Introduction
Ultraviolet (UV) radiation is considered a potent
agent for the induction of cell death (1). It has been reported that short
periods of UVB irradiation trigger apoptosis, whereas prolonged
exposure induces necrosis in various cell lines, including HL-60
cell lines, in vitro (2).
UV-induced apoptosis is principally attributed to DNA damage, death
receptor activation and reactive oxygen species (ROS) generation.
These initiate multiple signaling pathways, which result in tumor
suppressor gene p53 activation, regulation of Bcl-2 family
members and mitochondrial cytochrome c release (3–6).
Traditional UV lamps have gained popularity in
curing and disinfection applications for decades; however, due to
high energy demand and toxicity of mercury, other sources of UV
light are receiving more interest (7). Over the past few decades, UV
light-emitting diodes (LEDs) have received considerable attention
as an alternative UV source, due to a number of advantages over the
traditional UV lamps, including the absence of mercury, high energy
efficiency, increased operational flexibility and lifetime, and the
absence of the requirement of a warm-up period (8,9). UV
LEDs have consequently been recommended to replace traditional UV
lamps for numerous applications, such as sterilization, water
purification and medical treatment, including medical photo-therapy
for plaque-type psoriasis (10,11);
however, the effect of UV LED irradiation on human cells remains
poorly-defined. In the present study, the effect of 280 nm UV LED
irradiation on cultured HL-60 human leukemia cells and the
underlying mechanisms were examined.
Materials and methods
Cell culture
HL-60 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and cultured in Iscove's
modified Dulbecco's medium (Hyclone, Logan, UT, USA) supplemented
with 10% fetal bovine serum (Hyclone) in a humidified incubator
with 5% CO2 at 37°C. Cells were passaged three times
weekly, and exponentially growing cells were used for the
experiments. All experiments were performed in triplicate and
repeated three times.
Cell morphology
HL-60 cells were planted in a 24-well plate at a
density of 1×106 cells/well. Once cells settled to an
even monolayer, they were irradiated with UV LED at 0, 8, 15, 30
and 60 J/m2, and incubated for 2 h at 37°C in humidified
air with 5% CO2. Cell morphology was observed using
inverted microscopy (CKX41; Olympus Corporation, Tokyo, Japan) to
identify the biological characteristics of HL-60 cells.
Cell proliferation assay
HL-60 cells were planted in a 96-well plate at a
density of 4×104 cells/well. After cells had settled to
an even monolayer, they were irradiated with UV LED at 0, 8, 15, 30
and 60 J/m2 and maintained in the CO2
incubator for 2 h after irradiation. All samples were co-cultured
with cell counting kit-8 (CCK-8) solution (Dojindo Molecular
Technologies, Inc., Kyushu, Japan) for 3 h before the optical
density (OD) was measured at a wavelength of 450 nm using a
microplate reader (Multiskan FC; Thermo Fisher Scientific Inc.,
Waltham, MA, USA). The cell viability was calculated using the
following formula: Cell viability (%) = OD 450Test/OD
450Control × 100.
Flow cytometric analysis for the
detection of cell death
HL-60 cell death was detected by flow cytometry (FC
500 MPL; Beckman Coulter Inc., Fullerton, CA, USA) using
multicaspase assay kits (Guava Technologies, Burlingame, CA, USA).
HL-60 cells were planted in a 24-well plate at a density of
1×106 cells/well and irradiated with UV LED at 0, 8, 15,
30 and 60 J/m2. Following incubation for 2 h at 37°C,
the cells were harvested, washed with phosphate-buffered saline
(PBS) and stained with
sulforhodamine-valyl-alanyl-aspartyl-fluoromethyl-ketone
(SR-VAD-FMK) and 7-amino-actinomycin D (7-AAD), according to the
manufacturer's protocol. SR-VAD-FMK is a caspase inhibitor that
covalently binds to multiple active caspases during apoptosis, and
7-AAD is a nucleotide stain that only stains cells when membrane
integrity is compromised. A total of 5×103 cells per
analysis were examined using flow cytometry. Unstained cells, cells
stained with SR-VAD-FMK alone and cells stained with 7-AAD alone
were used as controls to set up compensation and quadrants.
SR-VAD-FMK positive/7-AAD negative cells (early apoptosis) and
double positive cells (late apoptosis) were considered as the
apoptotic cell population, while SR-VAD-FMK negative/7-AAD positive
cells as the necrotic cell population.
Cell cycle analysis
HL-60 cells were plated in a 24-well plate at a
density of 1×106 cells/well and exposed to UV LED
irradiation at 0, 8, 15 and 30 J/m2. Following
incubation for 2 h, cells were harvested and resuspended in PBS and
fixed in 70% ethanol at 4°C overnight. They were then washed twice
in cold PBS and incubated with propidium iodide staining solution
(Beyotime Institute of Biotechnology, Haimen, China) for 30 min at
room temperature. The percentage of cells at various phases of the
cell cycle, namely the G0/G1, S and G2/M phases, were determined by
flow cytometric analysis of 1×105 cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
HL-60 cells were plated in a 24-well plate at a
density of 1×106 cells/well and exposed to UV LED
irradiation (0, 8, 15 and 30 J/m2). Following incubation
for 2 h, total RNA was extracted from cells using RNAiso Plus
(Takara Bio, Inc., Shiga, Japan), according to the manufacturer's
protocol, and quantified by OD 260/280 ratio using a NanoDrop 2000C
spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE,
USA). Approximately 1 µg total RNA was reverse transcribed
into cDNA in a total volume of 20 µl using PrimeScript™ RT
reagent kit with gDNA Eraser (Takara Bio, Inc.). The final 20
µl PCR reaction mixture consisted of 10 µl of 2X SYBR
Premix Ex Taq (Takara Bio, Inc.), 0.8 µl PCR Forward Primer
(10 µM), 0.8 µl PCR Reverse Primer (10 µM),
2.0 µl template (≤100 ng) and 6.4 µl sterile
distilled water. RT-qPCR was performed in a Rotor-Gene RG-3000
cycler (Qiagen Pty, Ltd., Melbourne, Australia) under the following
conditions: 1 Cycle at 95°C for 30 sec, 40 cycles at 95°C for 5 sec
and 60°C for 20 sec. Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) was used as an internal control. The relative mRNA
expression of Bcl-2 was calculated by comparing their
Cq values with those of GAPDH using the 2–ΔΔCq
method (12). Statistical analysis
of Bcl-2 mRNA expression was performed using one-way
analysis of variance (ANOVA), followed by the Bonferroni correction
for multiple pairwise comparisons. The primer sequences used were
as follows: Forward, 5′-GTC CCA TCA AAA CTC CTG TCTT-3′ and
reverse, 5′-TTT CCA TCC GTC TGC TCTTC-3′ for Bcl-2;
and forward, 5′-TCA TGG GTG TGA ACC ATG AGAA-3′ and reverse, 5′-GGC
ATG GAC TGT GGT CAT GAG-3′ for GAPDH (Sangon Biotech Shanghai Co.,
Ltd., Shanghai, China).
Statistical analysis
Statistical analysis was conducted using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Comparisons among groups
were performed using one-way ANOVA, followed by the Bonferroni
correction for multiple pairwise comparisons. Data are presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
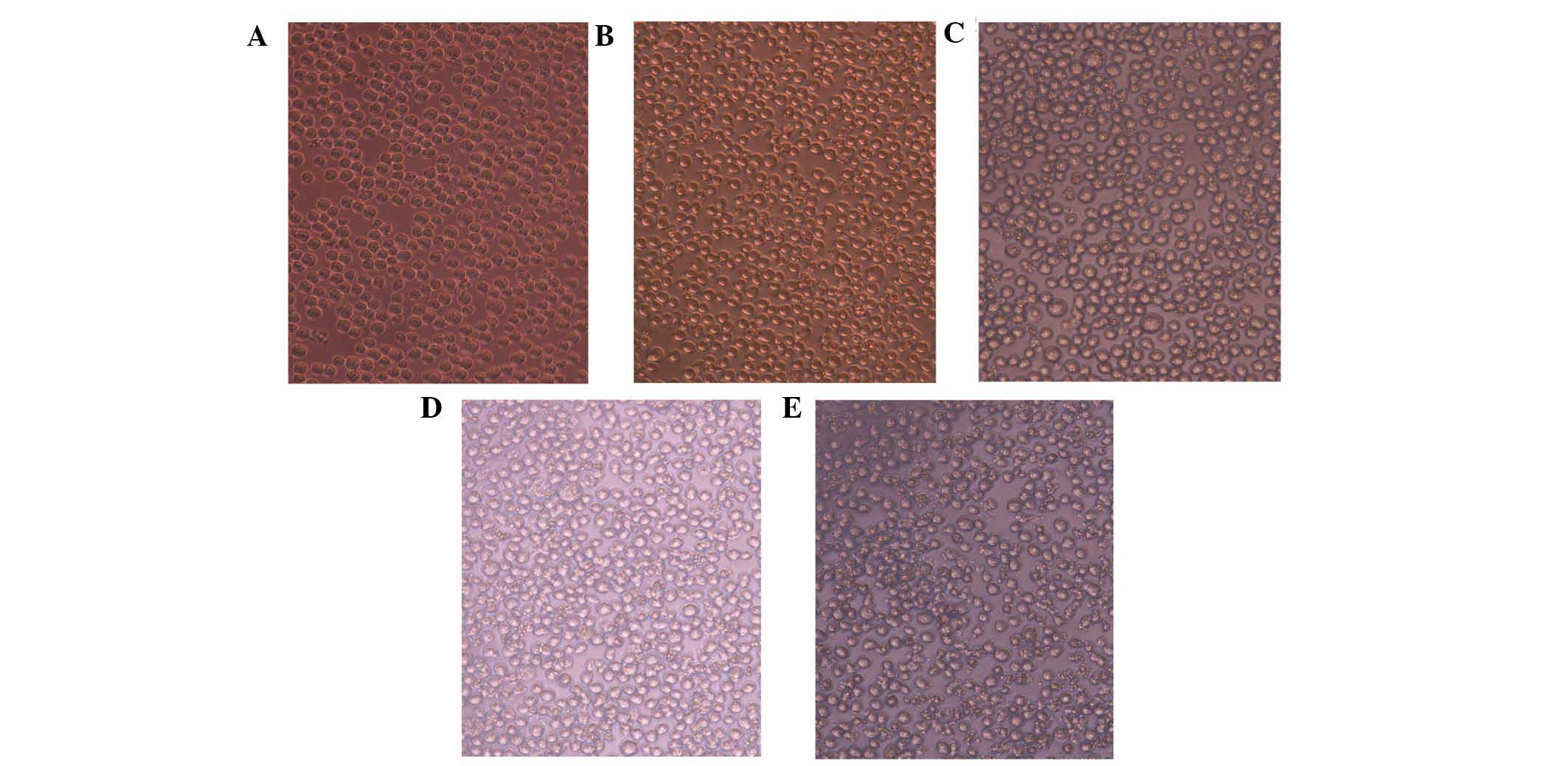
Cell morphology
Cell morphology was observed by microscopy to
identify the biological characteristics of HL-60 cells. The control
cells had a smooth membrane and were round and translucent,
arranged in an orderly manner, while the cells treated with UV LED
(Qingdao Ziyuan Photoelectronic Co., Ltd., Qingdao, China) were
found to be deformed and disordered. In addition, the transmittance
and density decreased as the dose of UV LED increased. When the
dose increased to 60 J/m2, swollen cells and cell debris
were clearly observed in the medium (Fig. 1).
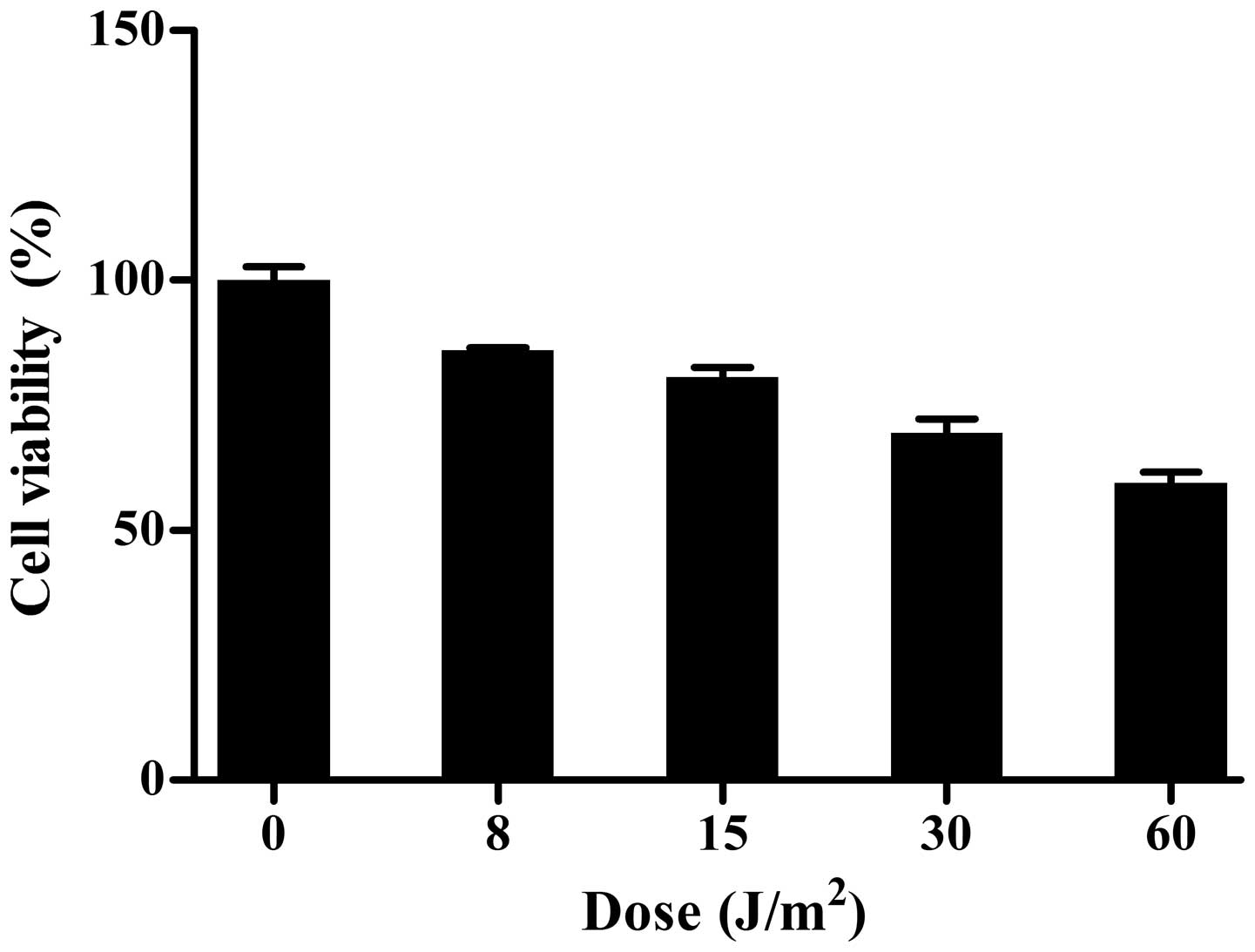
UV LED irradiation inhibits the
proliferation of HL-60 cells
The CCK-8 assay showed that, compared with the
control group, various doses of UV LED irradiation (8–60
J/m2) inhibited the proliferation of HL-60 cells in a
dose-dependent manner (Fig.
2).
UV LED irradiation induces apoptotic and
necrotic death of HL-60 cells
To understand the mechanism of the
anti-proliferative effects of UV LED irradiation on HL-60 cells,
flow cytometric analysis was performed using SR-VAD-FMK/7-AAD
double staining (Guava Technologies). The apoptotic cell ratios
gradually increased with the increase in dose (from 8 to 30
J/m2), indicating that UV LED at 8–30 J/m2
could induce apoptosis in a dose-dependent manner. However, when
cells were exposed to 60 J/m2 UV LED irradiation, the
necrotic cell ratio markedly increased, which demonstrated that UV
LED at 60 J/m2 principally induced necrosis rather than
apoptosis (Fig. 3).
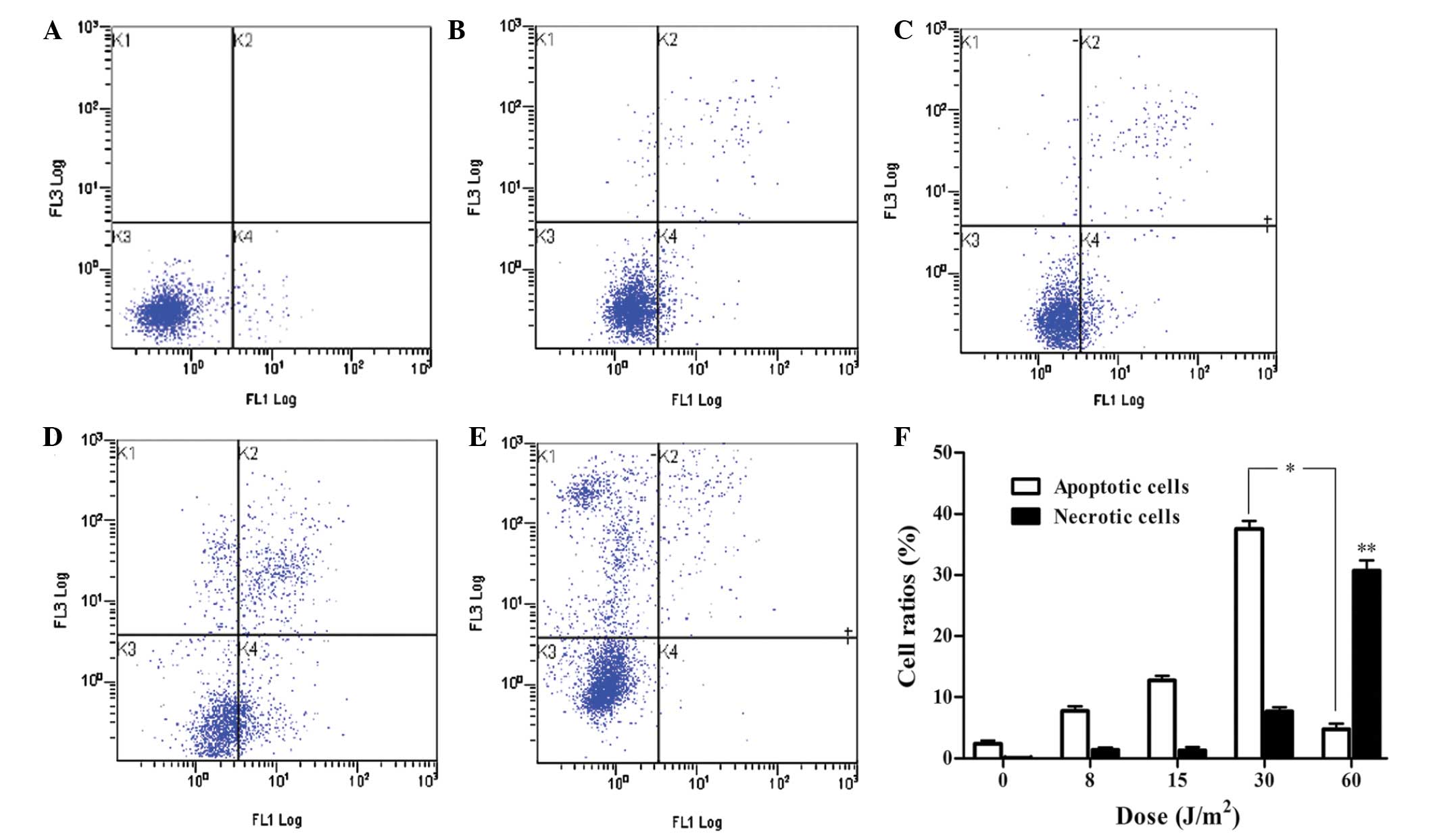 | Figure 3UV LED irradiation induces apoptotic
and necrotic death in HL-60 cells. HL-60 cells were irradiated with
(A) 0, (B) 8, (C) 15, (D) 30 and (E) 60 J/m2 and
incubated for 2 h. The four quadrants show the following: Lower
left, viable cells; lower right, early apoptotic cells; upper
right, late apoptotic cells; upper left, necrotic cells. (F)
Percentage of apoptotic and necrotic HL-60 cells exposed to UV LED
irradiation. P<0.01 for multiple pairwise comparisons of
apoptotic rate within the range of 0–30 J/m2.
*P<0.01 vs. 30 and 60 J/m2;
**P<0.01 vs. 0, 8, 15 and 30 J/m2. UV LED,
ultraviolet light-emitting diode irradiation. |
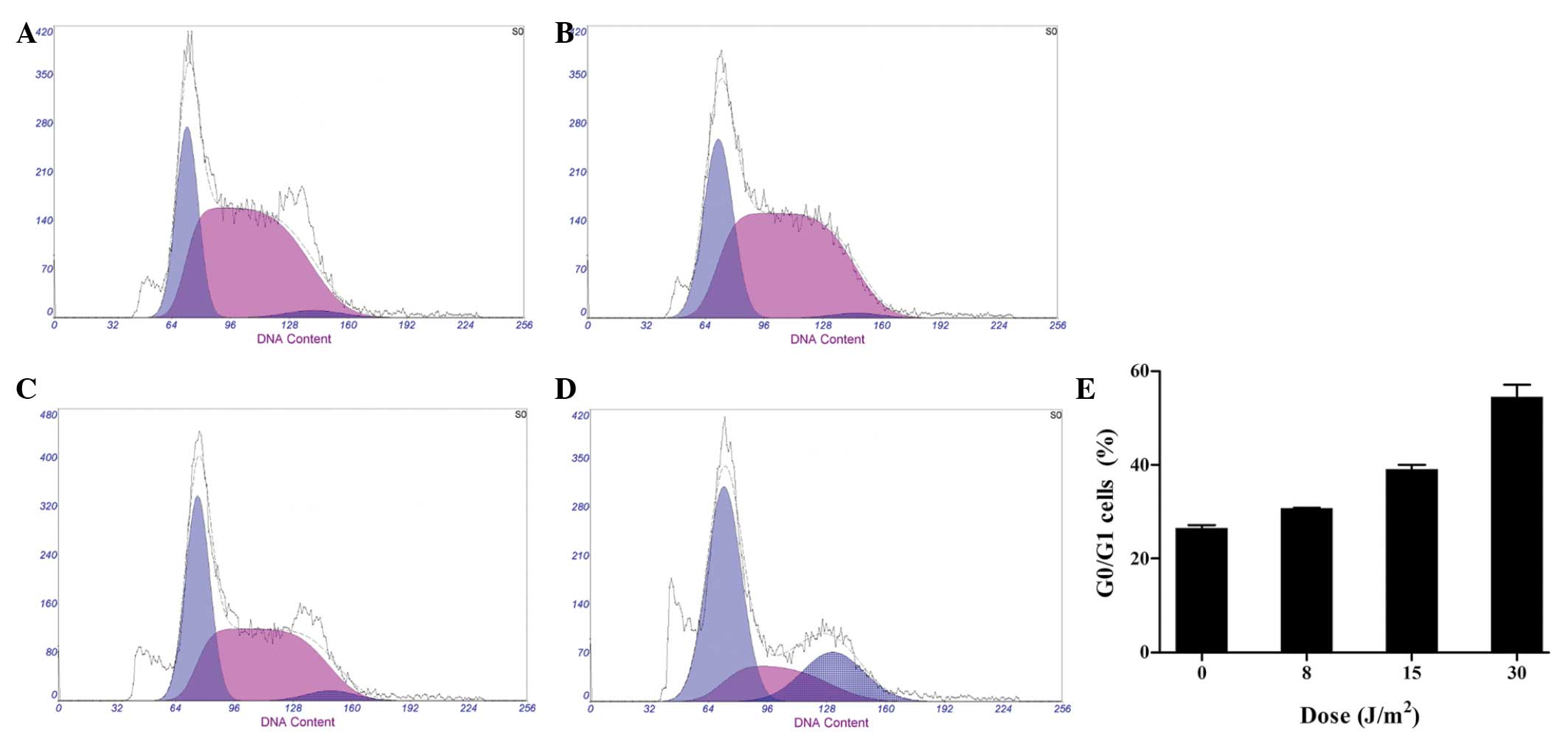
UV LED irradiation induces cell cycle
arrest of HL-60 cells
In an attempt to elucidate the mechanism underlying
the induction of apoptosis by UV LED irradiation, cell cycle
analysis was performed using flow cytometry. The percentages of
HL-60 cells in the G0/G1 phase were 27.18, 30.74, 38.23 and 54.72%
when cells were subjected to 0, 8, 5 and 30 J/m2 UV LED
irradiation, respectively. A dose-dependent increase was observed
in the percentage of G0/G1 cells at 8–30 J/m2,
indicating that UV LED irradiation was capable of inducing cell
cycle arrest at the G0/G1 phase (Fig.
4).
UV LED irradiation inhibits the mRNA
expression of Bcl-2
In order to further examine the induction of
apoptosis the mRNA expression of Bcl-2 was detected
by RT-qPCR. The results showed a decrease in the mRNA expression of
Bcl-2 at 8–30 J/m2, suggesting that UV LED
irradiation was able to downregulate the mRNA expression of
Bcl-2 (Fig. 5).
Discussion
UV radiation has been confirmed to induce apoptosis
through two biochemically and morphologically distinct processes,
apoptosis and necrosis (2).
Morphological features and chromatin changes show that various cell
lines undergo apoptosis following low doses of UVB irradiation,
whereas prolonged exposure induces necrosis (1). UV-induced apoptosis is a complex
event that involves multiple pathways. UV radiation primarily
induces DNA damage via the formation of ROS and DNA photoproducts,
predominantly cyclobutane pyrimidine dimers and
pyrimidine-pyrimidone photoproducts, that effectively block
replication and transcription mechanisms (4–6,13).
This results in p53 activation which either arrests the cell
cycle to enable DNA repair or triggers apoptosis (4,14–17).
Although DNA damage appears to be the main cause of the induction
of apoptosis (18,19), it has been reported that UV
radiation can either directly trigger clustering of death receptors
in a ligand-independent manner, or induce the release of their
natural ligands (20,21). Furthermore, UV-induced ROS are able
to directly cause early cytochrome c release due to the
mitochondrial membrane alterations, contributing independently to
the induction of apoptosis (22).
UV LEDs have become a viable option for the
replacement of conventional mercury lamps for water disinfection
(10). Despite the fact that UV
LEDs are efficient in the destruction of microorganisms, the effect
of UV LED irradiation on human cells remains to be elucidated. In
the present study, it was found that 280 nm UV LED irradiation
inhibited the proliferation of HL-60 cells in vitro. UV LED
irradiation at doses between 8–30 J/m2 was found to
induce dose-dependent apoptosis. However, a higher dose of UV LED
(60 J/m2) was found to induce necrosis, indicating the
toxic effect of UV LED irradiation at a high dose.
In response to UV-mediated DNA damage, the cell
cycle is arrested and repair mechanisms, such as nucleotide
excision repair, are activated (23). The presence of checkpoints allows
cells to accomplish DNA repair prior to DNA synthesis or mitosis,
thus reducing the incidence of DNA mutations; however, if DNA
damage is extensive and irreparable, proapoptotic genes are
targeted by p53 to initiate mitochondrial- and death
receptor-mediated apoptotic pathways, which ultimately activate a
cascade of caspases to execute apoptosis (24). The present results showed that
HL-60 cells underwent apoptosis and G0/G1 arrest when they were
subjected to 8–30 J/m2 UV LED irradiation, indicating
that apoptosis occurred when the cell entered the G1/S checkpoint
with damaged DNA.
Bcl-2 serves an important role in the
maintenance of mitochondrial membrane potential and calcium
homeostasis, in the blockage of Bax and Bak activation, and in ROS
generation, thus it acts as an antiapoptotic gene (25–27).
In the present study, UV LED irradiation at 8–30 J/m2
induced apoptosis and inhibited the mRNA expression of
Bcl-2. This suggested that the proapoptotic effect of
UV LED irradiation on HL-60 cells was associated with the
downregulation of Bcl-2 mRNA expression.
In conclusion, 280 nm UV LED irradiation inhibits
proliferation and induces apoptosis and necrosis in cultured HL-60
human leukemia cells. G0/G1 cell cycle arrest and down-regulation
of the mRNA expression of Bcl-2 are mechanisms
partially responsible for the occurrence of apoptosis. Further
research on other mechanisms is required in order to increase
understanding of the interplay between different apoptotic
pathways. This may provide an alternative way to enhance the
killing effect on tumor cells.
Abbreviations:
|
UV
|
ultraviolet
|
|
LED
|
light-emitting diode
|
|
CCK-8
|
cell counting kit-8
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
ROS
|
reactive oxygen species
|
|
OD
|
optical density
|
|
SR-VAD-FMK
|
sulforhodamine-valyl-alanyl-aspartyl-fluoromethyl-ketone
|
|
7-AAD
|
7-amino-actinomycin D
|
Acknowledgments
The authors thank Dr Lingling Cui from the Gout
Laboratory of the Affiliated Hospital of Qingdao University and Dr
Ke Lei from the Institute of Pediatrics of Affiliated Hospital of
Qingdao University for their technical assistance in the study.
References
|
1
|
Salucci S, Burattini S, Battistelli M,
Baldassarri V, Maltarello MC and Falcieri E: Ultraviolet B (UVB)
irradiation-induced apoptosis in various cell lineages in vitro.
Int J Mol Sci. 14:532–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin SJ and Cotter TG: Ultraviolet B
irradiation of human leukaemia HL-60 cells in vitro induces
apoptosis. Int J Radiat Biol. 59:1001–1016. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulms D, Zeise E, Pöppelmann B and Schwarz
T: DNA damage, death receptor activation and reactive oxygen
species contribute to ultraviolet radiation-induced apoptosis in an
essential and independent way. Oncogene. 21:5844–5851. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kulms D and Schwarz T: Molecular
mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol
Photomed. 16:195–201. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Timares L, Katiyar SK and Elmets CA: DNA
damage, apoptosis and langerhans cells-activators of UV-induced
immune tolerance. Photochem Photobiol. 84:422–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang YG, Jorgensen AG, Kaestel CG,
Wiencke AK, Lui GM, la Cour MH, Röpke CH and Nissen MH: Bcl-2, Bax
and c-Fos expression correlates to RPE cell apoptosis induced by
UV-light and daunorubicin. Current Eye Res. 20:25–34. 2000.
View Article : Google Scholar
|
|
7
|
Crawford MH, Banas MA, Ross MP, Ruby DS,
Nelson JS, Boucher R and Allerman AA: Final LDRD report:
ultraviolet water purification systems for rural environments and
mobile applications. Sandia Report. 1. pp. 352005
|
|
8
|
Würtele MA, Kolbe T, Lipsz M, Külberg A,
Weyers M, Kneissl M and Jekel M: Application of GaN-based
ultraviolet-C light emitting diodes-UV LEDs-for water disinfection.
Water Res. 45:1481–1489. 2011. View Article : Google Scholar
|
|
9
|
Chatterley C and Linden K: Demonstration
and evaluation of germicidal UV-LEDs for point-of-use water
disinfection. J Water Health. 8:479–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vilhunen S, Särkkä H and Sillanpää M:
Ultraviolet light-emitting diodes in water disinfection. Environ
Sci Pollut Res Int. 16:439–442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kemeny L, Csoma Z, Bagdi E, Banham AH,
Krenacs L and Koreck A: Targeted phototherapy of plaque-type
psoriasis using ultraviolet B-light-emitting diodes. Br J Dermatol.
163:167–173. 2010.PubMed/NCBI
|
|
12
|
Proietti De Santis L, Garcia CL, Balajee
AS, Latini P, Pichierri P, Nikaido O, Stefanini M and Palitti F:
Transcription coupled repair efficiency determines the cell cycle
progression and apoptosis after UV exposure in hamster cells. DNA
Repair (Amst). 1:209–223. 2002. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Ravanat JL, Douki T and Cadet J: Direct
and indirect effects of UV radiation on DNA and its components. J
Photochem Photobiol B. 63:88–102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy G, Young AR, Wulf HC, Kulms D and
Schwarz T: The molecular determinants of sunburn cell formation.
Exp Dermatol. 10:155–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cadet J, Sage E and Douki T: Ultraviolet
radiation-mediated damage to cellular DNA. Mutat Res. 571:3–17.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pattison DI and Davies MJ: Actions of
ultraviolet light on cellular structures. EXS. 131–157. 2006.
|
|
18
|
Dunkern TR, Fritz G and Kaina B:
Ultraviolet light-induced DNA damage triggers apoptosis in
nucleotide excision repair-deficient cells via Bcl-2 decline and
caspase-3/-8 activation. Oncogene. 20:6026–6038. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stege H, Roza L, Vink AA, Grewe M, Ruzicka
T, Grether-Beck S and Krutmann J: Enzyme plus light therapy to
repair DNA damage in ultraviolet-B-irradiated human skin. Proc Natl
Acad Sci USA. 97:1790–1795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang S and Kochevar IE: Ultraviolet A
radiation induces rapid apoptosis of human leukemia cells by Fas
ligand-independent activation of the Fas death pathways. Photochem
Photobiol. 78:61–67. 2003.PubMed/NCBI
|
|
21
|
Bang B, Gniadecki R, Larsen JK, Baadsgaard
O and Skov L: In vivo UVB irradiation induces clustering of Fas
(CD95) on human epidermal cells. Exp Dermatol. 12:791–798. 2003.
View Article : Google Scholar
|
|
22
|
Ali D, Verma A, Mujtaba F, Dwivedi A, Hans
RK and Ray RS: UVB-induced apoptosis and DNA damaging potential of
chrysene via reactive oxygen species in human keratinocytes.
Toxicol Lett. 204:199–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Costa RM, Chiganças V, Galhardo Rda S,
Carvalho H and Menck CF: The eukaryotic nucleotide excision repair
pathway. Biochimie. 85:1083–1099. 2003. View Article : Google Scholar
|
|
24
|
Benchimol S: p53-dependent pathways of
apoptosis. Cell Death Differ. 8:1049–1051. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rizzuto R, Pinton P, Ferrari D, Chami M,
Szabadkai G, Magalhães PJ, Di Virgilio F and Pozzan T: Calcium and
apoptosis: Facts and hypotheses. Oncogene. 22:8619–8627. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adams JM and Cory S: Life-or-death
decisions by the Bcl-2 protein family. Trends Biochem Sci.
26:61–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Assefa Z, Garmyn M, Vantieghem A, Declercq
W, Vandenabeele P, Vandenheede JR and Agostinis P: Ultraviolet B
radiation-induced apoptosis in human keratinocytes: Cytosolic
activation of procaspase-8 and the role of Bcl-2. FEBS Lett.
540:125–132. 2003. View Article : Google Scholar : PubMed/NCBI
|