Introduction
Intervertebral disc (IVD) degeneration has a
critical role in the pathogenesis of spinal disorders. In addition,
IVD degeneration is the main cause of lower back pain, and presents
a serious socioeconomic burden (1,2). The
treatment and prevention of degenerative disc disease is hampered
by a limited understanding regarding the mechanisms that regulate
IVD development, maintenance and degeneration. The cartilage
endplate (CEP) is the predominant source of nutrients for the IVD
(3,4). CEP degeneration markedly decreases
the biomechanical integrity and nutrition of IVDs, resulting in a
breakdown of the metabolic equilibrium of the extracellular matrix
(ECM) and subsequent acceleration of disc degeneration (5–8). The
prevention of CEP degeneration is a potential therapeutic strategy
for maintaining IVD health and preventing spondylopathy. It is well
known that the presence of apoptotic cells and loss of ECM are
characteristics of degenerated CEP; however, much remains to be
elucidated regarding the regulation of matrix protein biosynthesis
in cells of both the normal and degenerated disc.
The CCN family proteins, also known as extracellular
proteins, are dynamically expressed and have critical roles in
cardiovascular and skeletal development, injury repair, fibrotic
disease and cancer (9,10). The acronym CCN is derived from the
first three members of the family described, namely cysteine-rich
61/CCN1, connective tissue growth factor/CCN2, and nephroblastoma
overexpressed/CCN3. CCN proteins interact with various types of
molecules, including growth factors, ECM components and
cell-surface receptors, in order to regulate cell adhesion,
migration, proliferation, gene expression, differentiation,
apoptosis and survival (11). The
expression of CCN3 has previously been assessed in notochord
neonatal and mature rat IVDs, the results of which indicated that
CCN3 is involved in IVD development (12). In addition, recombinant CCN3
(rCCN3) has been demonstrated to act as a negative regulator; when
nucleus pulposus (NP) cells were treated with exogenous CCN3 in
vitro, cell proliferation was reduced, and the expression
levels of ECM components were decreased (12). A previous study reported that CCN3
expression was markedly upregulated in CCN2 deletion mice, which
was associated with impaired development of IVDs and markedly
accelerated age-associated IVD degeneration (13). The antiproliferative activities of
CCN3 have also been documented in embryonic fibroblasts,
chondrocytes, vascular smooth muscle cells, osteogenic mesenchymal
cells and NP cells (12,14,15).
Furthermore, CCN proteins are able to interact with tumor necrosis
factor (TNF) family cytokines to promote cell apoptosis (16–18).
CCN3 can unmask the cytotoxic potential of TNFα and lymphotoxin-α
(LTα), and promote the apoptotic activity of Fas ligand (FasL) and
TNF-related apoptosis-inducing ligand (TRAIL) in normal fibroblasts
(18). In addition, CCN2 can
increase the expression of ECM proteins (12), whereas CCN3 appears to be expressed
reciprocally and to act antagonistically to CCN2 (13,14,19).
The most prominent phenotype of CCN3 knockout mice involves
enhanced chondrogenesis and osteogenesis (20), whereas CCN2 mutant mice exhibit
severe chondrodysplasia (21).
However, whether CCN3 is associated with CEP homeostasis has yet to
be elucidated.
In the present study, a serum deprivation-induced
experimental model of apoptosis was used to explore the biological
effects of CCN3 on CEP cells (22), which mimicked the low nutrient
conditions in IVDs.
Materials and methods
Cell isolation and culture
All animal experiments were approved by the Ethics
Committee on Animal Experiments of Fudan University (Shanghai,
China). Two male Sprague-Dawley rats (age, ~12 weeks; weight, 400
g) were used in the present study and were supplied by the Chinese
Academy of Sciences (Shanghai, China). Animals were housed with
free access to food and water, under a 12-h light/dark cycle with a
constant temperature (20–23°C) and humidity (55±5%). The rats were
euthanized by cervical dislocation following anesthesia with
pelltobarbitalum natricum (50 mg/kg; Shanghai New Asia
Pharmaceutical Co., Ltd., Shanghai, China). All lumbar spines were
obtained within 1 h of sacrifice, and the discs were carefully
dissected under a microscope, in order to obtain only the CEP,
which was minced into small pieces (<0.3 mm3) under
aseptic conditions. To isolate the chondrocytes, the tissues were
sequentially treated with 0.25% trypsin (Sigma-Aldrich, St. Louis,
MO, USA) at 37°C for 120 min followed by 0.02% collagenase
(Sigma-Aldrich) at 37°C for 24 h. Following enzymatic digestion,
the tissues were filtered through a 70-µm mesh cell strainer
(Beyotime Institute of Biotechnology, Nantong, China) and were
washed with phosphate-buffered saline (PBS). Subsequently, the
cells were released from the matrix by centrifugation at 200 × g
for 5 min at room temperature, seeded into six-well plates at
2×104 cells/well, and maintained in Dulbecco's modified
Eagle medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Beyotime Institute of Biotechnology) at 37°C in a
humidified incubator containing 5% CO2. Primary
chondrocytes were maintained in a high-density monolayer culture
for 1 week, and toluidine blue staining (Shanghai Haoran
Biotechnology Co., Ltd., Shanghai, China) was used to confirm the
chondrocytic phenotype of the cells. The cells were then
trypsinized and subcultured into six-well plates, which were used
in the subsequent experiments as secondary cells.
Detection of apoptosis
After being cultured in either 1 or 10% FBS for 24
h, the cells were treated with a concentration gradient of
recombinant CCN3 (rCCN3; Sino Biological Inc., Beijing, China; 0,
0.5, 1 and 2 µg/ml) for 24 h. Subsequently, apoptosis was
determined by staining with annexin V fluorescein isothiocyanate
(FITC) and propidium iodide (PI) (BD Pharmingen, San Diego, CA,
USA), according to the manufacturer's protocol. To quantify
apoptosis, the cells were washed with cold PBS and suspended in
binding buffer. The cells were then stained with 5 µl
annexin V-FITC and 5 µl PI at 4°C for 15 min, and were
analyzed using FACScan flow cytometry (BD Biosciences, San Jose,
CA, USA) (22). The cells were
quantified as follows: i) Annexin V negative/PI negative (viable
cells); ii) annexin V positive/PI negative (cells in the initial
stages of apoptosis); iii) annexin V positive/PI positive (cells in
the advanced stages of apoptosis); and iv) annexin V negative/PI
positive (necrotic cells).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to detect the mRNA expression
levels of CCN3 in cells cultured with 1 or 10% FBS at 24, 36 and 48
h. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal standard control. In addition, after treating cells with 1
µg/ml rCCN3, the mRNA expression levels of aggrecan,
collagen II and CCN2 were assessed by RT-PCR at 8 and 24 h. Total
RNA was extracted from the cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Single-strand cDNA templates were prepared
from 1 µg total RNA using the RT-for-PCR kit (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. Specific cDNAs were subsequently amplified by PCR
using the following primers (Shanghai R&S Biotechnology Co.,
Ltd., Shanghai, China): Aggrecan, forward CAGAAAAACAGACTGAATGGGA,
reverse GCAAGTGAAGGTGTGTATGGC; collagen II, forward
GCAAGAATCCCGCTCGCA, reverse TGGGTTGGGGTAGACGCA; CCN3, forward
GCCCTTCCAGCCTACAGACC, reverse GGTGGATGGATTTCAGGGACT; CCN2, forward
GCTAATGGTGGACCGCAA, reverse CCAAGGTAACGCCAGGAAT; and GAPDH,
CCCCAATGTATCCGTTGTG, and reverse CTCAGTGTAGCCCAGGATGC. PCR
amplification from cDNA was performed using the Takara TP800
Thermal Cycler Dice (Takara Bio Inc., Shiga, Japan) in a final
volume of 20 µl [2X SYBR Green mix (10 µl; Toyobo
Co., Ltd., Tokyo, Japan) 1 µl primer mix, 1 µl
template DNA and 8 µl diethypyrocarbonate water] under the
following cycling conditions: Initial denaturation at 95°C for 2
min, denaturation at 95°C for 15 sec, annealing at 59°C for 20 sec,
elongation at 72°C for 20 sec and final extension at 72°C for 10
min; the optimal cycle number was 40 cycles. PCR products were
subjected to amplification curve analysis and were quantified using
SYBR Green (Invitrogen; Thermo Fisher Scientific, Inc.). The data
were normalized to GAPDH and were presented as ΔΔCt (22).
Western blot analysis
Cells cultured in 1% FBS were treated with 0 or 2
µg/ml rCCN3 for 24 h, and control group cells were cultured
in 10% FBS only. The protein expression levels of Fas, B-cell
lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) were
detected in the three groups by western blot analysis, according to
the manufacturer's protocol. Total protein was extracted from the
cells using protein loading buffer, and the total protein
concentration was determined by bicinchoninic acid assay
(Sigma-Aldrich). Protein extracts were separated by 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and were
transferred to polyvinylidene difluoride membranes (Millipore,
Bedford, MA, USA). The membranes were then blocked with 5% non-fat
dry milk in Tris-buffered saline with 0.1% Tween (TBST) for 1 h at
37°C, and incubated overnight at 4°C in TBST with polyclonal goat
anti-Fas (dilution 1:200; Sigma-Aldrich; cat. no. SAB2501317),
rabbit anti-Bcl-2 (dilution 1:500; cat. no. AB112; Beyotime
Institute of Biotechnology), mouse anti-Bax (dilution 1:500; cat.
no. AB026; Beyotime Institute of Biotechnology) and mouse
monoclonal anti-β-actin (dilution 1:2,000; cat. no. AF0003;
Beyotime Institute of Biotechnology) antibodies. Following further
incubation with a horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (dilution 1:5,000; cat. no.
A0208; Beyotime Institute of Biotechnology) for 1 h at room
temperature, the membranes were treated with
Electrochemiluminescence Plus (Tanon Science & Technology Co.,
Ltd., Shanghai, China), according to the manufacturer's protocol.
β-actin was used as a control to verify equal protein loading.
Statistical analysis
All measurements were carried out using the same
instrument under the same experimental conditions, and were
independently performed at least three times to ensure consistency.
Statistical analyses were performed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). Data are expressed as the mean ± standard
deviation. Significant differences were analyzed by one-way
analysis of variance among the groups and Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cells cultured from the CEP maintain a
chondrocytic phenotype
Toluidine blue staining was used to confirm the
phenotype of cells cultured from the CEP. The cells were observed
to be viable and possessed characteristic features of chondrocytes,
as evidenced by typical cell morphology and ECM staining (Fig. 1A).
Serum deprivation increases CCN3
expression
To investigate whether serum deprivation regulates
CCN3 expression, RT-qPCR was used to quantify the mRNA expression
levels of CCN3 in cells cultured in 1 or 10% FBS. As shown in
Fig. 1B, the mRNA expression
levels of CCN3 in the serum deprivation group were markedly
increased, as compared with in the control group at 24, 36 and 48
h. A marked increase was detected at 48 h in the serum deprivation
group; however, no obvious difference in the expression of CCN3 was
detected in the serum-deprived cells between 24 and 36 h.
CCN3 is involved in serum
deprivation-induced cellular apoptosis
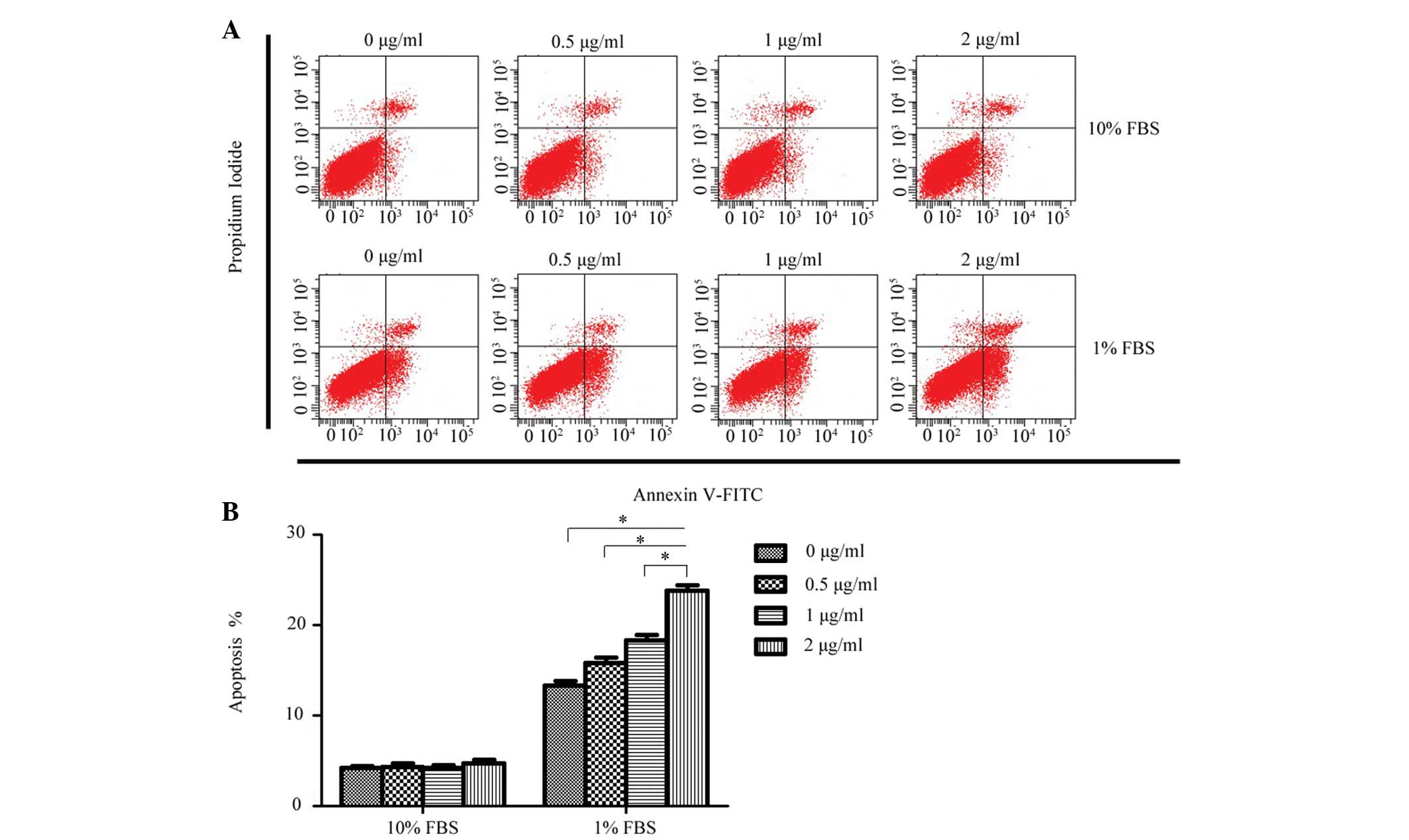
To determine whether exogenous rCCN3 had an effect
on the apoptosis of CEP cells, the apoptotic rate of cells stained
with annexin V-FITC and PI were quantitatively assessed following
treatment with a concentration gradient of rCCN3 for 24 h. As shown
in Fig. 2, a dose-dependent
increase of apoptosis was detected in the 1% FBS groups
(13.28±0.52, 15.86±0.58, 18.34±0.63 and 23.78±0.64%, respectively),
whereas rCCN3 did not exert a proapoptotic effect on cells cultured
in 10% FBS (4.22±0.24, 4.35±0.34, 4.26±0.30 and 4.75±0.42%,
respectively).
To explore the mechanism underlying the proapoptotic
effects of rCCN3, Fas, Bax and Bcl-2 expression levels were
detected by western blot analysis in cells cultured under 1 or 10%
FBS with or without rCCN3 (Fig.
3). The results demonstrated that Fas expression was markedly
upregulated following serum deprivation with or without rCCN3 at 24
h, and no obvious variation was detected following treatment with 2
µg/ml rCCN3. Furthermore, Bax expression was also markedly
increased, whereas Bcl-2 expression levels were decreased in the
cells under serum deprivation conditions, as compared with the
cells in the 10% FBS group. Notably, the results also demonstrated
that Bax and Bcl-2 were affected by exogenous rCCN3 treatment. The
differences in Bax and Bcl-2 were investigated and found to be
consistent with the effects on apoptosis following treatment with
rCCN3. These results suggest that rCCN3 may regulate cellular
apoptosis under serum deprivation conditions, and the mitochondrial
apoptotic pathway may be responsible for increased cellular
apoptosis under serum deprivation conditions.
CCN3 modulates the expression of critical
ECM genes
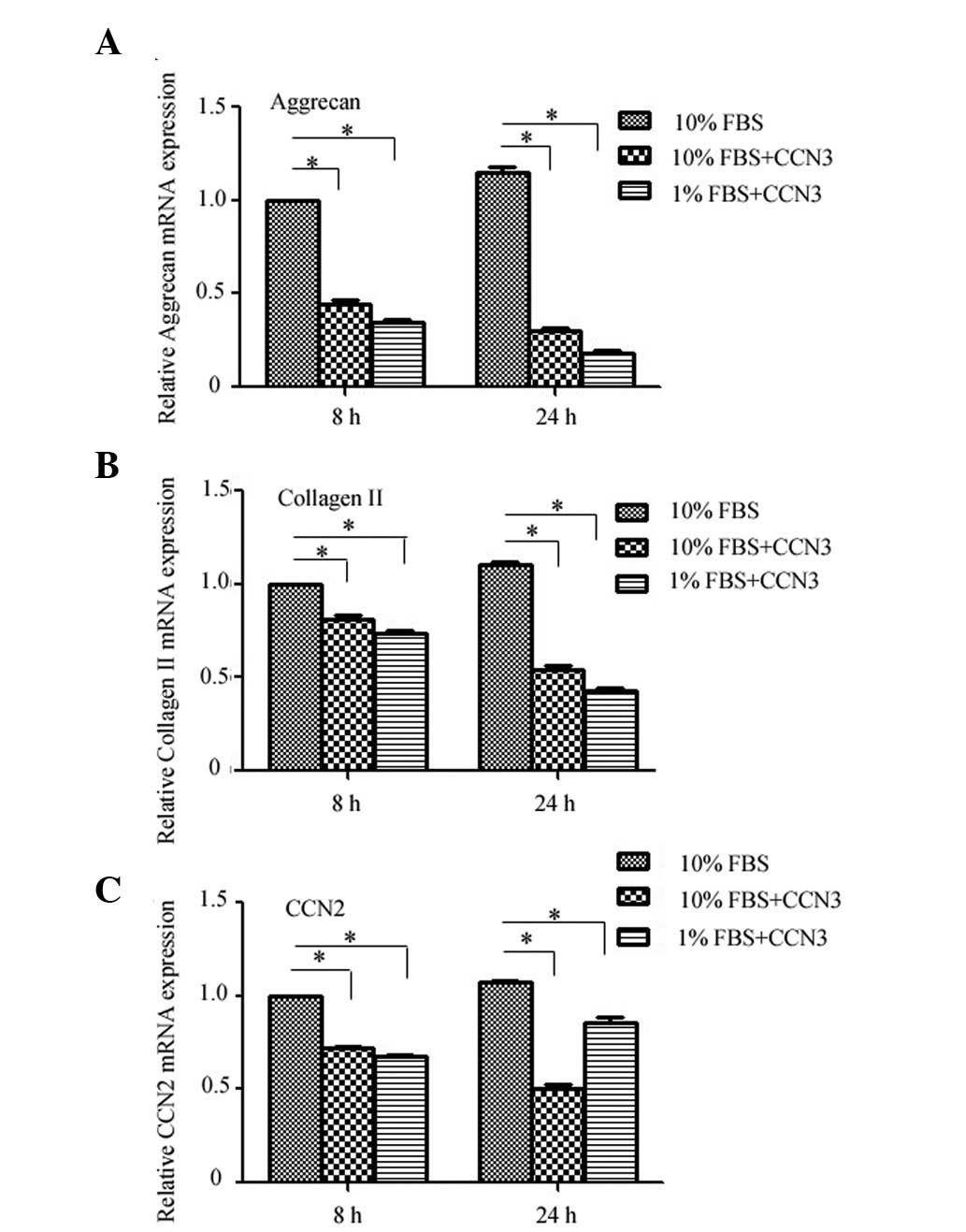
Finally, the effects of rCCN3 treatment on the
expression levels of critical ECM genes were determined by RT-qPCR
in CEP cells cultured under normal or serum deprivation conditions.
Treatment with rCCN3 decreased the expression levels of aggrecan
and collagen II under both normal and serum deprivation conditions
at 8 and 24 h, and a more obvious difference was detected under
serum deprivation conditions (Fig. 4A
and B). Furthermore, the mRNA expression levels of CCN2 were
decreased following treatment with rCCN3, whereas a decrease in
CCN2 expression was not as apparent in the 1% FBS group (Fig. 4C). These findings indicate that
CCN2 expression may also be influenced by serum deprivation.
Discussion
The CCN family of proteins has emerged as
dynamically expressed, multifunctional regulators of cellular
behavior, which maintain tissue homeostasis (11). Previous studies have demonstrated
that the CCN proteins are key mediators in the regulation of IVD
cell behavior and disc tissue homeostasis (13,23–25).
Bedore et al (13) revealed
that deletion of CCN2, accompanied by upregulated CCN3 expression,
induced a decrease in aggrecan and type II collagen expression in
NP cells, resulting in accelerated IVD degeneration. Transforming
growth factor-β may also modulate CCN expression in NP cells, thus
inhibiting CCN3 expression and increasing CCN2 expression, in order
to promote ECM deposition (12).
Of the CCN family, CCN2 appears to possess anabolic expression
patterns and activities, whereas CCN3 appears to be expressed
reciprocally and to act antagonistically to CCN2 (12). However, whether CCN3 may regulate
CEP cell behavior has yet to be explored.
The expression of CCN genes is particularly
sensitive to environmental perturbations, including the
availability of growth factors, hormones, cytokines, and exposure
to oxygen deprivation, UV and mechanical forces (26,27).
However, whether serum deprivation may regulate CCN3 expression has
yet to be reported. In the present study, rat CEP cells were
successfully isolated, cultured and confirmed to be of the
chondrocytic phenotype by toluidine blue staining. The mRNA
expression levels of CCN3 were markedly upregulated in response to
serum deprivation, particularly at 48 h. These results indicated
that CCN3 expression is sensitive to serum deprivation and that the
low nutrition condition of IVDs may regulate CCN3 expression, in
order to participate in the process of degeneration.
Western blot analysis demonstrated that high
expression levels of Fas could be detected under serum deprivation
conditions, with or without CCN3 treatment. A previous study
(16) reported that CCN proteins
are able to synergize with TNF family cytokines, including TNFα,
LTα, FasL and TRAIL, and enhance their cytotoxicity by binding
their receptors to promote the accumulation of reactive oxygen
species (ROS). In the present study, CCN3 and Fas expression were
simultaneously induced by serum deprivation, allowing them the
opportunity to interact with each other. As a result, it may be
hypothesized that CCN3 acts synergistically with Fas/FasL to
regulate serum deprivation-induced cellular apoptosis.
To determine whether exogenous rCCN3 was able to
promote the serum deprivation-induced apoptosis of CEP cells, cells
cultured under serum deprivation conditions were treated with a
dose-dependent concentration gradient of rCCN3. Flow cytometric
analysis detected a dose-dependent increase in apoptosis in
serum-deprived cells, whereas CCN3 did not exert a proapoptotic
effect on normal serum-cultured cells. These results suggested that
CCN3 can only promote cellular apoptosis induced by serum
deprivation, and cannot trigger apoptosis, which is consistent with
the findings on TNFα and FasL-induced fibroblast apoptosis
(17,18). The present study demonstrated that
CCN3 may influence CEP cell viability, and that serum
deprivation-induced abnormal expression of CCN3 may promote excess
cellular apoptosis and subsequent CEP degeneration.
The present study also detected the expression
levels of Bax and Bcl-2 by western blot analysis following
treatment with exogenous rCCN3. Bax expression was markedly
increased, whereas Bcl-2 expression was decreased under serum
deprivation conditions with or without rCCN3 treatment. These
results indicated that the mitochondrial apoptotic pathway was
involved in cellular apoptosis. Notably, the alterations in Bax and
Bcl-2 expression following treatment with rCCN3 were more obvious,
thus suggesting that CCN3 may engage in the cell apoptosis process
by regulating mitochondrial apoptosis-associated proteins.
Furthermore, Fas expression was detected at a high level under
serum deprivation conditions, and CCN3 was shown to synergize with
TNF family cytokines in order to promote cellular apoptosis by
recruiting ROS to increase cytochrome c release via an
imbalance in Bax and Bcl-2 levels (16). As a result, CCN3 may synergize with
cytokines and their receptors to promote serum deprivation-induced
apoptosis, as with Fas/FasL.
The effects of rCCN3 on the expression levels of
critical ECM genes were also examined. Aggrecan and type II
collagen are the most important components of endplate ECM, and a
decrease in these factors will result in the compression or
calcification of ECM, followed by accelerated disc degeneration
(28). Following treatment with
rCCN3, the expression levels of aggrecan and type II collagen were
inhibited not only under normal conditions but also under serum
deprivation conditions, and the decrease was more obvious under
serum deprivation conditions. These findings suggested that CCN3
was a harmful factor that acted antagonistically to aggrecan and
type II collagen expression, which is similar to previous in
vitro and in vivo findings in NP cells (12). The reciprocal effects of CCN2 were
also investigated following treatment with rCCN3. The decreases in
CCN2 expression in the 1 and 10% FBS groups were consistent with
the study results associated with other tissues (11,12).
However, the decrease in CCN2 expression was not as apparent under
serum deprivation, which may imply that CCN2 expression was also
affected by serum deprivation; however, this mechanism requires
further exploration.
In conclusion, the results of the present study
suggested that CCN3 may be associated with serum
deprivation-induced CEP cell apoptosis as a regulator, rather than
an initiator, and that CCN3 has a catabolic effect on the
modulation of ECM expression. Therefore, CCN3 may represent a novel
therapeutic target for the prevention of CEP degeneration.
Acknowledgments
The authors thank American Journal Experts for
English language editing. The present study was supported by the
Jinshan District (and Shanghai Municipal) Health Bureau (grant nos.
2012-694 and 2012-341).
References
|
1
|
Hoy D, Brooks P, Woolf A, Blyth F, March
L, Bain C, Baker P, Smith E and Buchbinder R: Assessing risk of
bias in prevalence studies: Modification of an existing tool and
evidence of interrater agreement. J Clin Epidemiol. 65:934–939.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Schepper EI, Damen J, van Meurs JB,
Ginai AZ, Popham M, Hofman A, Koes BW and Bierma-Zeinstra SM: The
association between lumbar disc degeneration and low back pain: The
influence of age, gender, and individual radiographic features.
Spine (Phila Pa 1976). 35:531–536. 2010. View Article : Google Scholar
|
|
3
|
Magnier C, Boiron O, Wendling-Mansuy S,
Chabrand P and Deplano V: Nutrient distribution and metabolism in
the intervertebral disc in the unloaded state: A parametric study.
J Biomech. 42:100–108. 2009. View Article : Google Scholar
|
|
4
|
Raj PP: Intervertebral disc:
Anatomy-physiology-pathophysiology-treatment. Pain Pract. 8:18–44.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grunhagen T, Shirazi-Adl A, Fairbank JC
and Urban JP: Intervertebral disk nutrition: A review of factors
influencing concentrations of nutrients and metabolites. Orthop
Clin North Am. 42:465–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirazi-Adl A, Taheri M and Urban JP:
Analysis of cell viability in intervertebral disc: Effect of
endplate permeability on cell population. J Biomech. 43:1330–1336.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding F, Shao ZW and Xiong LM: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang R, Li H, Ringgaard S, Rickers K, Sun
H, Chen M, Xie L and Bünger C: Interference in the endplate
nutritional pathway causes intervertebral disc degeneration in an
immature porcine model. Int Orthop. 38:1011–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kubota S and Takigawa M: The CCN family
acting throughout the body: Recent research developments. Biomol
Concepts. 4:477–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CC and Lau LF: Functions and
mechanisms of action of CCN matricellular proteins. Int J Biochem
Cell Biol. 41:771–783. 2009. View Article : Google Scholar :
|
|
11
|
Jun JI and Lau LF: Taking aim at the
extracellular matrix: CCN proteins as emerging therapeutic targets.
Nat Rev Drug Discov. 10:945–963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tran CM, Smith HE, Symes A, Rittié L,
Perbal B, Shapiro IM and Risbud MV: Transforming growth factor β
controls CCN3 expression in nucleus pulposus cells of the
intervertebral disc. Arthritis Rheum. 63:3022–3031. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bedore J, Sha W, McCann MR, Liu S, Leask A
and Séguin CA: Impaired intervertebral disc development and
premature disc degeneration in mice with notochord-specific
deletion of CCN2. Arthritis Rheum. 65:2634–2644. 2013.PubMed/NCBI
|
|
14
|
Ren Z, Hou Y, Ma S, Tao Y, Li J, Cao H and
Ji L: Effects of CCN3 on fibroblast proliferation, apoptosis and
extracellular matrix production. Int J Mol Med. 33:1607–1612.
2014.PubMed/NCBI
|
|
15
|
Janune D, Kubota S, Nishida T, Kawaki H,
Perbal B, Iida S and Takigawa M: Novel effects of CCN3 that may
direct the differentiation of chondrocytes. FEBS Lett.
585:3033–3040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CC and Lau LF: Deadly liaisons: Fatal
attraction between CCN matricellular proteins and the tumor
necrosis factor family of cytokines. J Cell Commun Signal. 4:63–69.
2010. View Article : Google Scholar :
|
|
17
|
Juric V, Chen CC and Lau LF: Fas-mediated
apoptosis is regulated by the extracellular matrix protein CCN1
(CYR61) in vitro and in vivo. Mol Cell Biol. 29:3266–3279. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen CC, Young JL, Monzon RI, Chen N,
Todorović V and Lau LF: Cytotoxicity of TNFalpha is regulated by
integrin-mediated matrix signaling. EMBO J. 26:1257–1267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tran CM, Schoepflin ZR, Markova DZ, Kepler
CK, Anderson DG, Shapiro IM and Risbud MV: CCN2 suppresses
catabolic effects of interleukin-1β through α5β1 and αVβ3 integrins
in nucleus pulposus cells: Implications in intervertebral disc
degeneration. J Biol Chem. 289:7374–7387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heath E, Tahri D, Andermarcher E,
Schofield P, Fleming S and Boulter CA: Abnormal skeletal and
cardiac development, cardiomyopathy, muscle atrophy and cataracts
in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev
Biol. 8:182008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ivkovic S, Yoon BS, Popoff SN, Safadi FF,
Libuda DE, Stephenson RC, Daluiski A and Lyons KM: Connective
tissue growth factor coordinates chondrogenesis and angiogenesis
during skeletal development. Development. 130:2779–2791. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding L, Wu JP, Xu G, Zhu B, Zeng QM, Li DF
and Lu W: Lentiviral-mediated RNAi targeting caspase-3 inhibits
apoptosis induced by serum deprivation in rat endplate chondrocytes
in vitro. Braz J Med Biol Res. 47:445–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tran CM, Markova D, Smith HE, Susarla B,
Ponnappan RK, Anderson DG, Symes A, Shapiro IM and Risbud MV:
Regulation of CCN2/connective tissue growth factor expression in
the nucleus pulposus of the intervertebral disc: Role of Smad and
activator protein 1 signaling. Arthritis Rheum. 62:1983–1992.
2010.PubMed/NCBI
|
|
24
|
Tran CM, Shapiro IM and Risbud MV:
Molecular regulation of CCN2 in the intervertebral disc: Lessons
learned from other connective tissues. Matrix Biol. 32:298–306.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bedore J, Leask A and Séguin CA: Targeting
the extracellular matrix: Matricellular proteins regulate
cell-extracellular matrix communication within distinct niches of
the intervertebral disc. Matrix Biol. 37:124–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kondo S, Kubota S, Mukudai Y, Moritani N,
Nishida T, Matsushita H, Matsumoto S, Sugahara T and Takigawa M:
Hypoxic regulation of stability of connective tissue growth
factor/CCN2 mRNA by 3′-untranslated region interacting with a
cellular protein in human chondrosarcoma cells. Oncogene.
25:1099–1110. 2006. View Article : Google Scholar
|
|
27
|
Kivelä R, Kyröläinen H, Selänne H, Komi
PV, Kainulainen H and Vihko V: A single bout of exercise with high
mechanical loading induces the expression of Cyr61/CCN1 and
CTGF/CCN2 in human skeletal muscle. J Appl Physiol (1985).
103:1395–1401. 2007. View Article : Google Scholar
|
|
28
|
Hee HT, Chuah YJ, Tan BH, Setiobudi T and
Wong HK: Vascularization and morphological changes of the endplate
after axial compression and distraction of the intervertebral disc.
Spine (Phila Pa 1976). 36:505–511. 2011. View Article : Google Scholar
|