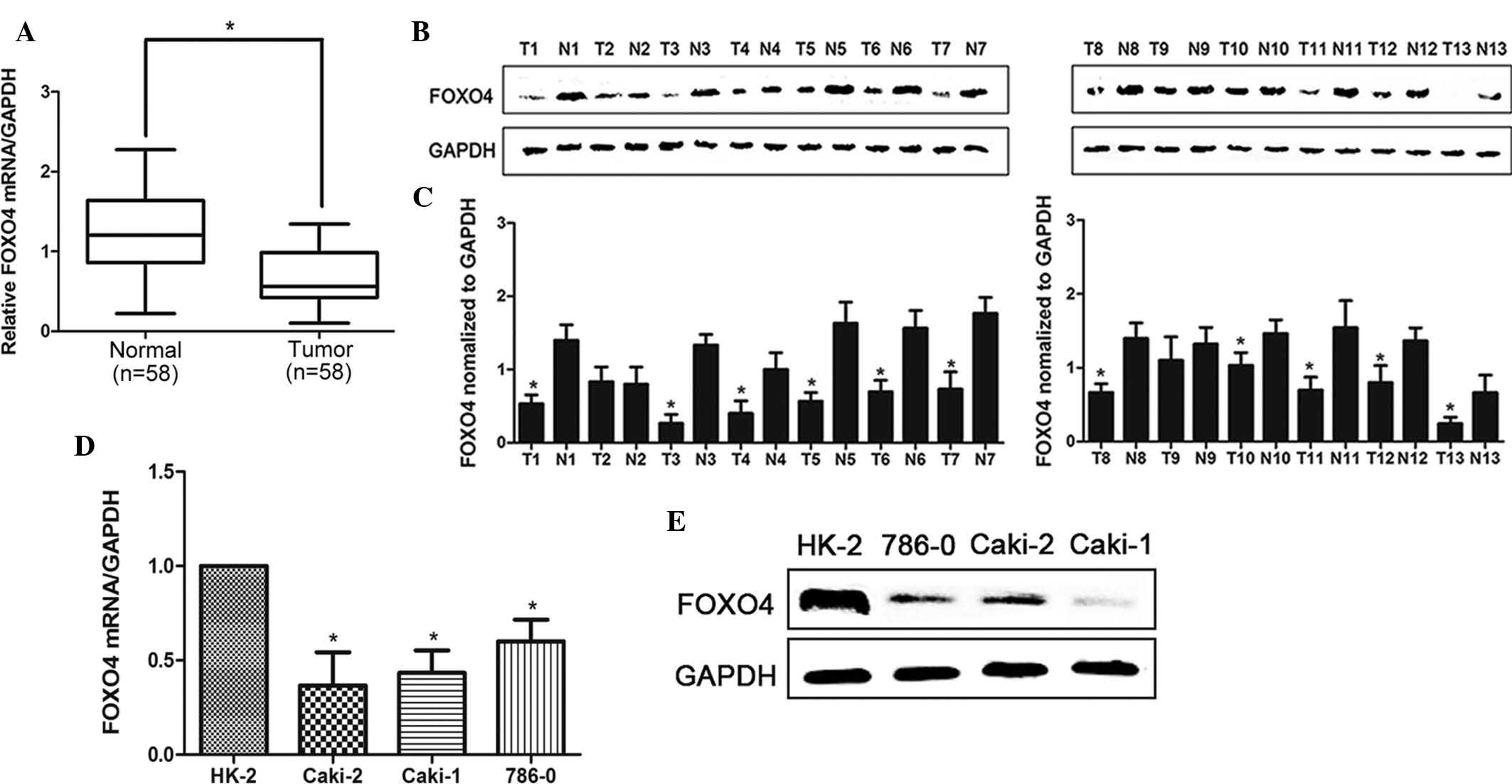
In order to examine expression levels of FOXO4, 58
clinical renal cancer samples were obtained and analyzed using
RT-qPCR. As shown in Fig. 1A,
FOXO4 expression was significantly reduced in 51 (87.9% of total)
human RCC tissues compared with that in their corresponding
adjacent non-tumorous tissues. Furthermore, the FOXO4 protein
expression was assessed in 13 pairs of randomly selected RCC
tissues and their adjacent normal tissues using western blot
analysis. As shown in Fig. 1B and
C, the protein levels of FOXO4 were significantly decreased in
RCC tissues compared with those in their corresponding normal
tissues in 11 out of 13 pairs (P<0.05). In addition, as shown in
Fig. 1D and E, the relative
expression levels of FOXO4 mRNA and protein in the three RCC cell
lines 786-0, Caki-1 and Caki-2 were significantly reduced compared
to those in the HK2 normal human renal tubular cell line.
Plasmid-mediated FOXO4 overexpression in
ccRCC cell lines
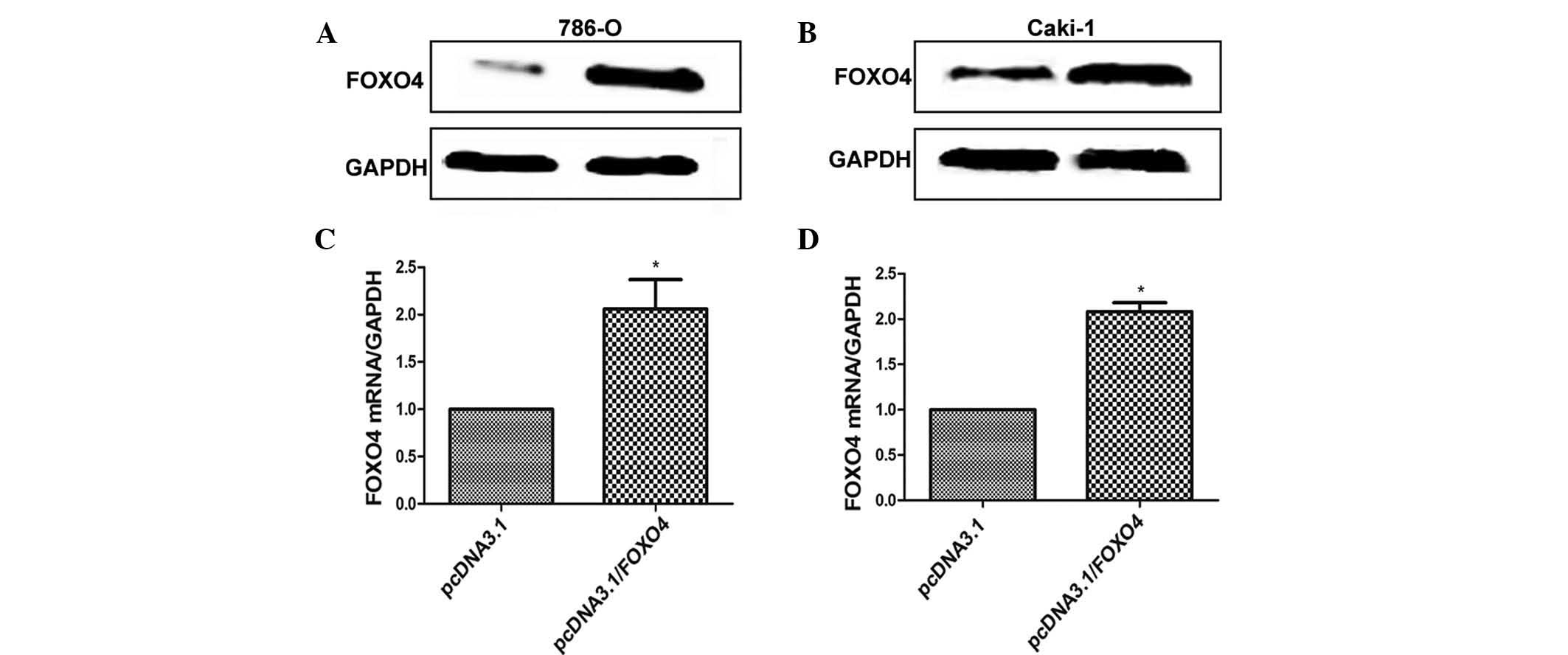
In order to clarify the role of FOXO4 in ccRCC
cells, two ccRCC cell lines were transfected with pcDNA3.1/FOXO4,
which resulted in high expression of FOXO4. As shown in Fig. 2, western blot and RT-qPCR indicated
that in 786-0 and Caki-1 cells, FOXO4 protein and mRNA expression
in the pcDNA3.1/FOXO4 plasmid group was upregulated compared with
that in the empty plasmid group.
FOXO4 overexpression induces apoptosis in
ccRCC cells
Following transfection with pcDNA3.1/FOXO4 plasmid
or pcDNA3.1 plasmid for 24 h, the apoptotic rate was assessed by
FACS using Annexin V/PI double staining. The results revealed that
overexpression of FOXO4 significantly increased the apoptotic rate
of 786-0 (25.0±8.0) and Caki-1 (15.9±4.1) cells, while the
apoptotic rate of the control-transfected cells was 3.1±0.2 in
786-0 cells and 1.3±0.2 in Caki-1 cells (Fig. 3A). To identify the mechanism of
FOXO4-induced apoptosis in 786-0 cells, FOXO4-overexpressing cells
were subjected to western blot analysis of apoptosis-associated
proteins at 24 h following transfection. As shown in Fig. 3B, Bax, cleaved-caspase 3,
cytochrome c and Bim were upregulated and Bcl-2 was
downregulated in 786-0 and Caki-1 cells transfected with
pcDNA3.1/FOXO4.
FOXO4 overexpression induces apoptosis of
786-0 cells partly through upregulation of Bim
In order to assess the role of Bim in FOXO4-induced
apoptosis, Bim siRNA was used to suppress the expression of Bim in
FOXO4-transfected 786-0 cells or control-transfected cells. As
shown in Fig. 4A, western blot
analysis indicated that Bim was downregulated after siRNA
treatment. Of note, knockdown of Bim expression led to a
substantial decrease in the apoptotic rate of FOXO4-overexpressing
786-0 cells (P<0.05) (Fig. 4B and
C). In addition, the expression of apoptosis-associated
proteins was altered following knockdown of Bim. In the
pcDNA3.1-FOXO4 + Bim-siRNA group, cleaved-caspase 3, Bax and
cytochrome c were significantly downregulated compared with
those in the pcDNA3.1-FOXO4 group. However, knockdown of Bim was
not able to rescue the levels of Bcl-2, which were significantly
downregulated in the pcDNA3.1-FOXO4 group (Fig. 4D).
Discussion
The present study was the first to demonstrate the
tumor-suppressor role of FOXO4 in ccRCC. FOXO4 was shown to be
downregulated in renal cancer tissues but to be highly expressed in
non-tumorous tissues. In accordance with the expression levels in
tissues, FOXO4 was also expressed at high levels in the HK-2 normal
human renal proximal tubular cell line, while its expression in
several renal cancer cell lines was low. These results suggested
that FOXO4 may act as a negative regulator in RCC. Of note,
vector-mediated overexpression of FOXO4 caused a significant level
of apoptosis in 786-0 and Caki-1 cells in vitro, alongside
decreased levels of Bax, cleaved-caspase 3, cytochrome c and
Bim, and increased expression of Bcl-2. Furthermore, knockdown of
Bim attenuated the apoptotic effects of FOXO4, indicating that
FOXO4-induced apoptosis is, at least partially, mediated via
Bim.
A number of studies have shown that phosphorylation
of FOXO proteins by Akt promote apoptosis in cancer cells (14–16).
Roy et al (14) reported
that the activation of FOXO transcription factor led to cell cycle
arrest and apoptosis in pancreatic cancer, suggesting its
tumor-suppressive effects. Furthermore, Nakayoshi et al
(17) found that FOXO4 knockdown
prevented early pro-angiogenic cells from oxidative stress-induced
apoptosis through downregulation of cleaved-caspase 3. This result
was in agreement with the results of the present study, which
revealed that FOXO4 activation caused apoptosis in RCCs. Current
research focuses on the tumor-suppressive roles of FOXOs and other
target genes in the induction of apoptosis and their utilization
for cancer therapy. FOXO3 was shown to bind to the promoter region
of the Bim gene and enhance its transcription to promote apoptosis
in breast (16) and hepatocellular
carcinoma cancer cells (18),
while downregulation of FOXO3 inhibits the transcription of its
target gene Bim to decrease apoptosis (16). As members of the FOXO family, FOXO4
and FOXO3 show similar biological behaviors. Overexpression of
FOXO4 was shown to activate the promoter of the Bim gene and
increase its expression, resulting in an elevation of the apoptotic
rate (16). Similarly,
phosphorylation of FOXO4 was shown to trigger apoptosis, depending
on the stimulation of pro-apoptotic protein Bim (19), which has a pivotal role in the
control of mitochondria-dependent apoptosis. Consistent with these
results, the present study also observed that FOXO4 caused
apoptosis in cancer cells, partly through enhancing the expression
of the Bim gene. Bim is a BH3-only protein, which can bind with all
five pro-survival proteins (Bcl-2, Bcl-extra large, Bcl-w, A1 and
myeloid cell leukemia 1), making it a more effective apoptosis
inducer than other BH3-only proteins (20). However, the underlying mechanism of
the interaction of FOXO4 with Bim requires further
investigation.
In conclusion, the present study indicated that the
knockdown of Bim in RCCs alleviated FOXO-induced
mitochondria-dependent apoptosis. Although it is well known that
FOXO4 exerts anti-tumor effects in numerous types of malignancy,
the present study was the first to demonstrate its apoptotic
effects in RCC. The present study enhanced the understanding of the
complex underlying mechanisms of FOXO4-induced apoptosis, which was
indicated to proceed via activation of the expression of the
pro-apoptotic protein Bim, inducing mitochondria-mediated
activation of the apoptotic cascade in RCC. Although it remains
elusive how FOXO4 activates Bim expression, it represents a novel
therapeutic target for RCC.
References
|
1
|
Chow WH and Devesa SS: Contemporary
epidemiology of renal cell cancer. Cancer J. 14:288–301. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moch H: An overview of renal cell cancer:
Pathology and genetics. Semin Cancer Biol. 23:3–9. 2013. View Article : Google Scholar
|
|
3
|
Atkins MB, Choueiri TK, Cho D, Regan M and
Signoretti S: Treatment selection for patients with metastatic
renal cell carcinoma. Cancer. 115(10 Suppl): S2327–S2333. 2009.
View Article : Google Scholar
|
|
4
|
van der Vos KE and Coffer PJ: The
extending network of FOXO transcriptional target genes. Antioxid
Redox Signal. 14:579–592. 2011. View Article : Google Scholar
|
|
5
|
Olmos Y, Sánchez-Gómez FJ, Wild B,
García-Quintans N, Cabezudo S, Lamas S and Monsalve M: SirT1
regulation of antioxidant genes is dependent on the formation of a
FoxO3a/PGC-1alpha complex. Antioxid Redox Signal. 19:1507–1521.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown J, Wang H, Suttles J, Graves DT and
Martin M: Mammalian target of rapamycin complex 2 (mTORC2)
negatively regulates Toll-like receptor 4-mediated inflammatory
response via FoxO1. J Biol Chem. 286:44295–44305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alikhani M, Roy S and Graves DT: FOXO1
plays an essential role in apoptosis of retinal pericytes. Mol Vis.
16:408–415. 2010.PubMed/NCBI
|
|
8
|
Cho EC, Kuo ML, Liu X, Yang L, Hsieh YC,
Wang J, Cheng Y and Yen Y: Tumor suppressor FOXO3 regulates
ribonucleotide reductase subunit RRM2B and impacts on survival of
cancer patients. Oncotarget. 15:4834–4844. 2014. View Article : Google Scholar
|
|
9
|
Yu DA, Yoon J, Ko YS, Park J, Kim SY, Kim
MA, Kim JH, Jung J, Cheon Y, Lee HS, et al: Forkhead transcription
factor FOXO1 inhibits nuclear factor-κB in gastric cancer. APMIS.
122:848–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Liang J, Castrillon DH, DePinho RA,
Olson EN and Liu ZP: FoxO4 regulates tumor necrosis factor
alpha-directed smooth muscle cell migration by activating matrix
metalloproteinase 9 gene transcription. Mol Cell Biol.
27:2676–2686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee MJ, Yu GR, Yoo HJ, Kim JH, Yoon BI,
Choi YK and Kim DG: ANXA8 down-regulation by EGF-FOXO4 signaling is
involved in cell scattering and tumor metastasis of
cholangiocarcinoma. Gastroenterology. 137:1138–1150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su L, Liu X, Chai N, Lv L, Wang R, Li X,
Nie Y, Shi Y and Fan D: The transcription factor FOXO4 is
down-regulated and inhibits tumor proliferation and metastasis in
gastric cancer. BMC cancer. 14:3782014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shukla S, Rizvi F, Raisuddin S and Kakkar
P: FoxO proteins' nuclear retention and BH3-only protein Bim
induction evokes mitochondrial dysfunction mediated apoptosis in
berberine-treated HepG2 cells. Free Radic Biol Med. 76:185–199.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv Y, Song S, Zhang K, Gao H and Ma R:
CHIP regulates AKT/FoxO/Bim signaling in MCF7 and MCF10A cells.
PLoS One. 8:e833122013. View Article : Google Scholar :
|
|
17
|
Nakayoshi T, Sasaki K, Kajimoto H, Koiwaya
H, Ohtsuka M, Ueno T, Chibana H, Itaya N, Sasaki M, Yokoyama S, et
al: FOXO4-knockdown suppresses oxidative stress-induced apoptosis
of early pro-angiogenic cells and augments their neovascularization
capacities in ischemic limbs. PLoS One. 9:e926262014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carbajo-Pescador S, Steinmetz C, Kashyap
A, Lorenz S, Mauriz JL, Heise M, Galle PR, González-Gallego J and
Strand S: Melatonin induces transcriptional regulation of Bim by
FoxO3a in HepG2 cells. Br J Cancer. 108:442–449. 2013. View Article : Google Scholar :
|
|
19
|
Urbich C, Knau A, Fichtlscherer S, Walter
DH, Brühl T, Potente M, Hofmann WK, de Vos S, Zeiher AM and
Dimmeler S: FOXO-dependent expression of the proapoptotic protein
Bim: Pivotal role for apoptosis signaling in endothelial progenitor
cells. FASEB J. 19:974–976. 2005.PubMed/NCBI
|
|
20
|
Dijkers PF, Medema RH, Lammers JW,
Koenderman L and Coffer PJ: Expression of the pro-apoptotic Bcl-2
family member Bim is regulated by the forkhead transcription factor
FKHR-L1. Curr Biol. 10:1201–1204. 2000. View Article : Google Scholar : PubMed/NCBI
|