Introduction
Macrovascular diseases, including atherosclerosis,
are the most common complications of diabetes (1). Diabetes mellitus impairs endothelial
function, which can be considered as the hallmark in the
development of cardiovascular diseases (2). The vascular endothelium is considered
to be important in diabetes-associated vascular dysfunction,
including atherosclerosis (3). The
endothelium is critical in the regulation of vascular function, and
in the development of physiological and pathophysiological
inflammation (4). Endothelial cell
injury is a critical component of atherosclerosis and hypertension
(5). Studies have indicated that
high glucose induces endothelial cell apoptosis, and can cause
cellular dysfunction and even cell death (6,7).
The Notch signaling pathway is one of the pathways
that is important in cell differentiation, acting primarily to
determine and regulate cell survival (8). In mammals, four receptors (Notch1-4)
and five ligands, including Jagged 1, Jagged 2, δ-like (Dll) 1,
Dll3 and Dll4, have been identified (9). Notch signaling affects cellular
activities, including proliferation, migration, growth,
differentiation and death (10).
In addition, Notch activity controls communication between cells,
signal transduction in the cytoplasm and gene transcription in the
nucleus (10). The genes
downstream of Notch signaling include Hairy and enhancer of split 1
(Hes1) and the Hairy-related transcription factor family (9). The binding of a ligand and receptor
induces a conformational change of the Notch receptor, which allows
an extracellular metalloprotease to cleave the receptor, inducing
the g-secretase-mediated protease to release the Notch
intracellular domain. Subsequently, the Notch intra-cellular domain
travels into the nucleus, where it activates the transcription of
downstream genes, including Hes1 (11). Previous studies have reported that,
in high glucose-induced cell apoptosis, the Notch signaling pathway
is upregulated (12), suggesting
that Notch inhibition may be a useful method to protect cells from
high glucose-induced apoptosis.
At present, the detrimental effects induced by high
glucose on human endothelial cells can be suppressed by several
types of plant-derived active substances, including radix hedysari
polysaccharide and (−)-epigallocatechin-3-gallate (12,13).
Vaccaria serum, the seeds of Vaccaria segatalis (Neck.)
Garcker. ex Asch. (Caryophyllaceae), is a well-known traditional
medicinal plant (14), which is
used for its effects on the circulatory system to promote
menstruation, improve blood circulation, regulate menstrual
disturbance and reduce edema (15,16).
It contains flavonoids, cyclic peptides, triterpene saponins,
lipids, aliphatic acids, monosaccharides, biotin and coumarin
(16,17). A number of these compounds exhibit
bioactive properties, including anti-angiogenesis, and
growth-inhibitory activity on luteal cells, HL-60 cells and
endothelial cells (18). Vaccarin
is a major flavonoid glycoside in Vaccariae semen. It is considered
one of the major active constituents, and has gained increased
attention in scientific investigations (19). The present study aimed to
investigate the protective effect of vaccarin (Fig. 1A) on the EA·hy926 human umbilical
vein endothelial cell line injured by high glucose in vitro.
The involvement of the Notch signaling pathway in the
vaccarin-induced protective effects observed during high
glucose-induced cellular injury remains to be studied. In order to
further understand whether vaccarin reduced Notch1 and apoptosis
in vitro, the role of vaccarin in the reduction of apoptosis
in EA.hy926 cells within the range of an effective concentration
was evaluated.
Materials and methods
Materials
Vaccarin was purchased from Shanghai Shifeng
Technology Co., Ltd. (Shanghai, China). Sulforhodamine B (SRB) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). An Annexin
V-Fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
Detection kit (cat. no. KGA104) was purchased from KeyGEN
Biotechnology, Co., Ltd. (Nanjing, China). A DAB kit (cat. no.
P0203) was purchased from Beyotime Institute of Biotechnology
(Jiangmen, China). Rabbit polyclonal notch1 (cat. no. ab52627;
1:500), rabbit polyclonal Hes1 (cat. no. ab108937; 1:500), rabbit
polyclonal caspase3 (cat. no. ab32351; 1:1,000) and rabbit
polyclonal β-tublin (cat. no. ab6046; 1:1,000) antibodies were
purchased from Abcam (HongKong, China). Polyclonal goat anti-rabbit
IgG secondary antibody (cat. no. ab10058; 1:2,000) was purchased
from Sangon Biotech Co., Ltd. (Shanghai, China). Kits used for the
measurement of lactate dehydrogenase (LDH; cat. no. 20130620),
methane dicarboxylic aldehyde (MDA; cat. no. 20130608), super
oxygen dehydrogenase (SOD; cat. no. 20130618) and bicinchoninic
acid (BCA; cat. no. 20130619) concentrations were purchased from
the Institute of Jiancheng Bioengineering (Nanjing, China). M-PER
Mammalian Protein Extraction Reagent was purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA).
Cell culture and treatment
Human EA·hy926 endothelial cells (cat. no. CRL-2922;
American Type Culture Collection, Manassas, VA, USA) were cultured
in Dulbecco's modified Eagle's medium (DMEM; (GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc.) and incubated at 37°C in a
humidified air containing 5% CO2.
Prior to induction with high glucose, the cells were
grown to 80–90% confluence and placed in 2% serum-containing media
for 12 h to achieve cell synchronization. The vaccarin solution was
diluted (3.44, 6.88 or 13.76 µM) with culture medium
immediately prior to the experiment. The cells were treated with
glucose in the absence or presence of vaccarin. The cell monolayers
were pretreated with vaccarin (3.44, 6.88 or 13.76 µM) at
37°C for 24 h, followed by being induced by glucose. Following
treatment with glucose, the cells were maintained in 10%
serum-containing media in a 5% CO2 atmosphere at 37°C
and used for further experiments.
Analysis of cell viability
An SRB assay was performed to assess EA·hy926 cell
viability (20). The EA·hy926
cells were seeded into 96-well culture plates, with four replicates
for each concentration. Culture of the cells in medium was
continued for 24 h, following which vaccarin solution at three
final concentrations (3.44, 6.88 and 13.76 µM), dissolved in
serum-free medium, were added to each well. Following 24 h
incubation at 37°C, glucose solution (90, 180 or 270 mM) dissolved
in serum-free medium were added to each well and continued to
culture for 12 and 24 h. The medium was then removed and 5%
trichloroacetic acid (TCA; Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China) was added to each well for 200 µl to fix
the cells for 40 min at 4°C. The TCA solution was then removed and
replaced with 100 µl SRB, and the cells were incubated at
30°C for 30 min. Following removal of the SRB, the samples were
washed with deionized water twice. Finally 10% tris hydroxymethyl
aminomethane (Tris; Sinopharm Chemical Reagent Co., Ltd.) was used
to dissolve the SRB, and the samples were shaken for 30 sec twice
at room temperature. The results were determined at 540 nm using a
Multiskan MK3 reader (Thermo Fisher Scientific, Inc.), with the
cell viability expressed as an optical density (OD) value. In
addition, the cell morphology was observed under an inverted/phase
contrast microscope, and images were captured at 200× magnification
using a Nikon Eclipse Ti microscope (Nikon, Toyko, Japan).
Wound healing assay
The migration rate of the cells was measured using a
wound healing assay (21).
Briefly, the EA•hy926 cells (8×104/well) were seeded
into wells and were cultured at 37°C in a saturated humidity
containing 5% CO2 for 24 h. When the cells had attached
completely, a line was scaped through the middle of the cell plate,
measuring ~1 mm in width, following treatment with vaccarin (6.88
and 13.76 µM). The cells were incubated, and images of
randomly-selected fields were captured at 100× magnification under
a microscope video system (Nikon Eclipse Ti; Nikon). The mean
distances between the two ends of the scratch were quantified by
manual measurements, and the migration rate was calculated with the
control defined as 100%.
Measurement of LDH release, and
intracellular SOD and MDA content
LDH, an indicator of cell injury, was detected using
an assay kit, according to the manufacturer's protocol, with the
activity of the enzyme expressed as units per liter, and the
absorbance was read at 450 nm using a Multiskan MK3 microplate
reader (Thermo Fisher Scientific, Inc.). As described previously
(22), the activities of SOD and
MDA were determined using commercially available kits, according to
the manufacturer's protocol, with enzyme activities expressed as
units/mg protein. A single unit of SOD activity was defined as the
quantity, which reduced the absorbance at 450 nm by 50%. The
measurement of BCA was performed prior to determining the SOD.
Levels of MDA were measured at 532 nm by its reaction with
thiobarbituric acid to form a stable chromophoric product, with MDA
levels expressed as nmol/mg protein.
Cellular apoptosis assay
The EA.hy926 cells were prepared for analysis,
according to the manufacturer's protocol of the Annexin V-FITC/PI
Apoptosis Detection kit. The stained cells were quantitatively
detected using a FACScan flow cytometer (BD Biosciences, San Jose,
CA, USA) in the FL1-H and FL2-H channels. Data were analyzed using
CellQuest Pro software version 6.0 (BD Biosciences), with a total
of 10,000 cells analyzed.
Hoechst staining
In a 24-well plate with cover slips,
6×104 EA•hy926 cells were seeded into each well and
cultured for 24 h, following treatment with different doses of
vaccarin (6.88 and 13.76 µM) for 24 h prior to being
subjected to 24 h glucose (180 mM) induction. Following removal of
the culture medium, the cells were fixed with 0.5 ml 4%
paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd.), and then
washed twice with phosphate-buffered saline (PBS). Following
treatment with Hoechst33342 (Wuhan Boster, China) for 10 min, the
cells were rinsed twice with PBS. The stained cells were
immediately observed under a fluorescence microscope (Nikon Eclipse
Ti, Nikon, Tokyo Japan).
Western blot analysis
Protein levels were analyzed using Western blot
analysis, as described previously (23). Briefly, total protein was extracted
using the BCA kit and 25 µg total protein/well was loaded
onto SDS-PAGE gels (Beyotime Institute of Biotechnology) following
denaturing in loading buffer at 100°C for 5 min. The protein
extracts were subjected to 8–12% SDS-PAGE and transferred onto a
nitrocellulose membrane (EMD Millipore, Billerica, MD, USA).
Following the transfer, the membranes were blocked at room
temperature for 2 h in 5% nonfat dry milk/Tris-buffered saline with
Tween 20 (TBST; Sinopharm Chemical Reagent Co., Ltd.) and then
incubated at 4°C overnight with the following primary antibodies:
Notch1 (1:500), Hes1 (1:500), caspase3 (1:1,000) and β-tublin
(1:1,000). The following day, the membranes were washed three times
with TBST for 10 min at room temperature, and subsequently
incubated in secondary antibody (anti-rabbit IgG; 1:2,000)
conjugated to horseradish peroxidase for 2 h at room temperature.
Following incubation, the membranes were washed, as above, and the
protein bands were visualized using a DAB Advanced Western Blotting
Detection kit (Beyotime Institute of Biotechnology). β-tublin was
used as the protein loading control following measurement of the
integral optical density.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance with SPSS 20.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
High glucose reduces cell viability, and
increases the expression levels of Notch1 and Hes1 in the EA·hy926
cells
In order to investigate the effect of high glucose
on EA·hy926 cells in the present study, the cells were induced by
high glucose (90, 180 or 270 mM) for 12 and 24 h, and cell
viability was examined using an SRB assay. As shown in Fig. 1B, treatment with high glucose alone
significantly reduced cell viability by >50% following 12 h
treatment at 270 mM, and following 24 h treatment with 180 and 270
mM glucose. The OD values in the control group were 0.885±0.005 and
1.022±0.020 following 12 and 24 h in culture, respectively. All the
groups had a significant decrease in viability, compared with these
normal control groups (P<0.01). These results resulted in the
selection of 24 h treatment with 180 mM glucose for the subsequent
experiments. In addition, the present study investigated the
expression levels of Notch1 and Hes1 following 24 h high glucose
culture and the results suggested that high glucose treatment
significantly increased the expression levels of Notch1 and Hes1 in
a dose-dependent manner (Fig.
1C).
Vaccarin increaes the viability and
migratory ability of high glucose-injured EA·hy926 cells
The effects of vaccarin on EA·hy926 cell
proliferation were examined following 12 and 24 h treatment with
180 mM glucose. As shown in Fig.
2A, the cell viability in the presence of vaccarin increased
significantly, compared with the groups without vaccarin treatment,
respectively (P<0.01). Vaccarin afforded dose-dependent
protection against the reduction in cell viability induced by high
glucose concentrations between 3.44 and 13.76 µM. As
observed under the microscope, high glucose treatment resulted in
significant cell shrinkage, compared with the control group.
However, pretreatment with three different vaccarin concentrations
(3.44, 6.88 and 13.76 µM) attenuated high glucose-injured
cell shrinkage (Fig. 2B). Based on
these results, pretreatment with 6.88 and 13.76 µM vaccarin
and 180 mM glucose for 24 h was selected for use in the subsequent
experiments. As shown in Fig. 2C,
following treatment with high glucose, the migratory ability of
cells decreased, resulting in a migration ratio of 23.16±2.87%
(P<0.01), compared with the normal cells. However, vaccarin at
concentrations of 6.88 and 13.76 µM significantly increased
the migration ratio (50.71±7.33 and 82.00±1.95%, respectively,
compared with the high glucose group (P<0.01).
 | Figure 2Effects of vaccarin on the viability,
morphology and migratory ability of high glucose-injured EA·hy926,
treated for 24 h (180 mM). (A) Viability of EA·hy926 cells was
assessed by performing a sulforhodamine B assay, and the viability
was expressed as an OD value. (B) Cell morphology was observed
under an inverted/phase contrast microscope, and images were
captured. Significant cell shrinkage was observed in the high
glucose group, and vaccarin treatment reduced the high
glucose-induced cell shrinkage. (C) Migratory ability of the
EA·hy926 was assessed by performing a wound healing assay. The
degree of migration was analyzed by averaging the width of each gap
in four places (magnification, ×100). The migration rate in the
control group was set to 100%. The results are expressed as the
mean ± standard deviation (n=4). **P<0.01, compared
with the control group, #P<0.05 and
##P<0.01, compared with the HG group;
$$P<0.01, compared with the HG+3.44 µM
vaccarin group; &P<0.05 and
&&P<0.01, compared with the HG+6.88 µM
vaccarin group. HG, 180 mM high glucose; OD, optical density. |
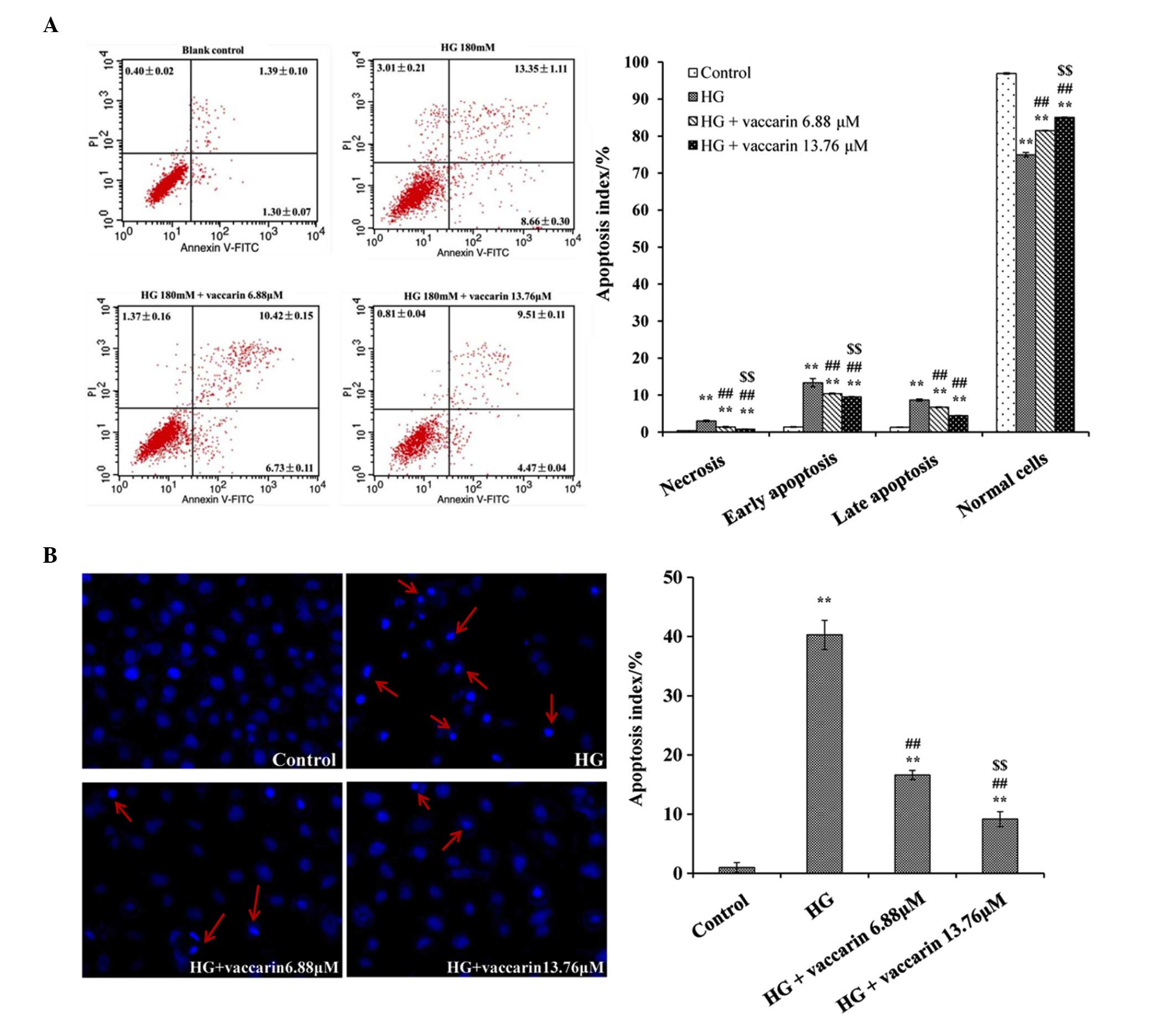
Vaccarin decreases the apoptotic index of
high glucose-injured EA·hy926 cells
The induction of apoptosis was measured by Annexin
V/PI double staining. The results of the flow cytometric analysis
in the high glucose group showed increase apoptosis. The ratio of
prophase and late apoptosis reached 8.66±0.30 and 13.35±1.11%,
respectively (P<0.01), compared with the control group. However,
the apoptotic ratio following treatment with vaccarin in the 6.88
and 13.76 µM groups significantly declined (P<0.01),
compared with the high glucose group (Fig. 3A).
In the Hoechst staining experiment, it was also
found that apoptosis was markedly higher in the high glucose group
(Fig. 3B). Following treatment
with vaccarin (6.88 and 13.76 µM), the apoptotic index was
significantly decreased (P<0.01), compared with the high glucose
group.
Effects of vaccarin on SOD, LDH release
and levels of MDA in high glucose-injured EA·hy926 cells
Treatment of the cells with high glucose for 24 h
decreased the levels of SOD, but increased LDH release and the
levels of MDA (P<0.01), compared with the control group. As
shown in Fig. 4, incubation of the
EA·hy926 cells in the presence of vaccarin (6.88 and 13.76
µM) with high glucose significantly increased SOD activity
(Fig. 4A) and decreased the level
of MDA and release of LDH, respectively (Fig. 4B and C). According to these
results, vaccarin significantly altered SOD activity, LDH leakage
and MDA levels in the high glucose-induced endothelial cells in a
concentration-dependent manner.
 | Figure 4Effects of vaccarin on the
intracellular levels of SOD and MDA, and LDH release, and the
effects of vaccarin on the expression levels of Notch1, Hes1 and
caspase 3 in high glucose-injured EA·hy926 cells. treated for 24 h.
(A) Release of LDH in EA·hy926 cells treated with high glucose in
the absence or presence of vaccarin. (B) Intracellular levels of
SOD in EA·hy926 cells treated with high glucose in the absence or
presence of vaccarin. (C) Intracellular levels of MDAin EA·hy926
cells treated with high glucose in the absence or presence of
vaccarin. (D) Representative images of Western blots are shown. The
results are expressed as the mean ± standard deviation (n=4).
**P<0.01, compared with the control group,
#P<0.05 and ##P<0.01, compared with the
HG group; $P<0.05 and $$P<0.01, compared with the
HG+6.88 µM vaccarin group. HG, high glucose; Hes1, Hairy and
enhancer of split 1; SOD, superoxide dismutase; MDA,
malondialdehyde; LDH, lactate dehydrogenase. |
Vaccarin increases the expression levels
of Notch1, Hes1 and caspase 3 in high glucose-injured EA·hy926
cells
To further investigate the effect and mechanism of
vaccarin of high glucose-injured EA·hy926 cells, the presented
study examined the expression levels of Notch1, Hes1 and caspase 3
using Western blot analysis. As shown in Fig. 4D, treatment with high glucose
significantly increased the expression levels of Notch1, Hes1 and
caspase 3, relative to the control group (P<0.01). By contrast,
in the cells were pretreated with vaccarin (6.88 and 13.76
µM), the expression levels of Notch1, Hes1 and caspase 3
decreased significantly (P<0.01), compared with the high glucose
group.
Discussion
Endothelial barrier dysfunction is pivotal in the
pathogenesis of diabetic vascular complications. Exposure of the
vascular endothelial tissue to high glucose causes endothelial
dysfunction and further complications of diabetes, including
cardiovascular diseases (24). A
previous report showed that high glucose induced the production of
reactive oxygen species (ROS), which can cause cellular
dysfunction, cell apoptosis and even cell death (6). The pathogenesis of diabetes mellitus
is complicated, and there are several signaling pathways involved
in the pathogenesis of diabetes mellitus, including the Notch
pathway (12). Notch signaling has
been widely implicated in endothelial to mesenchymal
transformation, endothelial cell proliferation and the control of
apoptosis (25). It has been
reported that, in a cultured renal proximal tubular cell model,
puromycin aminonucleoside triggers the upregulation of Notch1
signaling components, including Notch intracellular domain and the
downstream molecule, Hes1, which is accompanied by the
downregulation of Numb, an intrinsic Notch antagonist (26).
The results of the present study indicated that, in
human EA·hy926 endothelial cells, high glucose causes vascular
endothelial cell apoptosis via activating Notch1 and Hes1. In
addition, vaccarin was found to impart a protective effect against
high glucose induced endothelial injury, which was evidenced by
improved cell viability and migratory ability, and a decreased
apoptotic index. Therefore, the protective effects of vaccarin
against cell injury were, at least in part, dependent on Notch1
inhibition. There are several anti-high glucose-induced cell injury
drugs against apoptosis, which act through regulating the cell
apoptotic pathway (27). B cell
lymphoma (Bcl)2, Bcl-2-associated X protein, Bcl antagonist killer
1 and caspase 3, which are important in the process of cell
apoptosis, are all important members of the cell survival pathway
(28). Caspase 3 has a dominant
role in the execution of the apoptotic process, and the activation
of caspase 3 is the central link in apoptosis. Previous data have
demonstrated that caspase 3 may be an important target involved in
ROS-mediated high glucose-induced apoptosis in human endothelial
cells (29). In the present study,
the results showed that vaccarin effectively suppressed the
overexpression of caspase 3 under high glucose conditions.
High glucose-induced free radicals can have
irreversible effects on several biomolecules, including lipids,
which leads to lipid peroxidation. LDH leakage, corresponding to
membrane damage, and MDA, a by-product of lipid peroxidation
induced by excessive ROS, are widely used biomarkers of oxidative
stress injury (30). Antioxidants,
including SOD, are important in providing protection against high
glucose injury. In the present study, significant decreases in SOD
were observed in EA·hy926 cells following exposure to high glucose,
indicating the impairment in antioxidant defenses. In addition, a
marked elevation in MDA production was associated with an increase
in the release of LDH. Preincubation with vaccarin was found to
protect the EA·hy926 cells against high glucose-induced cellular
oxidative injury, as shown by inhibited levels of LDH and MDA, but
enhanced the activity of SOD. Notably, in addition to
downregulating high glucose-induced Notch signaling, vaccarin
treatment downregulated the high glucose-induced apoptotic
pathway-associated protein, caspase 3. Taken together, the results
of the present study suggested that the enhancement of endogenous
antioxidant preservation and attenuation of the cell apoptotic
pathway may represent a major mechanism of cellular protection by
vaccarin.
In conclusion, the present study demonstrated that
vaccarin prevented apoptosis of human EA·hy926 endothelial cells
induced by high glucose. These results indicated that vaccarin may
be a possible therapeutic in the prevention of diabetic vascular
lesions or atherosclerosis, although further investigations are
required.
Acknowledgments
This study was supported by the Fundamental Research
Funds for the Central Universities (grant no. JUSRP51412B).
References
|
1
|
Beckman JA, Creager MA and Libby P:
Diabetes and atherosclerosis: Epidemiology, pathophysiology and
management. JAMA. 287:2570–2581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malakul W, Thirawarapan S, Suvitayavat W
and Woodman OL: Type 1 diabetes and hypercholesterolaemia reveal
the contribution of endothelium-derived hyperpolarizing factor to
endothelium-dependent relaxation of the rat aorta. Clin Exp
Pharmacol Physiol. 35:192–200. 2008.
|
|
3
|
Vita JA: Endothelial function and clinical
outcome. Heart. 91:1278–1279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen CA, Wang TY, Varadharaj S, Reyes LA,
Hemann C, Talukder MA, Chen YR, Druhan LJ and Zweier JL:
S-glutathionylation uncouples eNOS and regulates its cellular and
vascular function. Nature. 468:1115–1118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu XG and Li L: Rosiglitazone suppresses
lipopolysaccharide-induced matrix metalloproteinase-2 activity in
rat aortic endothelial cells via Ras-MEK1/2 signaling. Intl J
Cardiol. 158:54–58. 2012. View Article : Google Scholar
|
|
6
|
Baumgartner-Parzer SM, Wagner L,
Pettermann M, Grillari J, Gessl A and Waldhausl W:
High-glucose-triggered apoptosis in cultured endothelial cells.
Diabetes. 44:1323–1327. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Deng WJ, Fan L, Tian LM, Jin L, Jin
Z, Guo Q, Xu Y and Li N: The role of radix hedysari polysaccharide
on the human umbilical vein endothelial cells (HUVECs) induced by
high glucose. Eur J Intern Med. 23:287–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cook KM and Figg WD: Angiogenesis
inhibitors: Current strategies and future. Prospects CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar
|
|
9
|
Juryńczyk M and Selmaj K: Notch: A new
player in MS mechanisms. J Neuroimmunol. 218:3–11. 2010. View Article : Google Scholar
|
|
10
|
Androutsellis-Theotokis A, Leker RR,
Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK,
Kittappa R and McKay RD: Notch signalling regulates stem cell
numbers in vitro and in vivo. Nature. 442:823–826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCright B: Notch signaling in kidney
development. Curr Opin Nephrol Hypertens. 12:5–10. 2003. View Article : Google Scholar
|
|
12
|
Gao F, Yao M, Shi Y, Hao J, Ren Y, Liu Q,
Wang X and Duan H: Notch pathway is involved in high
glucose-induced apoptosis in podocytes via Bcl-2 and p53 pathways.
J Cell Biochem. 114:1029–1038. 2013. View Article : Google Scholar
|
|
13
|
Yang J, Han Y, Chen C, Sun H, He D, Guo J,
Jiang B, Zhou L and Zeng C: EGCG attenuates high glucose-induced
endothelial cell inflammation by suppression of PKC and NF-κB
signaling in human umbilical vein endothelial cells. Life Sci.
92:589–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
China Pharmacopoeia Committee. Chinese
Pharmacopoeia 2010 Edition. China Medical Science and Technology
Press; Beijing, China: pp. 49–50. 2010, In Chinese.
|
|
15
|
Sang SM, Lao AN and Chen ZL: Chemistry and
bioactivity of the seeds of Vaccaria segetalis. Orient Foods Herbs.
21:279–291. 2000.
|
|
16
|
Li F and Liang JY: Research progress of
Vaccaria segetalis. Straits Pharm J. 3:1–5. 2007.In Chinese.
|
|
17
|
Li N, Ma CH and Liu D: Chemical
constituents analysis of fried vaccaria segetafis. Chin J Exptl
Tradit Med Form. 19:73–75. 2013.In Chinese.
|
|
18
|
Sun Y, Liang H, Zhang X, Tong H and Liu J:
Structural elucidation and immunological activity of a
polysaccharide from the fruiting body of Armillaria mellea.
Bioresour Technol. 100:1860–1863. 2009. View Article : Google Scholar
|
|
19
|
Meng H, Chen Y, Qin W, Tang X and Ye Z:
Determination of vaccarin in Vaccariae Semen by HPLC. Zhongguo
Zhong Yao Za Zhi. 35:2072–2074. 2010.In Chinese. PubMed/NCBI
|
|
20
|
Xia MX, Feng L and Zhang LF: Isolation,
purification and identification of chemical compound from semen
vaccariae to inhibit endothelial cells. Biotechnology Bulletin.
2:93–97. 2009.In Chinese.
|
|
21
|
Cheong SM, Choi H, Hong BS, Gho YS and Han
JK: Dab2 is pivotal for endothelial cell migration by mediating
VEGF expression in cancer cells. Exp Cell Res. 318:550–557. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su Y, Mao N, Li M, Dong X, Lin FZ, Xu Y
and Li YB: Sarpogrelate inhibits the expression of ICAM-1 and
monocyte-endothelial adhesion induced by high glucose in human
endothelial cells. Mol Cell Biochem. 373:195–199. 2013. View Article : Google Scholar
|
|
23
|
Li B, Qiu T, Zhang P, Wang X, Yin Y and Li
S: IKVAV regulates ERK1/2 and Akt signalling pathways in BMMSC
population growth and proliferation. Cell Prolif. 47:133–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng X, Li ZM, Zhou YJ, Cao YL and Zhang
J: Effect of the antioxidant alphalipoic acid on apoptosis in human
umbilical vein endothelial cells induced by high glucose. Clin Exp
Med. 8:43–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
MacKenzie F, Duriez P, Wong F, Noseda M
and Karsan A: Notch4 inhibits endothelial apoptosis via
RBP-Jkappa-dependent and -independent pathways. J Biol Chem.
279:11657–11663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding X, Zhu F, Li T, Zhou Q, Hou FF and
Nie J: Numb protects renal proximal tubular cells from puromycin
aminonucleoside-induced apoptosis through inhibiting Notch
signaling pathway. Intl J Biol Sci. 7:269–278. 2011. View Article : Google Scholar
|
|
27
|
Stampfer MJ, Hennekens CH, Manson JE,
Colditz GA, Rosner B and Willett WC: Vitamin E consumption and the
risk of coronary disease in women. N Engl J Med. 328:1444–1449.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen SD, Yin JH, Hwang CS, Tang CM and
Yang DI: Anti-apoptotic and anti-oxidative mechanisms of
minocycline against sphingomyelinase/ceramide neurotoxicity:
Implication in Alzheimer's disease and cerebral ischemia. Free
Radic Res. 46:940–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ho FM, Liu SH, Liau CS, Huang PJ and
Lin-Shiau SY: High glucose-induced apoptosis in human endothelial
cells is mediated by sequential activations of c-Jun NH
(2)-terminal kinase and caspase-3. Circulation. 101:2618–2624.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Y, Hu X, Yi B, Zhang T and Wen Z:
Glucagon-like peptide-1 receptor agonist protects against
hyperglycemia-induced cardiocytes injury by inhibiting high
mobility group box 1 expression. Molecular Biology Reports.
39:10705–10711. 2012. View Article : Google Scholar : PubMed/NCBI
|