Introduction
Macrophages are important cells in the immune system
that act as the first defense against invading agents (bacteria,
viruses, and fungi) by releasing cellular signaling molecules and
antimicrobial agents (1–4). Inflammatory responses in macrophages
are induced upon the stimulation of macrophages with external
stimuli, such as lipopolysaccharides (LPS). Inflammatory responses
include the expression of various pro-inflammatory mediators, such
as nitric oxide (NO), which is produced by inducible nitric oxide
synthase (iNOS), and prostaglandin E2 (PGE2),
which is produced by cyclooxygenase (COX)-2, in addition to the
expression of various pro-inflammatory cytokines, including
interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α
(5–10). Excessive amounts of these mediators
cause severe inflammatory diseases, including septic shock,
rheumatoid arthritis, systemic lupus erythematosus and inflammatory
bowel disease, although the enhanced production of inflammatory
mediators is important for the host defense against external
stimuli (10–16). Therefore, inhibiting the excessive
production of inflammatory mediators in macrophages by regulating
mRNA and protein expression levels may be a viable strategy to
develop effective anti-inflammatory agents.
A study from the World Health Organization has
indicated that greater than 80% of the population in developing
countries still use natural plants as their main medicinal source
(17). The Garcinia species
have been used traditionally for the treatment of inflammatory
diseases worldwide. The ethanol and dichloromethane extracts of the
fruit hull of Garcinia mangostana, a Thai traditional
medicine for the treatment of abscesses and skin infections
(18), have been reported to
exhibit anti-inflammatory activity (19) and potent antinociceptive effects in
mice (20). The ethyl acetate
extract of Garcinia hanburyi, a Thai folk medicine used to
treat infected wounds (21,22),
has been assessed for anti-inflammatory, analgesic and antipyretic
activities (23). The
hydroalcoholic extracts of the leaves from Garcinia
gardneriana, a plant traditionally used in southern Brazil to
treat skin disorders (24), have
shown anti-inflammatory activity in several experimental models
(25).
Polyphenols and their metabolites in Garcinia
plants are known to exhibit anti-inflammatory properties. Of these
poly-phenols, kolaviron, a biflavonoid complex from Garcinia
kola, and garcinol, a polyisoprenylated benzophenone derivative
isolated from Garcinia indica, have been applied to animal
models to evaluate their anti-inflammatory effects (26–29).
Garcinia subelliptica (G. subelliptica) is a
Garcinia species that is distributed widely from Okinawa
Island in Japan to Thailand, and contains secondary polyphenolic
metabolites which are known to exert anti-inflammatory effects
(30–32). Furthermore, G. subelliptica
has been reported to contain phloroglucinols and terpenoids that
exhibit anti-inflammatory properties similar to those of other
Garcinia plants (33).
Garcinielliptones exert anti-inflammatory effects by inhibiting
chemical mediators and xanthine oxidase in mast cells, neutrophils
and macrophages (33–35). However, systemic studies for the
anti-inflammatory effects of the ethanol extract of G.
subelliptica (EGS) have not been reported.
In the present study, the anti-inflammatory effects
of EGS exerted through the modulation of the production of
inflammatory mediators in LPS-stimulated RAW 264.7 macrophages
(ATSS, Manassas, VA, USA) were investigated to evaluate the
potential of EGS for the treatment of excessive inflammation.
Furthermore, the associated signaling pathways, such as the
phosphorylation of the inhibitor of κB (IκB) and the
mitogen-activated protein kinase (MAPK) pathway, were investigated
to assess the molecular targets of EGS.
Materials and methods
Cell culture and reagents
A 95% ethanol extract (Code no.: FBM124-035) of the
Garcinia subelliptica Merr. (Clusiaceae) was purchased from
the International Biological Material Research Center (Daejeon,
Korea). The RAW 264.7 macrophages, a mouse monocytic cell line,
were cultured in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific,
Inc.), 50 unit/ml penicillin, and 50 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in humidified air
containing 5% CO2. The cytokines that were produced,
including TNF-α and IL-6, were measured using enzyme-linked
immunosorbent assay (ELISA) Ready-SET-Go!® kits
(eBioscience, Inc., San Diego, CA, USA). Rabbit anti-IκBα (sc-371)
and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
sc-25778) polyclonal antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Mouse anti-phosphoylated
(p)-IκBα (Ser32/36; 9246) and anti-p-extracellular signal-regulated
kinase 1/2 (ERK 1/2; 9106) monoclonal antibodies; and rabbit
anti-iNOS (2982), anti-COX-2 (4842), anti-p-p38 (9211), anti-p38
(9212), anti-ERK 1/2 (9102), anti-p-c-Jun N-terminal kinase (JNK;
9251) and anti-JNK (9252) polyclonal antibodies; and goat
anti-rabbit horseradish peroxidase (HRP)-conjugated immunoglobulin
(Ig)G (7074) and horse anti-mouse HRP-conjugated IgG (7076) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell viability assay
RAW 264.7 macrophages were seeded in 96 well plates
(4×104/well). Following adhesion overnight, cells were
incubated with 20, 50, 100, 200, and 400 µg/ml EGS and 1
µg/ml LPS for 24 h. Following incubation, cell viability was
measured using an EZ-Cytox Cell Viability Assay kit (Daeil Lab
Services Co., Ltd., Seoul, Korea). Briefly, the EZ-Cytox solution
that contained a water soluble tetrazolium salt was added to each
well for 2 h at 37°C and 100 µl of supernatants were
transferred to 96 well plates. The absorbance was measured at 450
nm with a Synergy H1 Microplate Reader (BioTek Instruments, Inc.,
Winooski, VT, USA).
Nitrite assay
RAW 264.7 macrophages were incubated with 20, 50,
100 and 200 µg/ml EGS and 1 µg/ml LPS for 24 h.
Following incubation the levels of NO synthesis were determined by
assaying the culture supernatants for nitrite using the Griess
reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine
dihydrochloride, and 2.5% phosphoric acid). Nitrate is the stable
product of the reaction between NO and molecular oxygen. The
absorbance was measured at 540 nm, using a Synergy H1 Microplate
Reader, following incubation for 10 min.
ELISA
RAW 264.7 macrophages were stimulated with 1
µg/ml LPS and 20, 50, 100 and 200 µg/ml EGS for 24 h.
Following stimulation, the supernatants were obtained via
centrifugation at 2,339 × g for 3 min at 4°C, and a sandwich ELISA
was performed according to the manufacturer's instructions, using
monoclonal antibodies specific to each mediator to determine the
quantity of TNF-α and IL-6 in the culture supernatants. Prior to
the application of samples, the plate was pre-coated with the
coating antibody in the supplied buffer. Following incubation
overnight at 4°C, the plate was washed with 1X phosphate-buffered
saline (PBS)-Tween 20 (0.5%) and treated with 1X assay diluents for
1 h. Samples were loaded into each well and incubated for 2 h at
room temperature. Following washing, the plate was treated with a
biotinylated secondary antibody solution and HRP-streptavidin
solution for 1.5 h, and the substrate solution was added to the
washed-plate. Following 10 min incubation in dark conditions, 1 N
phosphoric acid (H3PO4) was added and the
optical density of the individual wells was measured at 450 nm
using a Synergy Microplate Reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RAW 264.7 macrophages were stimulated with 50 ng/ml
LPS and 20, 50, 100 and 200 µg/ml EGS for 6 h. Total RNA was
extracted from the cells via isoproponal precipitation using
Accuzol reagent (Bioneer Corporation, Daejeon, Korea),
reverse-transcribed into complementary DNA (cDNA) using a
TOPscript™ cDNA synthesis kit (Enzynomics, Daejeon, Korea) and then
PCR amplified. Quantification of mRNA was performed using a
real-time PCR reagent, iTaq™ Universal SYBR Green Supermix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's instructions. The PCR was run for 40 cycles of
denaturation at 94°C (5 sec) and annealing/extension at 60°C (30
sec) using a CFX Connect™ Real-Time Thermal Cycler (Bio-Rad
Laboratories, Inc.). According to the 2−∆∆Cq method
(36), the results were normalized
to the reference genes, β-actin and GAPDH, and were expressed as
the ratio of gene expressions to the LPS treated group (100%). PCR
primers were designed using Beacon Designer 7.0 (Premier Biosoft,
Palo Alto, CA, USA) and subsequently synthesized by Bioneer
Corporation. The sequences of PCR primers used are as follows:
Mouse iNOS, sense 5′-TGG CCA CCA AGC TGA ACT-3′ and antisense
5′-TCA TGA TAA CGT TTC TGG CTCTT-3′; COX-2, sense 5′-GAT GCT CTT
CCG AGC TGTG-3′ and antisense 5′-GGA TTG GAA CAG CAA GGA TTT-3′;
TNF-α, sense 5′-CTG TAG CCC ACG TCG TAGC-3′ and antisense 5′-TTG
AGA TCC ATG CCG TTG-3′; IL-6, sense 5′-TCT AAT TCA TAT CTT CAA CCA
AGAGG-3′ and antisense 5′-TGG TCC TTA GCC ACT CCTTC-3′; IL-1β,
sense 5′-TTG ACG GAC CCC AAA AGAT-3′ and antisense 5′-GAT GTG CTG
CTG CGA GATT-3′; β-actin, sense 5′-CGT CAT ACT CCT GCT TGCTG-3′ and
antisense 5′-CCA GAT CAT TGC TCC TCC TGA-3′; and GAPDH, sense
5′-GCT CTC TGC TCC TCC TGTTC-3′ and antisense 5′-ACG ACC AAA TCC
GTT GACTC-3′.
Semi-quantitative RT-PCR
PCR primers were designed using Primer3 software
(version 4.0.0), as previously described (37), and were subsequently synthesized by
Bioneer Corporation. The sequences of the PCR primers used study
are as follow: Mouse iNOS, sense 5′-GCA TGG AAC AGT ATA AGG CAA
ACA-3′ and antisense 5′-GTT TCT GGT CGA TGT CAT GAG CAA-3′; COX-2,
sense 5′-GCA TGG AAC AGT ATA AGG CAA ACA-3′ and antisense 5′-GTT
TCT GGT CGA TGT CAT GAG CAA-3′; TNF-α, sense 5′-GTG CCA GCC GAT GGG
TTG TACC-3′ and antisense 5-′AGG CCC ACA GTC CAG GTC ACTG-3′; IL-6,
sense 5′-TCT TGG GAC TGA TGC TGG TGAC-3′ and antisense 5′-CAT AAC
GCA CTA GGT TTG CCGA-3′; IL-1β, sense 5′-AGC TGT GGC AGC TAC
CTGTG-3′ and antisense 5′-GCT CTG CTT GTG AGG TGCTG-3′; and GAPDH,
sense 5′-GTC TTC ACC ACC ATG GAG AAGG-3′ and antisense 5′-CCT GCT
TCA CCA CCT TCT TGCC-3′. The PCR was run for 20–25 cycles of 94°C
(30 sec), 60°C (30 sec), and 72°C (30 sec) using a Genetouch
thermal cycler (Bioer Technology Co., Ltd., Hangzhou, China).
Following amplification, 10 µl of the RT-PCR products were
separated in 1.5% (w/v) agarose gels and stained with ethidium
bromide.
Preparation of total cell lysates
LPS-stimulated RAW 264.7 cells were treated with EGS
and 1 µg/ml LPS for 15 min and washed with ice-cold PBS. The
cells were lysed in lysis buffer containing 0.5% NP-40, 0.5% Triton
X-100, 150 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1 mM
ethylenediaminetetraacetic acid, 1% glycerol, 1 mM
phenylmethylsulfonyl fluoride and 1 µg/ml aprotinin, and
collected in microtubes prior to centrifugation at 15,814 x g for
30 min at 4°C. The supernatants were prepared in fresh
microtubes.
Immunoblot analysis
Protein concentration was measured using the
Bradford method (Bio-Rad Laboratories, Inc.). Aliquots (20
µg) of the cell lysates were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel in a Mini-Protein II gel apparatus (both
Bio-Rad Laboratories, Inc.) and transferred onto nitrocellulose
membranes (GE Healthcare Life Sciences, Pittsburgh, WI, USA) with
transfer buffer [192 mM glycine, 25 mM Tris-HCl (pH 8.8), and 20%
MeOH (v/v)]. Following blocking of non-specific sites with 5%
bovine serum albumin solution, the membrane was incubated overnight
at 4°C with the primary antibodies (all 1:1,000). Subsequently,
each membrane was incubated for 1 h at room temperature with
secondary HRP-conjugated goat anti-rabbit or horse anti-mouse IgG
(1:5,000). The target proteins were detected using an enhanced
chemiluminescence solution (Daeil Lab Services Co., Ltd.). Protein
levels were quantified by scanning the immunoblots and analyzing
them with LabWorks software (version 4.6; UVP, Inc., Upland, CA,
USA).
Statistical analysis and experimental
replicates
Data are presented as the mean ± standard error of
the mean. Statistical differences between each result were assessed
by comparing with the LPS-treated group using the Mann-Whitney U
test. Mann-Whitney U test was performed using Prism 3.0 (GraphPad
Software, Inc., San Diego, CA, USA) and P<0.01 was considered to
indicate a statistically significant difference. The data from nine
replicates were analyzed, including three independent experiments
with three replicates in each.
Results
Determination of the non-cytotoxic
concentration of EGS in macrophages
Selective regulation of inflammatory mediators and
the identification of specific target signaling molecules are
valuable strategies for the development of novel anti-inflammatory
reagents. Based on this concept, the current study investigated a
number of natural plant extracts, which are known traditionally to
have pharmacological effects, and evaluated their inhibitory
effects on inflammation by measuring the production of NO, a major
inflammatory mediator, in LPS-stimulated RAW 264.7 macrophages. Of
the extracts that exerted an inhibitory effect on NO production,
EGS was selected as Garcinia species have been used
traditionally to treat numerous inflammatory diseases. Considering
that the anti-inflammatory effects of EGS should be studied at
concentrations that do not influence cell viability, the
non-cytotoxic and maximally effective concentrations of EGS were
identified by the dose-dependent treatment of LPS-stimulated RAW
264.7 macrophages. Cell viability was determined by measuring the
ability of cells to metabolically reduce a water-soluble
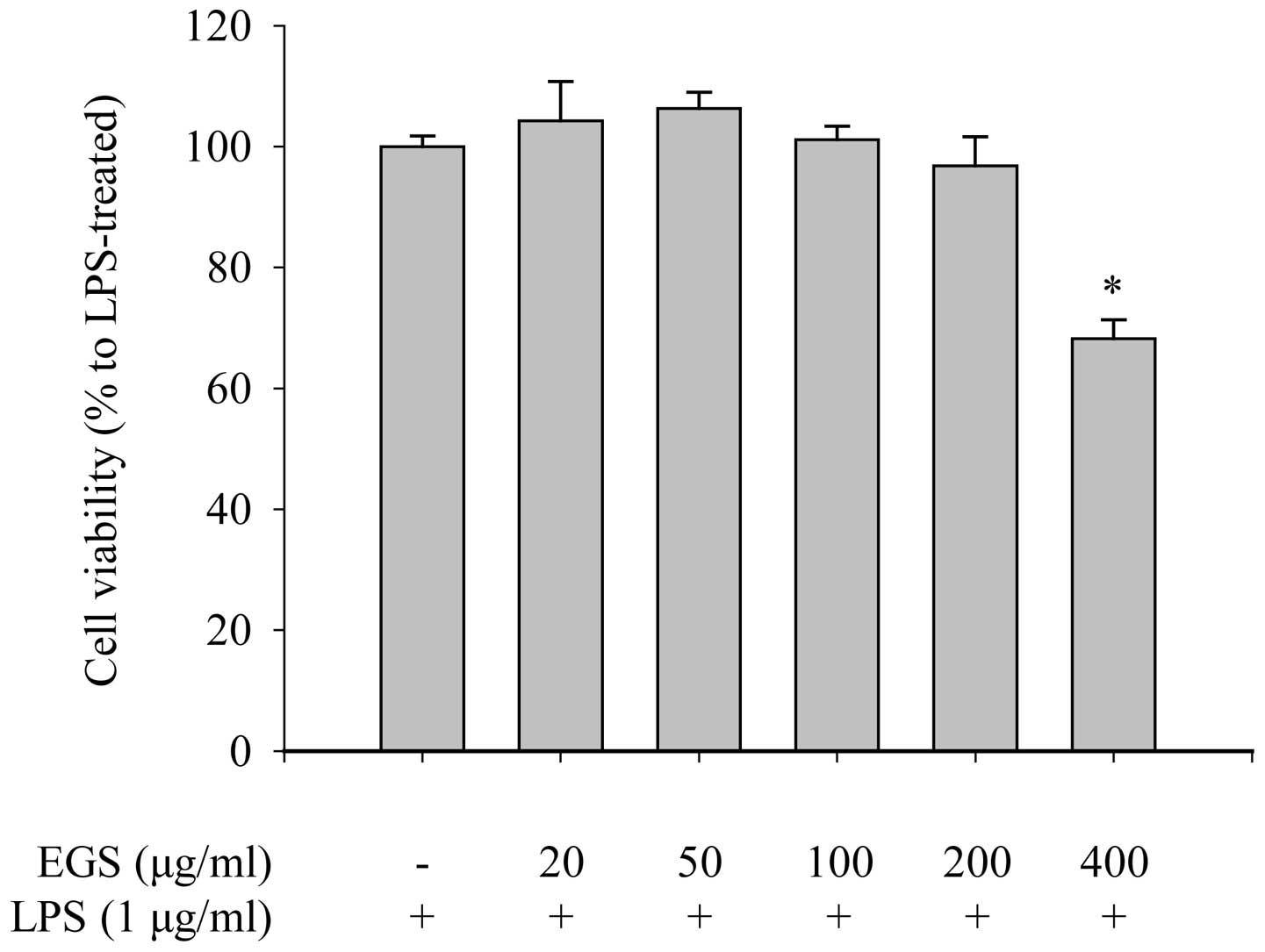
tetrazolium salt to a formazan dye. As shown in Fig. 1, the cell viability of the RAW
264.7 macrophages was not reduced by EGS treatment at doses of ≤200
µg/ml in the presence of 1 µg/ml LPS. However, a
number of dead cells (31.79±3.14%) were detected when the cells
were treated with 400 µg/ml EGS. These results suggest that
a low dose of EGS (200 µg/ml) does not affect the viability
of RAW 264.7 macrophages. Therefore, concentrations below 200
µg/ml were used in subsequent experiments.
Inhibitory effect of EGS on the
production of NO in LPS-stimulated macrophages
To evaluate the anti-inflammatory function of EGS in
macrophages, the profile of NO production was measured. As
expected, RAW 264.7 macrophages stimulated with LPS (1
µg/ml) produced a large quantity of NO compared with the
non-treated control group. When the cells were treated with EGS,
the enhanced production of NO by LPS was inhibited in a
dose-dependent manner, and the LPS-induced NO production was nearly
abolished at a dose of 200 µg/ml (Fig. 2A). Considering that NO production
is tightly regulated by iNOS expression, the effects of EGS on the
expression of iNOS mRNA and protein were investigated using PCR and
immunoblot analysis. The RT-qPCR results revealed that LPS notably
induces iNOS expression, which was inhibited by EGS in a
dose-dependent manner (Fig. 2B).
Semi-quantitative PCR data indicated a similar profile of
expression as the RT-qPCR data, which supported the inhibitory
effect of EGS on the expression of iNOS mRNA (Fig. 2C). In addition, LPS-induced
expression of iNOS protein was notably reduced by EGS in a
dose-dependent manner, and the maximum dose of EGS resulted in a
similar iNOS expression profile as the non-treated control group
(Fig. 2D). These results imply
that EGS inhibits NO production in activated macrophages by
modulating iNOS expression.
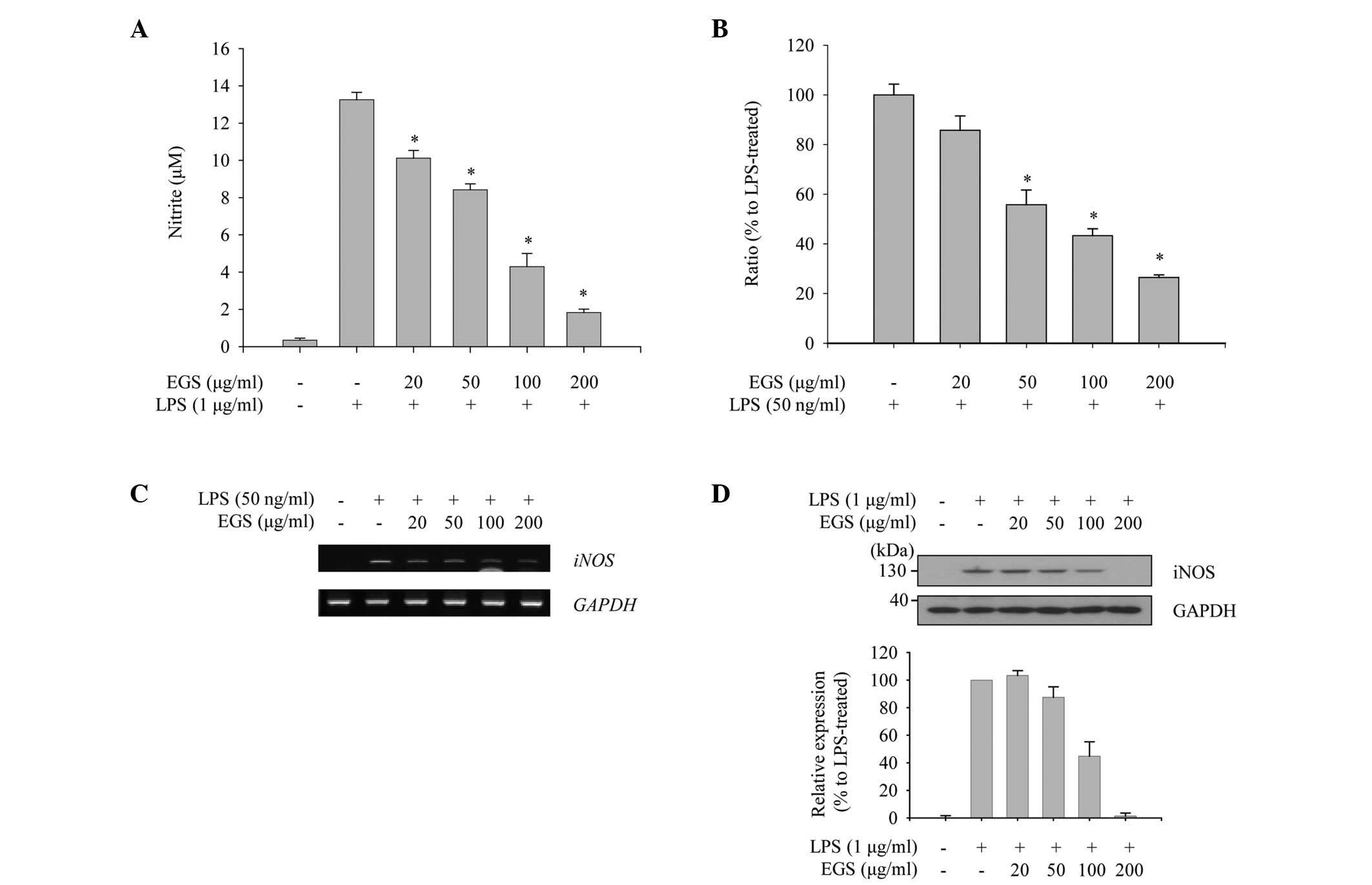 | Figure 2Inhibitory effects of EGS on the
production of NO. RAW 264.7 macrophages were treated simultaneously
with LPS and EGS (20, 50, 100 and 200 µg/ml) for the
indicated times. (A) Following 24 h stimulation, NO secretion in
the supernatants was measured using the Griess reagent. NO
secretion was calculated using a standard curve according to a
nitrite standard solution. (B) Following a 6 h stimulation, total
RNA was extracted and reverse transcribed to cDNA. iNOS was
amplified by reverse transcription-quantitative PCR, and the
expression of iNOS in each group was compared with the
LPS-treated group. (C) iNOS was amplified by PCR and
detected using a gel documentation system. GAPDH served as an
internal control. (D) Total cell lysates were prepared following 24
h stimulation and subjected to immunoblot analyses. The iNOS
protein expression was detected using an enhanced chemiluminescence
reagent. Expression levels were quantified by analysis with
LabWorks software and normalized to the corresponding GAPDH levels,
and are presented as relative expression levels. Data are presented
as the mean ± standard error. *P<0.01 vs. the
LPS-treated control group. EGS, ethanol extracts of Garcinia
subelliptica; NO, nitric oxide; LPS, lipopolysaccharide; iNOS,
inducible nitric oxide synthase; PCR, polymerase chain reaction;
GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
Inhibitory effect of EGS on the
expression of COX-2 in LPS-stimulated macrophages
As COX-2 is the enzyme responsible for the
production of PGE2 in activated macrophages, the present
study explored the effect of EGS on the expression levels of COX-2
mRNA and protein to measure its anti-inflammatory function. As
shown in Fig. 3A, induction of
COX-2 mRNA expression by LPS was detected by RT-qPCR
analysis. EGS treatment notably inhibited the enhanced expression
of COX-2 mRNA at concentrations of 100 and 200 µg/ml.
Furthermore, the inhibitory effect of EGS on the COX-2 mRNA
expression detected by RT-qPCR was supported by semi-quantitative
PCR (Fig. 3B). To examine the
effect of EGS on the expression of COX-2 protein in activated
macrophages, RAW 264.7 macrophages were treated with EGS in the
presence of LPS, and total cell lysates were prepared. The
increased levels of COX-2 protein expression mediated by LPS were
reduced markedly by EGS in a dose-dependent manner (Fig. 3C). From these data, COX-2
expression was observed to be downregulated by EGS, and the
subsequent production of PGE2 may therefore be regulated
by EGS.
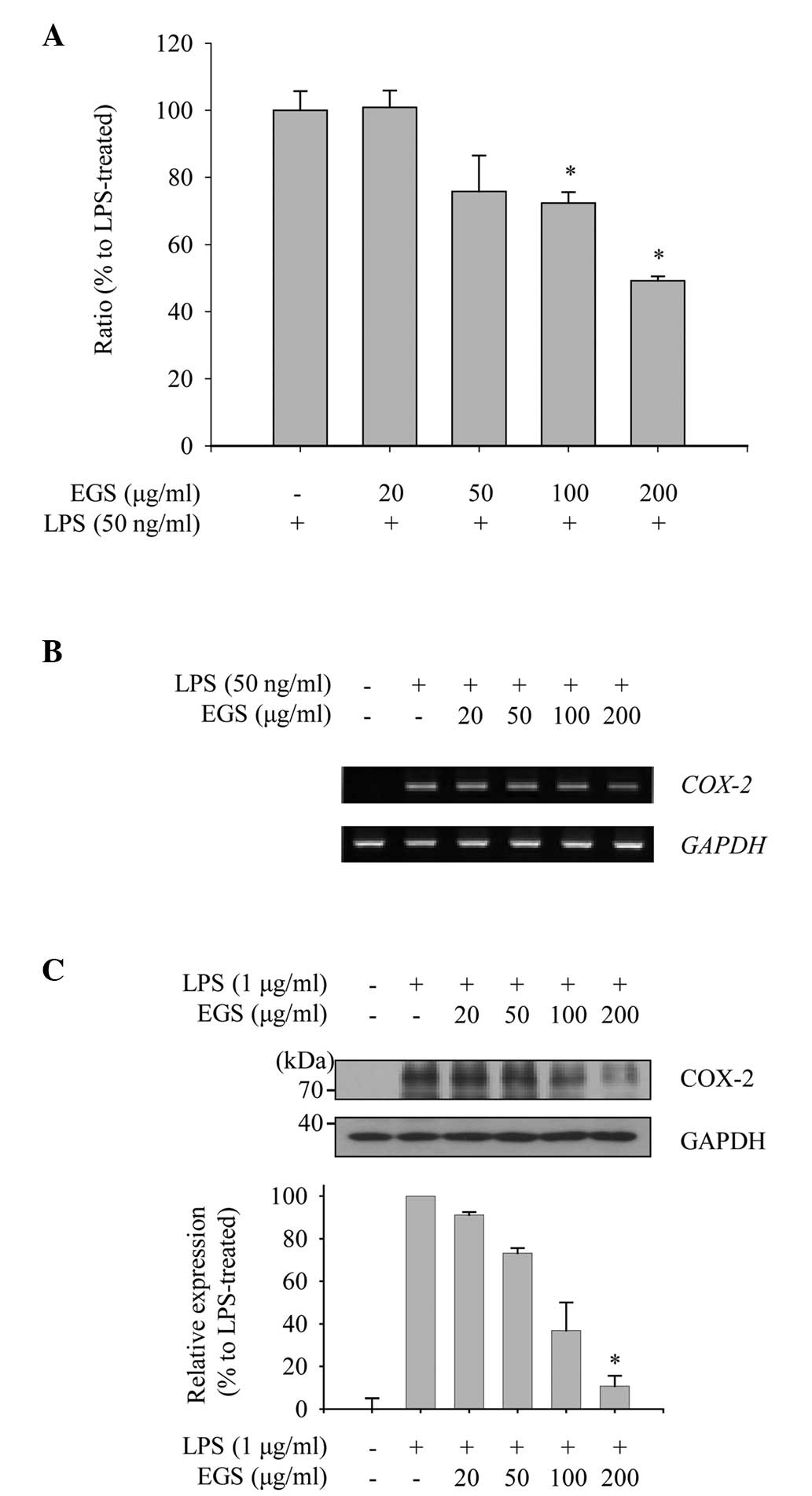 | Figure 3Effects of EGS on the expression of
COX-2. RAW 264.7 macrophages were treated simultaneously with LPS
and EGS (20, 50, 100 and 200 µg/ml) for the indicated times.
(A and B) Following 6 h stimulation, total RNA was extracted and
reverse transcribed to cDNA. (A) COX-2 was amplified by
reverse transcription-quantitative PCR, and the expression of
COX-2 in each group was compared with the LPS-treated group.
(B) COX-2 was amplified by PCR and detected using a gel
documentation system. GAPDH expression served as an internal
control. (C) Total cell lysates were prepared following 24 h
stimulation and subjected to immunoblot analyses. The COX-2 protein
expression was detected using an enhanced chemiluminescence
reagent, and expression levels were normalized to levels of the
GAPDH loading control, and are presented as relative expression
levels. Data are presented as the mean ± standard error.
*P<0.01 vs. the LPS-treated control group. EGS,
ethanol extracts of Garcinia subelliptica; COX-2,
cyclooxygenase 2; LPS, lipopolysaccharide; PCR, polymerase chain
reaction; GAPDH, glyceralde-hyde-3-phosphate dehydrogenase. |
Differential regulation of inflammatory
cytokine production by EGS in LPS-stimulated macrophages
To assess the anti-inflammatory properties of EGS in
activated macrophages, the effects of EGS on the production of
pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, were
measured in LPS-stimulated RAW 264.7 macrophages. Production of
IL-6 and TNF-α was increased notably following LPS stimulation
(Fig. 4A and B). However, EGS
treatment inhibited the production of IL-6 whilst not altering the
expression of TNF-α (Fig. 4A and
B). The mRNA expression of pro-inflammatory cytokines was
assessed to determine whether the production of pro-inflammatory
cytokines is regulated at the transcriptional level. As presented
in Fig. 4C, RT-qPCR analysis
indicated that EGS treatment inhibited the LPS-stimulated
expression of IL-6 and IL-1β but not TNF-α.
Similarly, semi-quantitative PCR analysis showed that EGS
selectively reduced the mRNA expression levels of IL-6 and
IL-1β (Fig. 4D). Taken
together, these results suggest that EGS negatively regulates the
LPS-induced production of pro-inflammatory cytokines by
transcriptional repression of IL-6 and IL-1β however,
not of TNF-α.
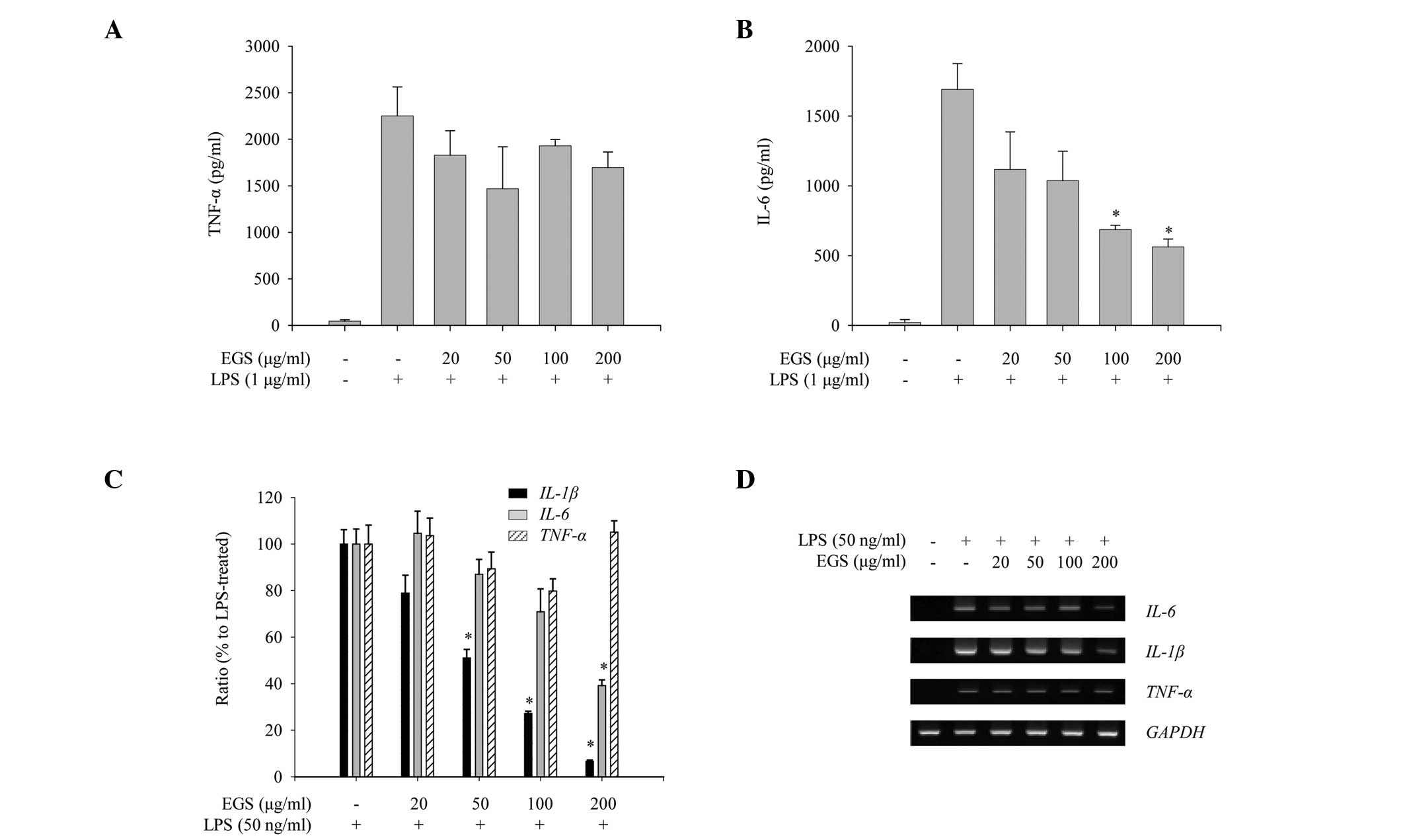 | Figure 4Inhibitory effects of EGS on the
production of pro-inflammatory cytokines. RAW 264.7 macrophages
were treated simultaneously with LPS and EGS (20, 50, 100 and 200
µg/ml) for the indicated times. Following 24 h stimulation,
enzyme-linked immunosorbent assays were used to measure the levels
of (A) TNF-α and (B) IL-6. The secretion of each cytokine was
determined using a standard curve. (C) Following a 6 h stimulation,
total RNA was extracted and reverse transcribed to cDNA.
IL-1β, TNF-α and, IL-6 were amplified by
reverse transcription-quantitative PCR, and the expression levels
of IL-1β, TNF-α and IL-6 in each group were
compared with those of the LPS-treated group. (D) IL-1β,
TNF-α and, IL-6 were amplified by PCR and detected
using a gel documentation system. GAPDH was used as a loading
control. Data are presented as the mean ± standard error.
*P<0.01 vs. the LPS-treated control group. EGS,
ethanol extracts of Garcinia subelliptica; LPS,
lipopolysaccharide; TNF-α, tumor necrosis factor α; IL,
interleukin; PCR, polymerase chain reaction; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
Inhibitory effect of EGS on the
activation of JNK in LPS-stimulated macrophages
The phosphorylation levels of JNK and IκB were
measured to investigate which inflammatory signaling pathways are
associated with the inhibitory effects of EGS in LPS-stimulated
macrophages. As shown in Fig. 5A,
LPS treatment altered the expression of p-IκBα and IκBα. However,
EGS did not affect the expression profiles of p-IκBα and IκBα. In
addition, the modulation of JNK phosphorylation by EGS was
measured. Immunoblot analyses revealed that EGS inhibited the
phosphorylation of JNK without affecting the protein levels of JNK.
EGS did not influence the phosphorylation or protein expression of
additional MAPK pathway mediators, including ERK and p38.
Collectively, these results suggest that EGS exerts
anti-inflammatory effects by inhibiting the activation of JNK.
 | Figure 5Inhibitory effects of EGS on NF-κB
and MAPK activation. RAW 264.7 macrophages were pretreated with
various concentrations of EGS (20, 50, 100 and 200 µg/ml)
for 1 h and then stimulated with LPS for 15 min. Total cell lysates
were prepared and subjected to immunoblot analyses. The expression
levels of (A) p-IκBα, IκBα, (B) p-JNK, JNK, p-ERK, ERK, p-p38 and
p38 were detected using specific antibodies. Relative expression
levels of IκBα and p-IκBα were normalized to GAPDH levels. Levels
of p-JNK and p-ERK were normalized to the JNK and ERK levels,
respectively. Quantitative analyses of phosphorylation and protein
levels are shown following normalization (lower panel). Data are
presented as the mean ± standard error. EGS, ethanol extracts of
Garcinia subelliptica; NF-κB, nuclear factor-κB; MAPK,
mitogen-activated protein kinase; LPS, lipopolysaccharide; p-,
phosphorylated; IκBα, inhibitor of κB protein; JNK, c-Jun
N-terminal kinase; ERK, extracellular signal-regulated kinase
TNF-α, tumor necrosis factor α; IL, interleukin; PCR, polymerase
chain reaction; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
Discussion
In the present study, EGS was observed to markedly
inhibit the production of IL-6 and IL-1β, however, not TNF-α, in
LPS-stimulated RAW 264.7 macrophages. Numerous reports have
indicated that interleukins and TNF-α are not simultaneously
inhibited by natural compounds (38,39).
One possibility for the differential regulation of pro-inflammatory
cytokines by EGS is that they possess different promoter binding
regions for transcription factors. Previous studies have
demonstrated that the signal transducer and activator of
transcription (STAT) protein-binding region is contained in the
IL-6 and IL-1β promoter regions, however, not in the TNF-α promoter
region (40). This implies that
the specific regulation of IL-6 and IL-1β production by EGS in
macrophages may be regulated through the inactivation of STAT
signaling.
In the present study, LPS treatment of murine
macrophages significantly enhanced the production of inflammatory
mediators via the stimulation of the MAPK pathway; thus, the
inhibition of p38, ERK and JNK phosphorylation may represent
potential target pathways for the alleviation of severe
inflammatory states (2,8,41).
However, numerous reports have suggested that MAPK signaling
cascades are differentially involved in the production of
inflammatory mediators by macrophages (42–44).
Watters et al (45)
suggested that the MAPK/ERK pathway is not essential for the
production of iNOS and IL-1β in macrophages. Caivano (46) and Paul et al (47) reported that the p38 and MAPK/ERK
pathways are not essential for the production of inflammatory
mediators. In the present study, EGS inhibited the LPS-induced
phosphorylation of JNK, however, the total MAPK levels, including
p38, ERK and JNK, were unaltered. Collectively, these results
suggest that the regulation of JNK activity, rather than total JNK
expression, is the key regulatory mechanism of EGS-mediated
inhibition of inflammatory mediators.
Considering that macrophages serve pivotal roles in
the pathogenesis of many inflammatory diseases, the EGS-mediated
selective regulation of inflammatory mediators suggests that EGS
may have therapeutic potential against inflammatory diseases.
However, further studies are required to analyze the major
components of EGS that are responsible for the reduction of
inflammatory mediators and to elucidate the exact mechanism
underlying the differences in the production of the
pro-inflammatory cytokines, IL-6 and TNF-α.
Acknowledgments
The current study was supported by a Chung-Ang
University Research Grant in 2014 and a National Research
Foundation of Korea (NRF) grant funded by the Ministry of
Education, Science and Technology (grant no.
NRF-2013R1A1A2062389).
References
|
1
|
Boscá L, Zeini M, Través PG and Hortelano
S: Nitric oxide and cell viability in inflammatory cells: A role
for NO in macrophage function and fate. Toxicology. 208:249–258.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujihara M, Muroi M, Tanamoto K, Suzuki T,
Azuma H and Ikeda H: Molecular mechanisms of macrophage activation
and deactivation by lipopolysaccharide: Roles of the receptor
complex. Pharmacol Ther. 100:171–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones BW, Heldwein KA, Means TK, Saukkonen
JJ and Fenton MJ: Differential roles of toll-like receptors in the
elicitation of proinflammatory responses by macrophages. Ann Rheum
Dis. 60(Suppl 3): iii6–iii12. 2001.
|
|
6
|
Jones BW, Means TK, Heldwein KA, Keen MA,
Hill PJ, Belisle JT and Fenton MJ: Different toll-like receptor
agonists induce distinct macrophage responses. J Leukoc Biol.
69:1036–1044. 2001.PubMed/NCBI
|
|
7
|
Rhee SH and Hwang D: Murine TOLL-like
receptor 4 confers lipopolysaccharide responsiveness as determined
by activation of NF kappa B and expression of the inducible
cyclooxygenase. J Biol Chem. 275:34035–34040. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schroder K, Sweet MJ and Hume DA: Signal
integration between IFNgamma and TLR signalling pathways in
macrophages. Immunobiology. 211:511–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stalińska K, Guzdek A, Rokicki M and Koj
A: Transcription factors as targets of the anti-inflammatory
treatment. A cell culture study with extracts from some
mediterranean diet plants. J Physiol Pharmacol. 56(Suppl 1):
S157–S169. 2005.
|
|
10
|
Szabó C and Thiemermann C: Regulation of
the expression of the inducible isoform of nitric oxide synthase.
Adv Pharmacol. 34:113–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clancy RM, Amin AR and Abramson SB: The
role of nitric oxide in inflammation and immunity. Arthritis
Rheumatol. 41:1141–1151. 1998. View Article : Google Scholar
|
|
12
|
Guadagni F, Ferroni P, Palmirotta R,
Portarena I, Formica V and Roselli M: Review. TNF/VEGF cross-talk
in chronic inflammation-related cancer initiation and progression:
An early target in anticancer therapeutic strategy. In Vivo.
21:147–161. 2007.PubMed/NCBI
|
|
13
|
Kröncke KD, Fehsel K and Kolb-Bachofen V:
Inducible nitric oxide synthase in human diseases. Clin Exp
Immunol. 113:147–156. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nathan C and Xie QW: Nitric oxide
synthases: Roles, tolls and controls. Cell. 78:915–918. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nathan C and Xie QW: Regulation of
biosynthesis of nitric oxide. J Biol Chem. 269:13725–13728.
1994.PubMed/NCBI
|
|
16
|
Nishimoto N and Kishimoto T: Interleukin
6: From bench to bedside. Nat Clin Pract Rheumatol. 2:619–626.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calixto JB: Efficacy, safety, quality
control, marketing and regulatory guidelines for herbal medicines
(phytotherapeutic agents). Braz J Med Biol Res. 33:179–189. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wutthithamavet W: Thai traditional
medicine. revised edition. Odean Store Press; Bangkok: 1997
|
|
19
|
Tewtrakul S, Wattanapiromsakul C and
Mahabusarakam W: Effects of compounds from Garcinia mangostana on
inflammatory mediators in RAW264.7 macrophage cells. J
Ethnopharmacol. 121:379–382. 2009. View Article : Google Scholar
|
|
20
|
Cui J, Hu W, Cai Z, Liu Y, Li S, Tao W and
Xiang H: New medicinal properties of mangostins: Analgesic activity
and pharmacological characterization of active ingredients from the
fruit hull of Garcinia mangostana L. Pharmacol Biochem Behav.
95:166–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duke JA: Handbook of medical herbs.
Library of congress; New York: 1985
|
|
22
|
Evan WC: Trease and Evans pharmacognosy.
Saunders Company Limited; Nottingham: 1996
|
|
23
|
Panthong A, Norkaew P, Kanjanapothi D,
Taesotikul T, Anantachoke N and Reutrakul V: Anti-inflammatory,
analgesic and antipyretic activities of the extract of gamboge from
Garcinia hanburyi Hook f. J Ethnopharmacol. 111:335–340. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guimarães CL, Otuki MF, Beirith A and
Cabrini DA: A review on the therapeutic potential of Garcinia
gardneriana. Dynamis. 12:6–12. 2004.In Portuguese.
|
|
25
|
Castardo JC, Prudente AS, Ferreira J,
Guimarães CL, Monache FD, Filho VC, Otuki MF and Cabrini DA:
Anti-inflammatory effects of hydroalcoholic extract and two
biflavonoids from Garcinia gardneriana leaves in mouse paw oedema.
J Ethnopharmacol. 118:405–411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ayepola OR, Chegou NN, Brooks NL and
Oguntibeju OO: Kolaviron, a Garcinia biflavonoid complex
ameliorates hyperglycemia-mediated hepatic injury in rats via
suppression of inflammatory responses. BMC Complement Altern Med.
13:3632013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Farombi EO, Adedara IA, Ajayi BO, Ayepola
OR and Egbeme EE: Kolaviron, a natural antioxidant and
anti-inflammatory phyto-chemical prevents dextran sulphate
sodium-induced colitis in rats. Basic Clin Pharmacol Toxicol.
113:49–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li F, Shanmugam MK, Chen L, Chatterjee S,
Basha J, Kumar AP, Kundu TK and Sethi G: Garcinol, a
polyisoprenylated benzophenone modulates multiple proinflammatory
signaling cascades leading to the suppression of growth and
survival of head and neck carcinoma. Cancer Prev Res (Phila).
6:843–854. 2013. View Article : Google Scholar
|
|
29
|
Tsai ML, Chiou YS, Chiou LY, Ho CT and Pan
MH: Garcinol suppresses inflammation-associated colon
carcinogenesis in mice. Mol Nutr Food Res. 58:1820–1829. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Minami H, Takahashi E, Fukuyama Y, Kodama
M, Yoshizawa T and Nakagawa K: Novel xanthones with superoxide
scavenging activity from Garcinia subelliptica. Chem Pharm Bull
(Tokyo). 43:347–349. 1995. View Article : Google Scholar
|
|
31
|
Minami H, Hamaguchi K, Kubo M and Fukuyama
Y: A benzophenone and a xanthone from Garcinia subelliptica.
Phytochemistry. 49:1783–1785. 1998. View Article : Google Scholar
|
|
32
|
Ito T, Yokota R, Watarai T, Mori K, Oyama
M, Nagasawa H, Matsuda H and Iinuma M: Isolation of six
isoprenylated biflavonoids from the leaves of Garcinia
subelliptica. Chem Pharm Bull (Tokyo). 61:551–558. 2013. View Article : Google Scholar
|
|
33
|
Weng JR, Tsao LT, Wang JP, Wu RR and Lin
CN: Anti-inflammatory phloroglucinols and terpenoids from Garcinia
subelliptica. J Nat Prod. 67:1796–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weng JR, Lin CN, Tsao LT and Wang JP:
Terpenoids with a new skeleton and novel triterpenoids with
anti-inflammatory effects from Garcinia subelliptica. Chemistry.
9:5520–5527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weng JR, Lin CN, Tsao LT and Wang JP:
Novel and anti-inflammatory constituents of Garcinia subelliptica.
Chemistry. 9:1958–1963. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
37
|
Koressaar T and Remm M: Enhancements and
modifications of primer design program Primer3. Bioinformatics.
23:1289–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Samavati L, Rastogi R, Du W, Hüttemann M,
Fite A and Franchi L: STAT3 tyrosine phosphorylation is critical
for interleukin 1 beta and interleukin-6 production in response to
lipopolysaccharide and live bacteria. Mol Immunol. 46:1867–1877.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu ZG, Jin H, Yu PJ, Tian YX, Zhang JJ
and Wu SG: Mollugin Inhibits the inflammatory response in
lipopolysaccharide-stimulated RAW264.7 macrophages by blocking the
Janus kinase-signal transducers and activators of transcription
signaling pathway. Biol Pharm Bull. 36:399–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee C, Lim HK, Sakong J, Lee YS, Kim JR
and Baek SH: Janus kinase-signal transducer and activator of
transcription mediates phosphatidic acid-induced interleukin
(IL)-1beta and IL-6 production. Mol Pharmacol. 69:1041–1047.
2006.
|
|
41
|
Cario E, Rosenberg IM, Brandwein SL, Beck
PL, Reinecker HC and Podolsky DK: Lipopolysaccharide activates
distinct signaling pathways in intestinal epithelial cell lines
expressing toll-like receptors. J Immunol. 164:966–972. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burk DR, Senechal-Willis P, Lopez LC,
Hogue BG and Daskalova SM: Suppression of
lipopolysaccharide-induced inflammatory responses in RAW 264.7
murine macrophages by aqueous extract of Clinopodium vulgare L.
(Lamiaceae). J Ethnopharmacol. 126:397–405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu H, Bargouti M, Zughaier S, Zheng Z,
Liu Y, Sangadala S, Boden SD and Titus L: Osteoinductive LIM
mineralization protein-1 suppresses activation of NF-kappaB and
selectively regulates MAPK pathways in pre-osteoclasts. Bone.
46:1328–1335. 2010. View Article : Google Scholar :
|
|
44
|
Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo
L and Yin Z: Chlorogenic acid inhibits lipopolysaccharide-induced
cyclo-oxygenase-2 expression in RAW264.7 cells through suppressing
NF-kappaB and JNK/AP-1 activation. Int Immunopharmacol.
9:1042–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watters JJ, Sommer JA, Pfeiffer ZA, Prabhu
U, Guerra AN and Bertics PJ: A differential role for the
mitogen-activated protein kinases in lipopolysaccharide signaling:
The MEK/ERK pathway is not essential for nitric oxide and
interleukin 1beta production. J Biol Chem. 277:9077–9087. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Caivano M: Role of MAP kinase cascades in
inducing arginine transporters and nitric oxide synthetase in
RAW264 macrophages. FEBS Lett. 429:249–253. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Paul A, Cuenda A, Bryant CE, Murray J,
Chilvers ER, Cohen P, Gould GW and Plevin R: Involvement of
mitogen-activated protein kinase homologues in the regulation of
lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not
nitric oxide synthase in RAW 264.7 macrophages. Cell Signal.
11:491–497. 1999. View Article : Google Scholar : PubMed/NCBI
|