Introduction
Osteoarthritis (OA) is a type of chronic,
progressive and common clinical articular cartilage disease. It is
also termed hypertrophic arthritis or degenerative arthritis; the
pathological characteristics of the disease include degeneration of
articular cartilage, sclerosis of subcartilaginous bone, reactive
hyperplasia of subcartilaginous bone and osteophyte formation
(1). Primary OA predominantly
occurs in middle-aged and elderly people, and the disease results
from the disturbance of catabolism and anabolism in chondrocytes
and the extracellular matrix with the common effects of biological
and mechanical factors (2). The
practice of high-loaded and antagonistic types of physical exercise
has been increasing, such that the young population are inflicting
excessive pressure on their knee joints, which may lead to serious
knee joint injuries (3). Excessive
wear and injury of articular cartilage, which is predominantly
mediated by mechanical factors, may result in the aging and
degeneration of articular cartilage, which gives rise to pain,
lesions and dysfunctions of the joints (4,5). At
present, the pathogenesis of exercise-induced OA in the area of
sports medicine remains unknown, therefore, it is of significant
importance to investigate its pathogenesis.
Exercise-induced OA is a type of OA, where the
inflammatory signaling pathway-associated cytokines, including
interleukin 1β, tumor necrosis factor α, serve an important role in
the disturbance of catabolism and anabolism in chondrocytes and the
extracellular matrix. It is the inflammatory cytokines that alter
the metabolism of chondrocytes, the histological structures of the
extracellular matrix and the activation of matrix
metalloproteinase, which lead to disturbance in the synthesis and
degradation of cartilage matrix, thus resulting in the development
of OA (6,7). However, how the cytokines transduce
signaling into the chondrocytes and regulate the inflammatory genes
remains unclear.
β-catenin is a multifunctional protein in the
cytoplasm, and a key molecule in the Wnt signaling pathway for
regulating gene transcription (8).
The canonical Wnt/β-catenin pathway serves a key role in the
process of formation and differentiation of chondrocytes and the
metabolism in the extracellular matrix. The inactivation of
β-catenin may lead to the differentiation of chondrocytes and cell
death (9). However, the
association between the canonical Wnt/β-catenin pathway and the
pathogenesis of exercise-induced OA remains to be fully
elucidated.
The present study aimed to investigate the molecular
pathogenesis of the canonical Wnt/β-catenin pathway in
exercise-induced OA by establishing a exercise-induced OA rat
model, and detecting the key genes and proteins of the canonical
Wnt/β-catenin pathway by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), western blotting and
immunohistochemical staining.
Materials and methods
Experimental animals
A total of 30 healthy male Sprague-Dawley rats (age,
5–6 weeks; weight, 160–230 g) were were provided by the Laboratory
Animal Center of Xi'an Jiaotong University Health Science Center
(Xi'an, China) for use in the present study. The rats were raised
in individual cages and had ad libitum access to food and
water. The feed-stuff was provided by the Laboratory Animal Center
of Xi'an Jiaotong University Health Science Center, according to
national standards of rodent animal feed. Indoor temperature
25±3°C, relative humidity 55–75%. The present study was approved by
the ethics committee of Langfang Teachers University (Langfang,
China).
Experimental methods
Animal groups
Following feeding for 5 days in order to adapt to
the environment, the 30 Sprague-Dawley rats were randomly divided
into three groups (n=10/group), the control, normal
exercise-induced OA and injured exercise-induced OA groups. The
rats in the control group did not undergo operation nor
training.
Establishment of the exercise-induced
OA rat model
In each rat of the injured exercise-induced OA model
group, the anterior and posterior cruciate ligaments of their right
knee joints were cut. Firstly, the rats were anesthetized by
intraperitoneal injection with 10% chloral hydrate (0.3 ml/100 g;
Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and hair
around the surgical area was shaved and washed. The operative area
was then sterilized with iodine (GE Healthcare Life Sciences,
Logan, UT, USA), after which an incision into the skin and
articular cavity of the medial side of the right knee joint was
made. The patella was turned inside-out, with the knee flexed as to
expose the articular cavity and enable the anterior and posterior
cruciate ligaments to be cut. The above steps were conducted with
care to ensure there was no injury to the cartilage. Finally, the
articular capsules and the skin were sutured closed subsequent to
washing of the joint with normal saline, then the wounds were
cleaned with iodine. Each rat in this group received an
intramuscular injection of 200,000 U penicillin (GE Healthcare Life
Sciences) for anti-inflammation. Subsequent to 7 days of wound
healing, the rats in the injured exercise-induced OA model and
normal exercise-induced OA model groups underwent training with the
animal experimental treadmill to establish the exercise-induced OA
model. The first week involved the rats adapting to the treadmill
and the exercise load; the exercise intensity was gradually
increased (the speed of treadmill was gradually increased from 10
m/min to 16 m/min). The formal training commenced in the second
week; the speed of the treadmill was maintained at 16 m/min and the
exercise duration was 30 min. Training lasted two weeks, and each
week there were six training days and one rest day.
Tissue preparation and cell
culture
Subsequent to establishment of the exercise-induced
OA model, the rats were anesthetized by intraperitoneal injection
with 10% chloral hydrate (0.3 ml/100 g), prior to being sacrificed
by decapitation. The right knee cartilage of each rat was
collected. Samples were sequentially digested by trypsin (0.5%) and
type II collagenase (0.2%; both GE Healthcare Life Sciences). Cells
were seeded at a density of 1.5×104 cells/cm2
in Dulbecco's modified Eagle's medium/F12 with 10% fetal bovine
serum in 25 cm2 culture flasks and were cultured until
they reached confluence.
RT-qPCR
In order to determine the mRNA expression levels of
catenin (cadherin-associated protein) β1 (Ctnnb1), bone
morphogenetic protein 2 (BMP-2), sex determining region Y-box 9
(Sox-9) and runt-related transcription factor 2 (Runx-2) in three
groups of chondrocytes, RT-qPCR was conducted. Total RNA was
isolated from chondrocytes using TRIzol reagent (Baosheng Biology
Co., Ltd., Dalian, China), and mRNA (0.1 µg) was converted
to cDNA using the RevertAid™ First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. Primer sequences are listed in Table I. All primer and probe sets were
supplied by TaqMan® Gene Expression Assays (Applied
Biosystems; Thermo Fisher Scientific, Inc.). qPCR was performed
using 2.0 µl cDNA, the SYBR® Premix Ex Taq™ II
kit (Takara Bio, Inc., Otsu, Japan) and the FTC 3000 Real-Time PCR
System (Funglyn Biotech Inc., Toronto, ON, Canada). The PCR cycling
conditions were as follows: 95°C for 30 sec, followed by 40 cycles
of 95°C for 5 sec and 60°C for 30 sec. The relative fold-change for
each individual gene, as normalized to the reference gene
(β-actin), was calculated using the 2−ΔΔCq method
(10). The PCR results were
analyzed using the Bio-Rad iQ5 software, version 2.1 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All assays were performed
in triplicate.
 | Table IPrimers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Name | 5′ Primer
sequence | 3′ Primer
sequence | Amplicon size
(bp) |
|---|
| Runx-2 |
GCCCAGGCGTATTTCAGATG |
GGTAAAGGTGGCTGGGTAGT | 193 |
| BMP-2 |
GCAAGAACAAAGCAGGACCA |
TGCTTCTTTATGAGGGCCCA | 212 |
| Ctnnb1 |
AGACAGCTCGTTGTACTGCT |
GTGTCGTGATGGCGTAGAAC | 150 |
| Sox-9 |
CAAGAACAAGCCACACGTCA |
GTGGTCTTTCTTGTGCTGCA | 141 |
| β-Actin |
CGGAGTCAACGGATTTGGTCGTAT |
AGCCTTCTCCATGGTGGTGAAGAC | 120 |
Western blot analysis
Proteins were extracted from chondrocytes using the
Tissue Protein Extraction kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The concentration of
total protein was quantified using the NanoDrop ND-1000 (Thermo
Fisher Scientific, Inc., Wilmington, DE, USA). A total of 12
µl total protein was loaded per lane and electrophoresed in
12% sodium dodecyl sulfate-polyacrylimide gel electrophoresis using
a constant voltage of 80 V for 2 h. The proteins were then
transferred onto a 0.45-nitrocellulose membrane (Hybond-ECL; GE
Healthcare Life Sciences) at 350 mA for 90 min and blocked with 5%
nonfat milk at room temperature for 2 h. The membranes were
incubated overnight at 4°C with mouse anti-rat collagen II
monoclonal antibody (mAb; 1:500; hm1062; Abgent, Inc., San Diego,
CA, USA), mouse anti-rat Wnt-3a polyclonal antibody (1:50; 2391S;
Cell Signaling Technology, Inc., Danvers, MA, USA), mouse anti-rat
β-catenin mAb (1:50; sc-59737; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), mouse anti-rat Mmp-13 polyclonal antibody (1:50;
bs668; Bioworld Technology, Inc., St. Louis Park, MN, USA) and
mouse anti-rat β-actin monoclonal antibody (1:3,000; CW0096;
Abgent, Inc.). Subsequently the membranes were washed three times
with Tris-buffered saline containing 5% Tween-20 (Sinopharm
Chemical Reagent Co., Ltd.) for 5 min each, prior to incubation
with horseradish peroxidase-conjugated goat anti-mouse polyclonal
IgG (1:4,000; bs-0296p; BIOSS, Beijing, China) at 4°C for 2 h,
immunoreactive proteins were visualized on an X-ray film (Sinopharm
Chemical Reagent Co., Ltd.,) with an Enhanced Chemiluminescence kit
(NEN Life Science Products, Inc., Boston, MA, USA). Quantification
of band intensity was conducted using Gel-Pro Analyzer 4.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Immunohistochemical staining of Wnt-3a
and β-catenin
Immunohistochemical staining was performed to
investigate the expression of key proteins involved in the
canonical Wnt/β-catenin pathway (i.e. Wnt-3a and β-catenin) in knee
cartilage from rats in the normal exercise-induced OA, injured
exercise-induced OA and control groups. Paraffin-embedded
(Sinopharm Chemical Reagent Co., Ltd.) 5-µm cartilage
sections were prepared using a histotome (Sinopharm Chemical
Reagent Co., Ltd.), and were then deparaffinized in xylene
(Sinopharm Chemical Reagent Co., Ltd.) and rehydrated in a graded
ethanol series and water. Sections were treated with 3% hydrogen
peroxide (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) for 10 min, washed with phosphate-buffered saline
(PBS; Sinopharm Chemical Reagent Co., Ltd.) and incubated in 10 M
urea solution (GE Healthcare Life Sciences) and trypsin at 37°C for
20 min to unmask the antigens. Subsequent to blocking with 5% goat
serum (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 20
min at room temperature, mouse polyclonal Wnt-3a and mouse
monoclonal β-catenin antibody (1:50 dilution), as well as the
anti-mouse IgG (1:4,000) antibody, were applied and incubated
overnight at 4°C. Subsequent to washing with PBS, sections were
incubated using the SAP kit (Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.). The substrate 3,3′-diaminobenzidine
(Sinopharm Chemical Reagent Co., Ltd.) was added to stain sections
with hematoxylin (Sinopharm Chemical Reagent Co., Ltd.)
counter-staining. Finally, sections were dehydrated and mounted
under coverslips. Wnt-3a and β-catenin localization in each
cartilage zone was assessed systematically by counting the number
of positive cells under the SZX16 Stereo Microscope (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
Data were presented as the mean ± standard
deviation, and tested for normality and equal variance. Statistical
analysis was performed using SPSS software, version 18.0 (SPSS,
Inc., Chicago, IL, USA). Differences in the means were determined
by one-way analysis of variance for multiple comparisons followed
by the least significant difference test for two-group comparisons
with multiple comparisons. The Student's t-test was applied to
determine differences between two groups. The normality and
homogeneity of variance of data were tested prior to statistical
analysis. The nonparametric test (Kruskal Wallis) was used when the
conditions for normality were not fulfilled. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of Runx-2, BMP-2, Ctnnb1 and
Sox-9 mRNA in the cartilage of the three groups
The mRNA expression levels of Runx-2, BMP-2, Ctnnb1
and Sox-9 were detected using RT-qPCR. As presented in Fig. 1, the results demonstrated that the
mRNA expression levels of Runx-2, BMP-2 and Ctnnb1 were
significantly increased in the injured exercise-induced OA model
and the normal exercise-induced OA model groups, as compared with
the control group. In addition, the mRNA expression levels of
Runx-2, BMP-2 and Ctnnb1 were significantly increased in the
injured exercise-induced OA model group, as compared with the
normal exercise-induced OA model group. Conversely, the mRNA
expression levels of Sox-9 were significantly reduced in the
injured exercise-induced OA model and the normal exercise-induced
OA model groups, as compared with control group. No significant
differences were observed between the injured exercise-induced OA
model and normal exercise-induced OA model groups in the expression
of Sox-9 mRNA.
 | Figure 1mRNA expression levels of Runx-2,
BMP-2, Ctnnb1 and Sox-9 in groups A, B and C. *P<0.05
and &P<0.01 between groups. Runx-2, runt-related
transcription factor 2; BMP-2, bone morphogenetic protein 2;
Ctnnb1, catenin (cadherin-associated protein) β1, Sox-9, sex
determining region Y-box 9; group A, injured exercise-induced
osteoarthritis group; group B, normal exercise-induced
osteoarthritis group; group C, control group. |
Expression of collagen II, Mmp-13, Wnt-3a
and β-catenin at the protein level in the three groups
Western blot analysis was conducted to determine the
protein expression levels of collagen II, Mmp-13, Wnt-3a and
β-catenin. As presented in Fig. 2,
there was a significantly reduced level of collagen II expression
in the injured exercise-induced OA model and normal
exercise-induced OA model groups, as compared with the control, and
expression in the injured exercise-induced OA model group was
significantly reduced compared with the normal exercise-induced OA
model group (P<0.05). Wnt-3, β-catenin, Mmp-13 were all
significantly overexpressed in the injured exercise-induced OA
model group and the normal exercise-induced OA model groups, as
compared with the normal control. In the expression of Wnt-3 and
Mmp-13, the injured exercise-induced OA model group was observed to
exhibit significantly greater expression, as compared with the
normal exercise-induced OA model group (Fig. 2; Table II).
 | Table IIProtein expression levels of
chondrocytes in three groups, as measured by western blotting. |
Table II
Protein expression levels of
chondrocytes in three groups, as measured by western blotting.
| Protein | Expression in
chondrocytes (densitometry, X/β-actin)
|
|---|
| Group A | Group B | Group C |
|---|
| Collagen II | 0.34 (0.25–0.43) | 0.56 (0.42–0.71) | 0.68 (0.27–1.09) |
| Mmp-13 | 1.92 (1.49–2.34) | 1.35 (1.12–1.57) | 0.74 (0.54–1.15) |
| β-Catenin | 0.95 (0.53–1.37) | 0.89 (0.78–1.00) | 0.47 (0.22–0.73) |
| Wnt-3a | 0.62 (0.38–0.86) | 0.34 (0.17–0.43) | 0.23 (0.17–0.30) |
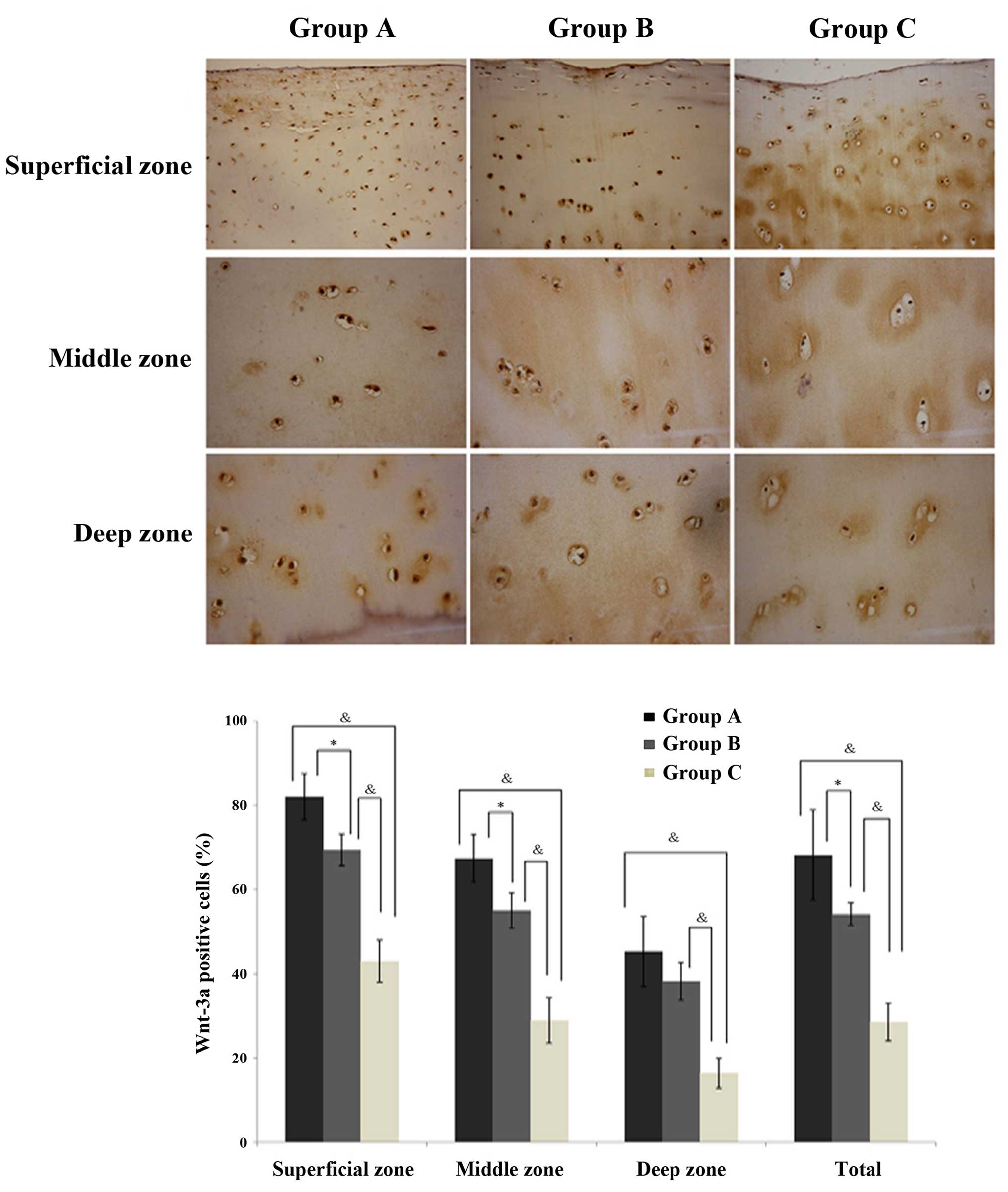
Immunostaining of Wnt-3a and
β-catenin
In the cartilage, Wnt-3a (Fig. 3) and β-catenin (Fig. 4) expression levels were observed to
be significantly increased in the normal exercise-induced OA and
injured exercise-induced OA groups, as compared with the control
group. Additionally, the expression was identified to be
significantly greater in the injured exercise-induced OA group, as
compared with the normal exercise-induced OA group (Figs. 3 and 4; Table
III). The same trends were observed in every zone, a gradual
reduction in protein expression in the superficial, middle and deep
zones across the injured exercise-induced OA, normal
exercise-induced OA and control group cartilages. Thus, it was
established that Wnt-3a and β-catenin are highly expressed in the
cartilages of the normal exercise-induced OA and injured
exercise-induced OA groups.
 | Table IIIProtein expression in chondrocytes in
the three groups, as determined using immunohistochemistry. |
Table III
Protein expression in chondrocytes in
the three groups, as determined using immunohistochemistry.
| Protein | Expression in
chondrocytes (positive cell rate)
|
|---|
| Group A | Group B | Group C |
|---|
| Wnt-3a | 0.62 (0.35–0.88) | 0.54 (0.50–0.58) | 0.28 (0.17–0.29) |
| β-Catenin | 0.68 (0.38–0.86) | 0.50 (0.47–0.54) | 0.28 (0.17–0.29) |
Discussion
OA is a type of chronic arthritis that is
characterized by degeneration and deficiency of articular cartilage
and hyperostosis of subchondral bone and the edges of joints
(11,12). OA is the most common in the knee,
and this is representative of OA, manifesting with biochemical and
metabolic disturbances and the damage of the articular cartilage
structure. Local structural cartilage damage combines inflammation
of the synovial membrane and fibroplasia of the joint capsule,
which finally lead to pain, swelling and dysfunction of knee
joints, potentially resulting in disability (13–15).
OA associated with exercise predominantly occurs as a result of
excessive abrasiveness and may lead to joint aging and early joint
degeneration (7,16,17).
The present study aimed to investigate the molecular mechanisms of
the key genes and proteins in the canonical Wnt/β-catenin pathway
in the sport injury-induced OA by establishing the exercise-induced
OA rat model.
Wnt signaling pathway regulates numerous processes,
including growth, development, disease, aging and death, cellular
morphology and function (18,19).
The embryonic development process of higher animals involves
cellular proliferation, differentiation, apoptosis and
anti-apoptosis, and the Wnt/β-catenin pathway is the most
well-known pathway in mediating this process (20). β-catenin is a multifunctional
protein which may regulate cell adhesion and signal transduction,
and it is the most important component of the Wnt/β-catenin pathway
(21,22).
Mmps and cytokines serve an important role in the
damage of articular cartilage (23), however the mechanisms remain
unclear. Previous studies have, however, demonstrated that the
Wnt/β-catenin pathway regulated the expression levels of Mmps and
the BMP gene families (24). Zhu
et al (21) identified that
the overexpression of β-catenin may lead to OA-like alterations in
the knee joints of mice, and increases in the gene expression of
Mmp-9, Mmp-13 and BMP-2. The activity of gelatinase and the gene
expression of Mmp-3 and Mmp-13 has been previously identified to be
increased subsequent to the activation of the Wnt/β-catenin pathway
by Wnt-3a in the articular cartilage of rabbit (25). Based on the abovementioned studies,
the Wnt/β-catenin pathway is suggested to serve a key role in the
mechanism of OA. In the present study, the expression of BMP-2 and
Mmp-13, the marker genes of chondrocyte maturation and the
downstream genes in the Wnt/β-catenin pathway (20), were significantly different among
the three groups.
BMP is involved in the regulation of the
differentiation, proliferation, maturity and apoptosis and may
increase the expression of Runx-2 downstream (26). The BMP content was significantly
increased in the synovium and cartilage tissue in the mouse model
of OA induced by papain, and it was observed that the BMP inhibitor
was able to reverse the formation of osteophytes in OA (27). When the process of endochondral
ossification is completed, overexpression of BMP leads to
hypertrophy, differentiation of chondrocytes and the formation of
osteophytes (28). The
overexpression of BMP-2 mRNA has been previously identified in the
chondrocytes of OA knees in the patients with mechanical injury
(28). A previous study identified
that the expression of BMPs and Runx-2 were increased significantly
in the peripheral blood of patients with OA, therefore BMPs and
Runx-2 may be considered as markers for the activity of disease and
reaction to treatment (29). In
the current study, Runx-2 and BMP-2 mRNA expression levels were
identified to be upregulated, and the Runx-2 and BMP-2 protein
expression levels were increased in the injured exercise-induced OA
model group and exercise-induced OA model group compared with the
control group. This indicated that the normal exercise-induced OA
group had similar alterations in gene and protein expression, as
compared with the injured exercise-induced OA group; however
whether these alterations were induced by the Wnt/β-catenin pathway
requires further investigation.
Mmp-13 is the most effective collagen II degradation
enzyme and the overexpression of Mmp-13 results in damage to the
articular cartilage (30). A
previous study additionally identified that the high expression
levels of Mmp-13 in transgenic mice could result in the appearane
of OA-like pathological alterations (30). The Wnt/β-catenin signaling pathway
was suggested to serve an important role in this process;
overexpression of β-catenin can lead to increases in Mmp-13 gene
expression, which contributes to the degradation of cartilage
matrix and damage of articular cartilage (21,31).
Sox-9 regulates the development of cartilage in the
early phase and sustains the specialty of chondrocytes (32). Sox-9 serves a key regulating role
in controlling differentiation and hypertrophy of chondrocytes
(32). Additionally, it has been
reported that Wnt/β-catenin signaling pathway does not serve a role
in differentiation and hypertrophy of chon-drocytes if the
expression of Sox-9 is reduced, indicating that the Wnt/β-catenin
signaling pathway-mediated promotion of chondrocyte differentiation
is dependent on the expression of Sox-9 (32,33).
Sox-9 has also been reported to regulate the expression of collagen
II, and it is notable that the Wnt/β-catenin signaling pathway may
regulate the upstream Sox-9 gene as part of a feedback regulation
(34). The results of the current
study identified that the expression of Sox-9 was reduced, and the
expression of β-catenin was increased at mRNA and protein levels,
thus indicating that increased expression levels of β-catenin
regulate Sox-9 via feedback regulation in this exercise-induced OA
rat model.
In the present study, upregulation of β-catenin and
Wnt-3a was identified in the injured exercise-induced OA model and
exercise-induced OA model groups. Therefore, β-catenin and Wnt-3a
may participate in the pathogenesis of exercise-induced OA by
abnormally activating the Wnt/β-catenin pathway as a result of
frequently excessive pressure in sports. This may provide an
improved understanding of the molecular pathogenesis of
exercise-induced OA, thus providing a basis for future
research.
Acknowledgments
The authors would like to thank the Laboratory
Animal Center of Xi'an Jiaotong University Health Science Center
for their support and cooperation in the collection of animals and
the experimental methods. They would also like to thank Mr. Shen
Wan, Mr. Shuilang He, Miss. Chuiyan Wen, Dr Chunying Liu and Mr.
Yang Wen for their technical assistance.
References
|
1
|
Buckwalter JA, Mankin HJ and Grodzinsky
AJ: Articular cartilage and osteoarthritis. Instr Course Lect.
54:465–480. 2005.
|
|
2
|
Ramos YF, Bos SD, Lakenberg N, Böhringer
S, den Hollander WJ, Kloppenburg M, Slagboom PE and Meulenbelt I:
Genes expressed in blood link osteoarthritis with apoptotic
pathways. Ann Rheum Dis. 73:1844–1853. 2014.
|
|
3
|
Ren YH: The epidemiological investigation
of sports injury in elite athletes. Zhong guo yundong yi xue.
19:377–386. 2000.In Chinese.
|
|
4
|
Minns RJ and Muckle DS: The role of the
meniscus in an instability model for osteoarthritis in the rabbit
knee. Br J Exp Pathol. 63:18–24. 1982.
|
|
5
|
Setton LA, Mow VC, Müller FJ, Pita JC and
Howell DS: Mechanical behavior and biochemical composition of
canine knee cartilage following periods of joint disuse and disuse
with remobilization. Osteoarthritis Cartilage. 5:1–16. 1997.
|
|
6
|
Buckwalter JA and Martin JA: Sports and
osteoarthritis. Curr Opin Rheumatol. 16:634–639. 2004.
|
|
7
|
Lequesne M: Sport practice and
osteoarthritis of the limbs. Science & Sports. 19:281–285.
2004.In French.
|
|
8
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005.
|
|
9
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487.
2004.
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001.
|
|
11
|
Aigner T, Rose J, Martin J and Buckwalter
J: Aging theories of primary osteoarthritis: From epidemiology to
molecular biology. Rejuvenation Res. 7:134–145. 2004.
|
|
12
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012.
|
|
13
|
Benito MJ, Veale DJ, Fitzgerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005.
|
|
14
|
Sellam J and Berenbaum F: The role of
synovitis in patho-physiology and clinical symptoms of
osteoarthritis. Nat Rev Rheumatol. 6:625–635. 2010.
|
|
15
|
Ma CH, Lv Q, Cao Y, Wang Q, Zhou XK, Ye BW
and Yi CQ: Genes relevant with osteoarthritis by comparison gene
expression profiles of synovial membrane of osteoarthritis patients
at different stages. Eur Rev Med Pharmacol Sci. 18:431–439.
2014.
|
|
16
|
Eckstein F, Hudelmaier M and Putz R: The
effects of exercise on human articular cartilage. J Anat.
208:491–512. 2006.
|
|
17
|
Almekinders LC and Garrett WE: Sports
injuries. Curr Probl Surg. 37:331–338. 2000.
|
|
18
|
Strutt D: Frizzled signalling and cell
polarisation in Drosophila and vertebrates. Development.
130:4501–4513. 2003.
|
|
19
|
Luyten FP, Tylzanowski P and Lories RJ:
Wnt signaling and osteoarthritis. Bone. 44:522–527. 2009.
|
|
20
|
Wodarz A and Nusse R: Mechanisms of Wnt
signaling in development. Annu Rev Cell Dev Biol. 14:59–88.
1998.
|
|
21
|
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C,
Rosier RN, O'Keefe RJ, Zuscik M and Chen D: Activation of
beta-catenin signaling in articular chondrocytes leads to
osteoarthritis-like phenotype in adult beta-catenin conditional
activation mice. J Bone Miner Res. 24:12–21. 2009.
|
|
22
|
Piters E, Boudin E and Van Hul W: Wnt
signaling: A win for bone. Arch Biochem Biophys. 473:112–116.
2008.
|
|
23
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006.
|
|
24
|
Kawaguchi H: Regulation of osteoarthritis
development by Wnt-beta-catenin signaling through the endochondral
ossification process. J Bone Miner Res. 24:8–11. 2009.
|
|
25
|
Yuasa T, Otani T, Koike T, Iwamoto M and
Enomoto-Iwamoto M: Wnt/β-catenin signaling stimulates matrix
catabolic genes and activity in articular chondrocytes: Its
possible role in joint degeneration. Lab Invest. 88:264–274.
2008.
|
|
26
|
Guohui Y: Bone morphogenetic protein in
the repair of articular cartilage injury. J Clin Rehabil Tissue Eng
Res. 46:8669–8672. 2010.
|
|
27
|
Scharstuhl A, Vitters EL, van der Kraan PM
and van den Berg WB: Reduction of osteophyte formation and synovial
thickening by adenoviral overexpression of transforming growth
factor beta/bone morphogenetic protein inhibitors during
experimental osteoarthritis. Arthritis Rheum. 48:3442–3451.
2003.
|
|
28
|
Dell'Accio F, De Bari C, El Tawil NM,
Barone F, Mitsiadis TA, O'Dowd J and Pitzalis C: Activation of WNT
and BMP signaling in adult human articular cartilage following
mechanical injury. Arthritis Res Ther. 8:R1392006.
|
|
29
|
Grcevic D, Jajic Z, Kovacic N, Lukic IK,
Velagic V, Grubisic F, Ivcevic S and Marusic A: Peripheral blood
expression profiles of bone morphogenetic proteins, tumor necrosis
factor-superfamily molecules and transcription factor Runx2 could
be used as markers of the form of arthritis, disease activity, and
therapeutic responsiveness. J Rheumatol. 37:246–256. 2010.
|
|
30
|
Neuhold LA, Killar L, Zhao W, Sung ML,
Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, et
al: Postnatal expression in hyaline cartilage of constitutively
active human collagenase-3 (MMP-13) induces osteoarthritis in mice.
J Clin Invest. 107:35–44. 2001.
|
|
31
|
Nakashima A and Tamura M: Regulation of
matrix metallopro-teinase-13 and tissue inhibitor of matrix
metalloproteinase-1 gene expression by WNT3A and bone morphogenetic
protein-2 in osteoblastic differentiation. Front Biosci.
11:1667–1678. 2006.
|
|
32
|
Topol L, Chen W, Song H, Day TF and Yang
Y: Sox9 inhibits Wnt signaling by promoting beta-catenin
phosphorylation in the nucleus. J Biol Chem. 284:3323–3333.
2009.
|
|
33
|
Hwang SG, Yu SS, Lee SW and Chun JS:
Wnt-3a regulates chon-drocyte differentiation via c-Jun/AP-1
pathway. FEBS Lett. 579:4837–4842. 2005.
|
|
34
|
Miclea RL, Karperien M, Bosch CA, van der
Horst G, van der Valk MA, Kobayashi T, Kronenberg HM, Rawadi G,
Akçakaya P, Löwik CW, et al: Adenomatous polyposis coli-mediated
control of beta-catenin is essential for both chondrogenic and
osteogenic differentiation of skeletal precursors. BMC Dev Biol.
9:262009.
|