Introduction
Atherosclerosis is a chronic vascular inflammatory
disease, characterized by narrowing and rigidity of the lumen as a
result of cholesterol and lipid accumulation (1,2).
Abnormal proliferation of intimal vascular smooth muscle cells
(VSMCs) leads to intimal thickening of the aorta, and has an
important role in initiation and amplification of atherogenesis
(3). Oxidized low-density
lipoprotein (ox-LDL) is a mitogen in VSMCs and stimulates the
proliferation of VSMCs and activation of the extracellular
signal-regulated protein kinase (ERK)1/2 signaling pathway.
Therefore, the ox-LDL-induced proliferation of VSMCs in the intima
of the arterial wall is considered to be an important factor in
atherosclerotic plaque development (4).
Epimedium brevicornum
Maxim, a traditional Chinese herbal medicine, has
been widely used for tonifying kidneys and strengthening bone for
thousands of years in China, Korea and Japan (5–7).
Icariin (C33H40O15; molecular
weight, 676.67; Fig. 1), a
flavonoid isolated from Epimedium brevicornum Maxim, is
considered as the main pharmacological active constituent (8,9) and
has been reported to possess various pharmacological effects,
including anti-inflammatory, anti-osteoporosis (10), anti-tumor (11), immunoregulatory (12) and anti-oxidative actions (13). In addition, icariin has been shown
to have beneficial effects on cardiovascular diseases such as
atherosclerosis (14,15). However, the potential mechanisms of
action of icariin against atherosclerosis have remained to be fully
elucidated. In view of this, the present study was designed to
elucidate whether icariin can attenuate the initiation and
progression of atherosclerosis. The effects of icariin on
ox-LDL-induced proliferation of VSMCs were assessed, and the
results indicated that they are mediated via suppression of
cell-cycle regulatory protein proliferating cell nuclear antigen
(PCNA) and deactivation of ERK1/2.
Materials and methods
Cell culture
Human aortic vascular smooth muscle cells (HA-VSMCs)
were obtained from the Chinese Academy of Sciences Cell Bank
(Shanghai, China) and cultured in 100-mm dishes in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Sijiqing Bioengineering Material Co., Ltd.
Hangzhou, China) and 1% penicillin/streptomycin (Sigma-Aldrich, St
Louis, MO, USA) at 37°C in a humidified atmosphere containing 5%
CO2.
Cell viability assay
VSMCs in the logarithmic growth phase were seeded
into 96-well plates at a density of 1×104 cells per well
and incubated for 24 h at 37°C in an atmosphere containing 5%
CO2. After pre-treatment with the indicated
concentrations of icariin (purity, >98%; Vic's Biological
Technology Co., Ltd, Sichuan, China; 0, 10, 20 or 40 µm) for
24 h prior to stimulation with oxidized low-density lipoprotein
(ox-LDL; Yiyuan Biotechnologies Co., Ltd, Guangzhou, China; 50
µg/ml) for the indicated times (24 or 48 h). Subsequently,
the medium was discarded and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT;
Sigma-Aldrich) solution was added at a final concentration of 0.5
mg/ml, followed by incubation for 4 h at 37°C. The MTT solution was
carefully removed and 150 µl dimethyl sulfoxide
(Sigma-Aldrich) was added to each well followed by a 15-min
incubation. The absorbance of each well was measured using a
microplate reader (Multiskan MK3; Thermo Fisher Scientific, Inc.)
with a reference wave length of 490 nm. The initial absorbance at 0
h, prior to icariin treatment, was also measured.
Cell cycle analysis
VSMCs in the logarithmic growth phase were seeded
into six-well plates at a density of 1×104 cells per
well and then incubated for 24 h at 37°C in an atmosphere
containing 5% CO2. After pre-treatment with the
indicated concentrations of icariin (0, 10, 20 or 40 µm) for
24 h, cells were incubated with or without ox-LDL (50 µg/ml)
for a further 24 h. The cells were trypsinized, collected and
washed twice with ice-cold phosphate-buffered saline (PBS) prior to
fixing in 70% cold ethanol at 4°C overnight. Next, the fixed cells
were re-suspended in PBS containing 100 µg/ml RNase A
(Sigma-Aldrich) and 50 µg/ml propidium iodide (PI;
Sigma-Aldrich) for 30 min at room temperature. Cells were then
analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San
Jose, CA, USA). The percentages of cells in G0/G1, S and G2/M
phases were determined using ModFit LT V3.3.11 software (Verity
Software House Inc., Topsham, ME, USA).
Western blot analysis
VSMCs in the logarithmic growth phase were seeded
into six-well plates for incubation for 24 h at 37°C in an
atmosphere containing 5% CO2. After pre-treatment with
the indicated concentrations of icariin (0, 10, 20 or 40 µm)
for 24 h, the cells were incubated with or without ox-LDL (50
µg/ml) for another 24 h. Subsequently, VSMCs were scraped in
ice-cold PBS and lysed in cold lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). After centrifugation at 13,000 × g,
the supernatant (total protein extract) was separated and
quantified using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Equal amounts of protein samples (50
µg) were loaded onto 10% SDS-PAGE gels (Applygen
Technologies, Inc., Beijing, China) and transferred to
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 5% fat-free milk in Tris-buffered
saline containing 0.05% Tween 20 (TBST; Zhongtian Jingwei
Technologies, Inc., Beijing, China) and incubated with the primary
rabbit anti-human monoclonal antibodies against ERK1/2 (dilution,
1:1,000; Abcam, Cambridge, MA, USA; cat. no. ab36911),
anti-phosphorylated-ERK1/2 (dilution, 1:1,000; Abcam; cat. no.
50011) or anti-PCNA (dilution, 1:1,000; Abcam; cat. no. ab92552) or
anti-GAPDH (dilution, 1:1,000; Sigma-Aldrich; cat. no. sab4300645)
at 4°C overnight. Following incubation, the membranes were washed
three times in TBST for 15 min and the membranes were incubated
with horseradish-peroxidase-labeled goat anti-rabbit secondary
antibody for 1 h at room temperature (dilution, 1:5,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-45101). Following
three further washes in TBST, the protein expression levels were
visualized using the enhanced chemiluminescence kit, BeyoECL Plus
(Beyotime Institute of Biotechnology), images of the blots were
captured on X-ray films (GE Healthcare, Little Chalfont, UK) and
were analyzed using ImageJ version 1.46 (National Institutes of
Health, Bethesda, MD, USA). GAPDH was used as the protein loading
control.
Statistical analysis
Statistical analysis was performed using the SPSS
17.0 statistical package (SPSS, Inc., Chicago, IL, USA). Values are
expressed as the mean ± standard deviation. One-way analysis of
variance was applied for multiple comparisons and the least
significant difference test was applied for intra-group comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Icariin inhibits ox-LDL-induced VSMC
proliferation
To evaluate the effects of icariin on the
proliferation of VSMCs induced by ox-LDL, the cell viability was
assessed using an MTT assay. As shown in Fig. 2, exposure of the cells to ox-LDL
for 24 or 48 h significantly increased the cell viability compared
with that in the control group. However, icariin inhibited
ox-LDL-induced VSMC proliferation in a concentration-dependent
manner.
Icariin reduces ox-LDL-induced cell cycle
progression and PCNA expression
To clarify the effect of icariin on cell-cycle
regulation, the cell cycle distribution of ox-LDL-induced VSMCs was
assessed using flow cytometry. As shown in Fig. 3, treatment with ox-LDL markedly
increased the percentage of VSMCs in S and G2/M phases and
correspondingly decreased the percentage of cells in G0/G1 phase.
However, pre-treatment with icariin significantly reversed these
effects in a concentration-dependent manner. To assess whether the
effects of icariin on the cell cycle were associated with the
expression of PCNA, western blot analysis was performed. As shown
in Fig. 4, the protein expression
of PCNA was markedly increased following ox-LDL treatment, which
was inhibited by icariin in a dose-dependent manner. The western
blot results were in line with the findings of the cell cycle
analysis, as ox-LDL enhanced the population of cells in S phase,
which was accompanied an increased expression of PCNA, while
icariin pre-treatment was able to inhibit these effects in a
concentration-dependent manner. These results suggested that
icariin inhibits ox-LDL-induced VSMC proliferation by blocking cell
cycle progression.
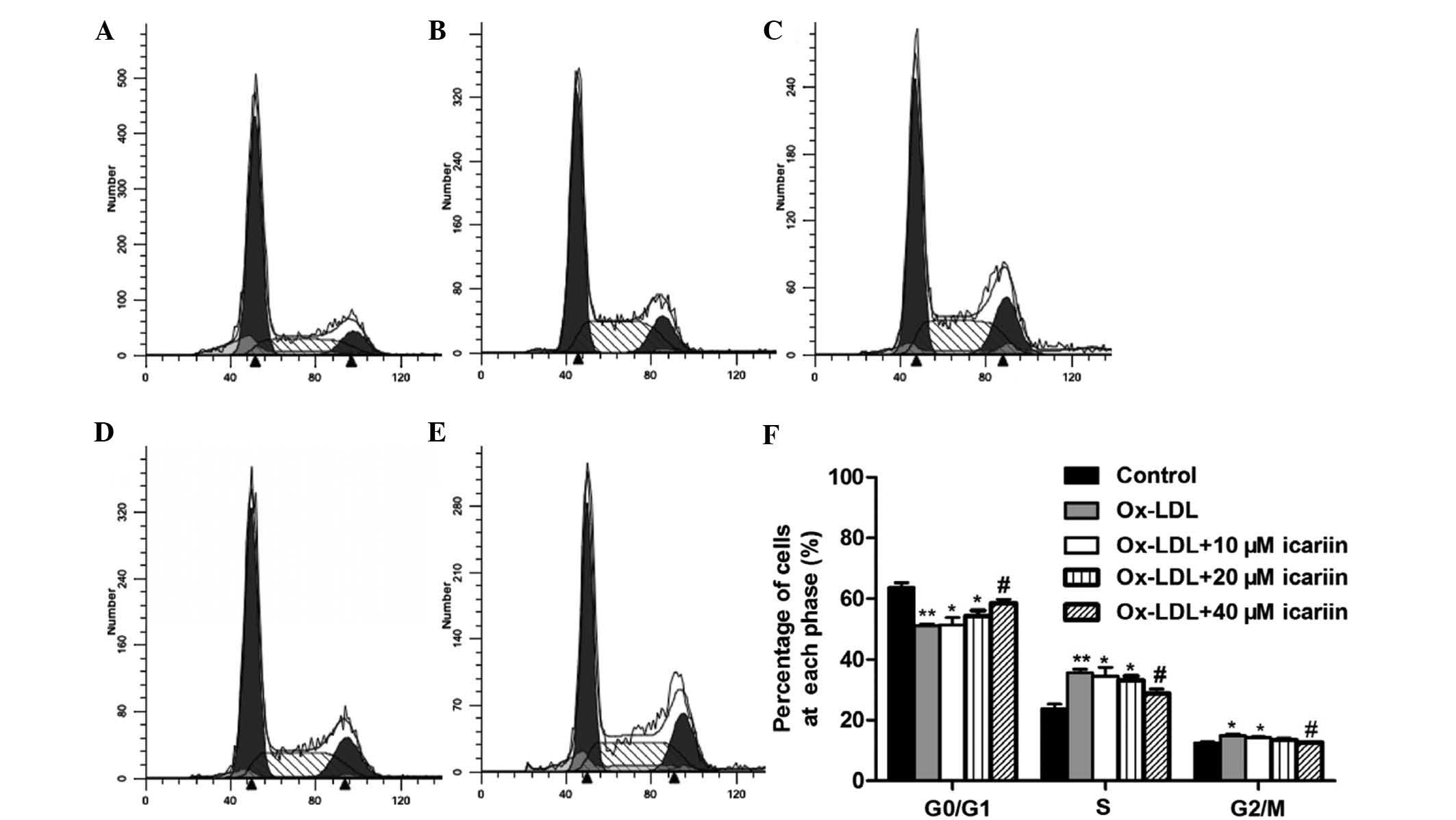 | Figure 3Effects of icariin on the cell cycle
of VSMCs induced by ox-LDL. VSMCs were pre-treated with various
concentrations of icariin (0, 10, 20, 40 µm) and then
incubated with ox-LDL (50 µg/ml) for another 24 h. The cell
cycle distribution was determined by assessing the individual
nuclear DNA content reflected by the fluorescence intensity of
incorporated propidium iodide. Flow cytometric analysis of (A)
VSMCs without any treatment (control), (B) VSMCs induced by ox-LDL
and (C–E) VSMCs induced by ox-LDL following pre-treatment with
icariin at 10, 20 or 40 µm, respectively. (F) Percentages of
cells in G0/G1, S and G2/M phases after the indicated treatments.
Representative flow cytometry graphs are shown and results are
expressed as the mean ± standard deviation from three independent
experiments. *P<0.05 and **P<0.01 vs.
the control group, #P<0.05 vs. the ox-LDL-simulated
group. VSMC, vascular smooth muscle cell; ox-LDL, oxidized
low-density lipoprotein. |
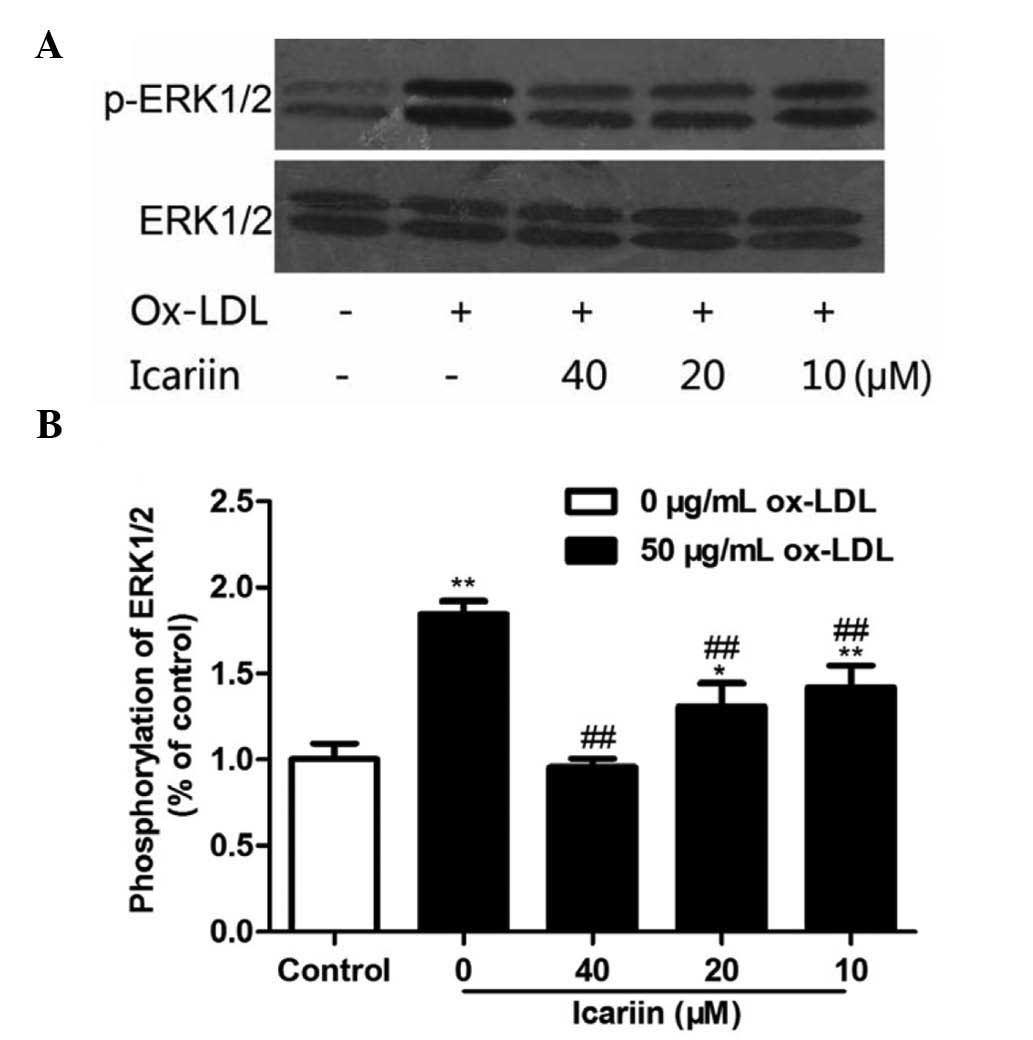
Icariin inhibits ox-LDL-induced
phosphorylation of ERK1/2
To demonstrate whether icariin inhibited
ox-LDL-induced VSMC proliferation by inhibiting the activation of
ERK1/2, the phosphorylation levels of ERK1/2 were examined. As
shown in Fig. 5, ox-LDL and
icariin had no effect on the levels of total ERK1/2. VSMCs
incubated with ox-LDL for 24 h showed markedly enhanced ERK1/2
phosphorylation, which was significantly and dose-dependently
inhibited by icariin pre-treatment. These results suggested that
icariin may reduce ox-LDL-induced proliferation of VSMCs, at least
in part, via inhibition of ox-LDL-induced ERK1/2
phosphorylation.
Discussion
Atherosclerosis is a chronic vascular inflammatory
disease (16). It is characterized
by the formation of atherosclerotic plaques consisting of foam
cells, leukocytes, platelets, inflamed smooth muscle cells and
endothelial cells (17). It is
increasingly recognized that ox-LDL has a critical role in the
promotion of atherosclerosis initiation, progression and plaque
destabilization (18). Several
studies have indicated that ox-LDL can stimulate the proliferation
of VSMCs and the activation of ERKs (4,19).
Therefore, VSMC proliferation induced by ox-LDL in the intima of
the arterial wall is thought to have a critical role in the
development of atherosclerotic lesions (4). However, inhibition of VSMC
proliferation represents a potentially important therapeutic
strategy for the prevention and treatment of atherosclerosis
(20). The present study showed
that ox-LDL induced VSMC proliferation and provided the first
evidence that icariin inhibited ox-LDL-stimulated VSMC
proliferation in a concentration-dependent manner.
The cell cycle is a highly regulated process that
involves a complex cascade of events to regulate cell proliferation
(21–23). It comprises three distinct phases:
The G0/G1 phase, the DNA synthesis-associated S phase and the G2/M
phase (23,24). Under normal conditions, VSMCs
proliferate at low rates, largely remaining in the G0/G1 phase of
the cell cycle. Following proliferative stimulation, VSMCs re-enter
the cell cycle (3,25). In the present study,
flow-cytometric analysis indicated that ox-LDL treatment promoted
the proliferation of VSMCs and increased the S-phase population
with a simultaneous decrease in the G0/G1-phase population, while
pre-treatment with icariin significantly reversed these effects.
The results suggested that icariin may reduce ox-LDL-induced
proliferation of VSMCs by inhibiting their transition from G0/G1
phase to S phase.
PCNA is involved in a number of essential cellular
processes, including DNA repair, DNA replication and cell-cycle
regulation (26), and is regulated
by a variety of mechanisms that act at the transcriptional as well
as the post-transcriptional level (27). PCNA is required for G0/G1-to-S
phase transition and its synthesis is tightly regulated during the
cell cycle (4). The present study
found that the percentage of VSMCs in S and G2/M phase increased
after treatment with ox-LDL, which was inhibited by pre-treatment
with icariin. These findings were in line with the effects of
ox-LDL and icariin on the protein expression of PCNA: Ox-LDL
treatment enhanced the expression of PCNA, while icariin
dose-dependently inhibited these increases. It is therefore likely
that icariin inhibited ox-LDL-induced VSMC proliferation through
inhibition of PCNA expression.
ERK is a widely expressed protein kinase and an
intracellular signaling molecule that is involved in cell
proliferation (28). Previous
studies have indicated that ox-LDL induces VSMC proliferation
through activation of the ERK pathway (19,29,30).
In line with these results, the present study revealed that the
phosphorylation of ERK1/2 in VSMCs was enhanced by ox-LDL, which
was inhibited by pre-treatment with icariin. These results
suggested that icariin significantly inhibited ox-LDL-induced
proliferation of VSMCs by blocking cell-cycle progression, partly
via inhibiting the ox-LDL-induced activation of ERK1/2.
In conclusion, the present study demonstrated that
icariin inhibited the proliferation of VSMCs stimulated by ox-LDL
via decreasing the S-phase population of the cell cycle.
Ox-LDL-induced phosphorylation of ERK1/2 and the expression of PCNA
were also suppressed by icariin. These findings suggested that
icariin may inhibit ox-LDL-induced proliferation of VSMCs by
inactivating the ERK1/2 signaling pathway and by suppressing the
expression of PCNA. Icariin may therefore be able to reduce the
development of atherosclerosis.
Acknowledgments
The present study was supported by the Jilin Natural
Science Foundation (grant no. 20150101221JC) and by the Applied
Research Project of Tonghua Normal University (grant no.
2014096).
References
|
1
|
Dell'omo G, Penno G, Pucci L, Lucchesi D,
Fotino C, Del Prato S and Pedrinelli R: ACE gene insertion/deletion
polymorphism modulates capillary permeability in hypertension. Clin
Sci (Lond). 111:3573–64. 2006. View Article : Google Scholar
|
|
2
|
Zhu F, Li C, Jin XP, Weng SX, Fan LL,
Zheng Z, Li WL, Wang F, Wang WF, Hu XF, et al: Celastrol may have
an anti-atherosclerosis effect in a rabbit experimental carotid
atherosclerosis model. Int J Clin Exp Med. 7:1684–1691.
2014.PubMed/NCBI
|
|
3
|
Boehm M and Nabel EG: Cell cycle and cell
migration: New pieces to the puzzle. Circulation. 103:2879–2881.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang WC, Yu YM, Chiang SY and Tseng CY:
Ellagic acid suppresses oxidised low-density lipoprotein-induced
aortic smooth muscle cell proliferation: Studies on the activation
of extracellular signal-regulated kinase 1/2 and proliferating cell
nuclear antigen expression. Br J Nutr. 99:709–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY,
Yao XS, Leung PC and Wong MS: The osteoprotective effect of Herba
epimedii (HEP) extract in vivo and in vitro. Evid Based Complement
Alternat Med. 2:353–361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hidaka S, Okamoto Y, Yamada Y, Kon Y and
Kimura T: A Japanese herbal medicine, Chujo-to, has a beneficial
effect on osteoporosis in rats. Phytother Res. 13:14–19. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakamoto S, Sassa S, Kudo H, Suzuki S,
Mitamura T and Shinoda H: Preventive effects of a herbal medicine
on bone loss in rats treated with a GnRH agonist. Eur J Endocrinol.
143:139–142. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao-Hong D, Chang-Qin X, Jian-Hua H,
Wen-Jiang Z and Bing S: Icariin delays homocysteine-induced
endothelial cellular senescence involving activation of the
PI3K/AKT-eNOS signaling pathway. Pharm Biol. 51:433–440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng KW, Fu H, Liu GX and Wang XM: Icariin
attenuates lipo-polysaccharide-induced microglial activation and
resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and
JNK/p38 MAPK pathways. Int Immunopharmacol. 10:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang G, Qin L, Sheng H, Yeung KW, Yeung
HY, Cheung WH, Griffith J, Chan CW, Lee KM and Leung KS:
Epimedium-derived phytoestrogen exert beneficial effect on
preventing steroid-associated osteonecrosis in rabbits with
inhibition of both thrombosis and lipid-deposition. Bone.
40:685–692. 2007. View Article : Google Scholar
|
|
11
|
Zhang DC, Liu JL, Ding YB, Xia JG and Chen
GY: Icariin potentiates the antitumor activity of gemcitabine in
gallbladder cancer by suppressing NF-κB. Acta Pharmacol Sin.
34:301–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yap SP, Shen P, Li J, Lee LS and Yong EL:
Molecular and pharmacodynamic properties of estrogenic extracts
from the traditional Chinese medicinal herb, Epimedium. J
Ethnopharmacol. 113:218–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Huang JH, Ning Y and Shen ZY:
Icariin and its pharmaceutical efficacy: Research progress of
molecular mechanism. J Chin Integr Med. 9:1179–1184. 2011.In
Chinese. View Article : Google Scholar
|
|
14
|
Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao
YX and Dong JC: Icariin attenuates LPS-induced acute inflammatory
responses: Involvement of PI3K/Akt and NF-kappaB signaling pathway.
Eur J Pharmacol. 642:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu HB and Huang ZQ: Vasorelaxant effects
of icariin on isolated canine coronary artery. J Cardiovasc
Pharmacol. 49:207–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nurgeldiyeva MJ, Hojakuliyev BG and
Muhammedov MB: Correlation of atherogenesis with an infection of
Candida albicans. Int J Clin Exp Med. 7:2137–2143. 2014.PubMed/NCBI
|
|
17
|
Butcher MJ, Herre M, Ley K and Galkina E:
Flow cytometry analysis of immune cells within murine aortas. J Vis
Exp. pii: 2848. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Wang Y, Li H, Shen G and Hu S:
Ox-LDL influences peripheral Th17/Treg balance by modulating Treg
apoptosis and Th17 proliferation in atherosclerotic cerebral
infarction. Cell Physiol Biochem. 33:1849–1862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang CM, Chien CS, Hsiao LD, Pan SL, Wang
CC, Chiu CT and Lin CC: Mitogenic effect of oxidized low-density
lipoprotein on vascular smooth muscle cells mediated by activation
of Ras/Raf/MEK/MAPK pathway. Br J Pharmacol. 132:1531–1541. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abate-Daga D, Hanada K, Davis JL, Yang JC,
Rosenberg SA and Morgan RA: Expression profiling of TCR-engineered
T cells demonstrates overexpression of multiple inhibitory
receptors in persisting lymphocytes. Blood. 122:1399–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian R, Li Y and Gao M: Shikonin causes
cell cycle arrest and induces apoptosis by regulating the
EGFR/NF-κB signaling pathway in human epidermoid carcinoma A431
cells. Biosci Rep. 35:pii: e00189. 2015. View Article : Google Scholar
|
|
22
|
Li QY, Zhu YF, Zhang M, Chen L, Zhang Z,
Du YL, Ren GQ, Tang JM, Zhong MK and Shi XJ: Chlorogenic acid
inhibits hypoxia-induced pulmonary artery smooth muscle cells
proliferation via c-Src and Shc/Grb2/ERK2 signaling pathway. Eur J
Pharmacol. 751:81–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu W, Du S and Wang J: Berberine inhibits
the proliferation of prostate cancer cells and induces
G0/G1 or G2/M phase arrest at
different concentrations. Mol Med Rep. 11:3920–3924.
2015.PubMed/NCBI
|
|
24
|
Elledge SJ: Cell cycle checkpoints:
Preventing an identity crisis. Science. 274:1664–1672. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JY, Kim KH, Lee WR, An HJ, Lee SJ, Han
SM, Lee KG, Park YY, Kim KS and Lee YS: Apamin inhibits
PDGF-BB-induced vascular smooth muscle cell proliferation and
migration through suppressions of activated Akt and Erk signaling
pathway. Vascul Pharmacol. 70:8–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He H, Tan CK, Downey KM and So AG: A tumor
necrosis factor alpha- and interleukin 6-inducible protein that
interacts with the small subunit of DNA polymerase delta and
proliferating cell nuclear antigen. Proc Natl Acad Sci USA.
98:11979–11984. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li YY, Wang L and Lu CD: An E2F site in
the 5′-promoter region contributes to serum-dependent up-regulation
of the human proliferating cell nuclear antigen gene. Febs Lett.
544:112–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roskoski R Jr: ERK1/2 MAP kinases:
Structure, function and regulation. Pharmacol Res. 66:105–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Zhang M, Li Y, Liu S, Ping S,
Wang J, Ning F, Xie F and Li C: Simvastatin inhibits the additive
activation of ERK1/2 and proliferation of rat vascular smooth
muscle cells induced by combined mechanical stress and oxLDL
through LOX-1 pathway. Cell Signal. 25:332–340. 2013. View Article : Google Scholar
|
|
30
|
Farrokhi E, Samani KG and Chaleshtori MH:
Oxidized low-density lipoprotein increases bone sialoprotein
expression in vascular smooth muscle cells via runt-related
transcription factor 2. Cell Signal. 349:240–243. 2015.
|