Introduction
Bone morphogenetic proteins (BMPs) belong to the
transforming growth factor-β super family and were originally
identified to induce ectopic bone growth and cartilage formation as
osteoinductive cytokines (1,2).
Subsequently, previous studies have demonstrated that the BMPs
perform multiple functions in the regulation of cell growth,
differentiation and apoptosis in various cell types (3–5).
BMP9, a member of the BMP family, is a secreted protein that is
expressed in the liver (6). BMP9
induces ectopic bone growth and hypertrophic chondrocyte formation,
in addition to supporting the differentiation of mesenchymal cells
(MSCs) into cartilage (7). Animal
experiments have demonstrated that BMP9 has an increased capacity
for the induction of osteogenesis in MSCs when compared with BMP2
and BMP7, which have been used in clinical therapy for bone defects
and additional orthopedic diseases (8,9).
MicroRNAs (miRNAs) are a class of endogenous
noncoding RNAs, of approximately 22 nucleotides in length, which
act as post-transcriptional regulators in gene expression, via
combining to the 3′-untranslated region (UTR) region of the
targeted mRNA, causing it to degrade and inhibiting its translation
(10). miRNAs are involved in
diverse physiological and pathological processes and previous
studies have identified that certain miRNAs may positively or
negatively regulate osteogenesis and osteoclastogenesis (10–12).
These results indicated that miRNAs may be therapeutic targets in
the treatment of bone defects and additional orthopedic
diseases.
In the present study, the expression of miR-23b was
analyzed and the function in osteogenesis of BMP9-induced C2C12
myoblasts was investigated via the transfection of exogenous mimics
and inhibitors of miR-23b.
Materials and methods
Cell culture
The C2C12 myoblast, HCT116 and HEK293T cell lines
were purchased from the American Type Culture Collection (Manassas,
VA, USA), and were maintained in complete Dulbecco's modified
Eagle's medium (DMEM; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). The
cells were incubated at 37°C and a 5% CO2 in a
humidified atmosphere.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from each sample using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Quality of the RNA
was evaluated using the NanoDrop 1000 UV-Vis spectrophotometer
(Thermo Fisher Scientific, Inc.) and first-strand cDNA was
synthesized using the PrimeScript™ RT reagent kit (Takara, Bio,
Inc., Otsu, Japan). For miR-23b, the reverse transcription primer
was used. SYBR Green II dye-based qPCR analysis was conducted using
the Rotor-Gene 6000 Real-Time PCR machine (Corbett Research,
Mortlake, Australia). The amplification program was set as follows:
Initial denaturation at 94°C for 10 min; 35 cycles of denaturation
at 94°C for 1 min, annealing at 55°C for 15 sec and extension at
70°C for 15 sec; and a final extension step at 70°C for 5 min. The
RT-qPCR was conducted using the 2−∆∆Ct method and each
sample of 0.1 µg of cDNA was tested in triplicate (13). Glyceraldehyde 3-phosphate
dehydrogenase and U6 were used as endogenous normalization
controls. The primer sequences of the genes are presented in
Table I.
 | Table IPrimer sequences of genes. |
Table I
Primer sequences of genes.
| Gene | Primer sequence
(5′-3′) |
|---|
| Runx2 | F:
GGTGAAACTCTTGCCTCGTC |
| R:
AGTCCCAACTTCCTGTGCT |
| ALP | F:
TGCCTACTTGTGTGGCGTGAA |
| R:
TCACCCGAGTGGTAGTCACAATG |
| OCN | F:
TCTGACAAAGCCTTCATGTCC |
| R:
AAATAGTGATACCGTAGATGCG |
| OPN | F:
ACACTTTCACTCCAATCGTCC |
| R:
TGCCCTTTCCGTTGTTGTCC |
| miR-23b | RT:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAATCA |
| F:
GAGGGTTCCTGGCATGC |
| R:
GTGCAGGGTCCGAGGT |
| GAPDH | F:
AATGTGTCCGTCGTGGATCTGA |
| R:
AGTGTAGCCCAAGATGCCCTTC |
| U6 | RT:
AAAATATGGAACGCTTCACGAATTTG |
| F:
CTCGCTTCGGCAGCACATATACT |
| R:
ACGCTTCACGAATTTGCGTGTC |
Plasmids construction
The 3′UTR constructs of runt-related transcription
factor 2 (Runx2) were chemically synthesized, and were then cloned
into the pMIR-REPORT miRNA Expression Reporter Vector System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) between the
Hind III and Pme I (Promega Corporation, Madison, WI,
USA) restriction enzyme sites subsequent to annealing by mixing
with primers in boiling water for 10 min and allowed to cool. The
3′-UTR region fragments of the Runx2 containing the predicted
binding sites of mmu-miR-23b and its mutant sequence were
synthesized as presented in Table
II, and the β-galactosidase (β-gal) reporter plasmid
(Invitrogen; Thermo Fisher Scientific, Inc.) as a control.
 | Table IISynthesized sequences of Runx2-luc
reporter. |
Table II
Synthesized sequences of Runx2-luc
reporter.
| Gene | Sequence |
|---|
| Wild type |
5′-CTAGTCGGAAATATTGCTAAGCAATCTCAATTCCTTCAGGCATAATGTGATTTT-3′ |
|
3′-AGCTAAAATCACATTATGCCTGAAGGAATTGAGATTGCTTAGCAATATTTCCGA-5′ |
| Mutant |
5′-CTAGTCGGAAATATTGCTAAGCAATCTCAATTCCTTCAGGCATAACTTGATTTT-3′ |
|
3′-AGCTAAAATCAAGTTATGCCTGAAGGAATTGAGATTGCTTAGCAATATTTCCGA-5′ |
Transfection of miRNA mimics, inhibitors
and plasmids
The miRNA mimics (miR-23b mimic), inhibitors
(miR-23b inhibitor) and negative controls of miR-23b were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China) and
transfected into C2C12 at final concentrations of 50 nM per well in
a 24-well plate with Entranster-R transfection reagent (Engreen,
Beijing, China) following the manufacturer's instructions. The
component was mixed in serum-free DMEM, and then the transfection
was conducted in complete DMEM and refreshed 6 h subsequent to
transfection. The plasmids were transfected into cells using
Lipofectamine 2000 Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) as described previously (14).
Preparation of BMP9-conditioned
medium
The recombinant adenovirus expressing BMP9 (Ad-BMP9)
and short interfering (si)Runx2 (Ad-siRunx2) were generated
previously using the AdEasy system, as developed by Dr T.C. He (The
University of Chicago, Chicago, IL, USA) (15). BMP9-conditioned medium was prepared
as previously described (16).
Briefly, HCT116 cells were infected with an optimal titer of 0.2
µl Ad-BMP9. A total of 6 h later, the culture medium was
refreshed with serum-free DMEM. The BMP9-conditioned medium was
collected at 24 and 48 h subsequent to the exchange of the medium
and it was used immediately with another stored at 4°C.
Western blotting
Western blotting was performed according to a
standard protocol as previously described (17). C2C12 cells in dishes were lysed
with radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China), and the total protein
was separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and electro-blotted onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, CA, USA). The
membranes were then incubated with an optimal concentration of the
following primary antibodies: Anti-Runx2 (1:1,000) and anti-β-actin
(1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Protein
bands were visualized using the Quantity One software 4.5.2
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Alkaline phosphatase (ALP), Alizarin Red
S (ARS) staining and the quantitative ALP assay
ALP activity was assessed using a modified Great
EscAPe SEAP Chemiluminescence kit (Clontech Laboratories, Inc.,
Mountain View, CA, USA) and/or histochemical staining assay [using
a mixture of 0.1 mg/ml naphthol AS-MX phosphate (Sigma-Aldrich, St.
Louis, MO, USA) and 0.6 mg/ml Fast Blue BB salt (Sigma-Aldrich)] as
described (18). For quantitative
ALP measurement, the ALP activity was determined at a wavelength of
405 nm (E6080; Promega Corporation) in triplicate and the results
were repeated in at least three independent experiments.
ARS staining was conducted to evaluate mineralized
matrix nodules as described previously (19). Briefly, cells were cultured in the
presence of ascorbic acid (50 µg/ml; Beijing Solarbio
Science and Technology Co., Ltd., Beijing, China) and
β-glycerophosphate (10 mmol/l; Beijing Solarbio Science and
Technology Co., Ltd.). Cells were fixed with 0.05% (v/v)
glutaraldehyde (Chongqing Chuandong Chemical Group Co., Ltd.,
Chongqing, China) at room temperature for 10 min. Subsequent to
washing with distilled water, the fixed cells were incubated with
0.4% ARS (Sigma-Aldrich) for 5 min, followed by extensive washing
with distilled water. The staining of calcium mineral deposits was
recorded under bright field microscopy (T-DH; Nikon Corporation,
Tokyo, Japan).
Dual luciferase reporter assay
A total of 0.4 µg β-gal reporter plasmid or
pMir-Runx2 plasmid and 0.3 µg mimics were co-transfected
into 293T cells using Lipofectamine 2000 reagent. Cells were
harvested at 48 h subsequent to transfection and assayed for
firefly luciferase activity using the Dual-Glo™ Luciferase Assay
system (Promega Corporation). Firefly luciferase activity was
normalized to β-gal.
Bio-informatics prediction
To predict the target genes of miR-23b during the
C2C12 myoblast differentiation induced by BMP9, the following three
miRNA target prediction databases were used: TargetScan (http://www.targetscan.org), PicTar (http://www.pictar.org) and miRbase (http://www.mirbase.org).
Statistical analysis
The results represent the average of three
independent experiments and the data are presented as the mean ±
standard deviation. Statistical significance was determined using
Student's paired t-test, and P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-23b expression reduces in the early
stage of BMP9-induced osteoblast differentiation of C2C12
myoblasts
Several miRNAs including miR-21, miR-23b and miR-155
have been previously identified to be expressed during the early
stages of MSC osteogenesis by microarray data analysis (data not
shown). Given that the role of miR-23b in the regulation of
osteogenesis remains to be defined, the current study investigated
whether miR-23b serves a role in regulating BMP9-induced osteogenic
differentiation. By RT-qPCR, it was identified that the expression
levels of miR-23b were downregulated during the early stages of
BMP9-induced C2C12 osteogenic differentiation, which reached its
minimum on day 3 (Fig. 1A). To
further confirm the effect of BMP9, RT-qPCR was used to detect the
mRNA expression levels of osteoblast-specific genes over 7 days,
including ALP (Fig. 1B), OCN
(Fig. 1C), OPN (Fig. 1D) and Runx2 (Fig. 1E). Similar to the results of a
previous study, BMP9 was observed to promote the expression of
these genes in C2C12 myoblasts (20).
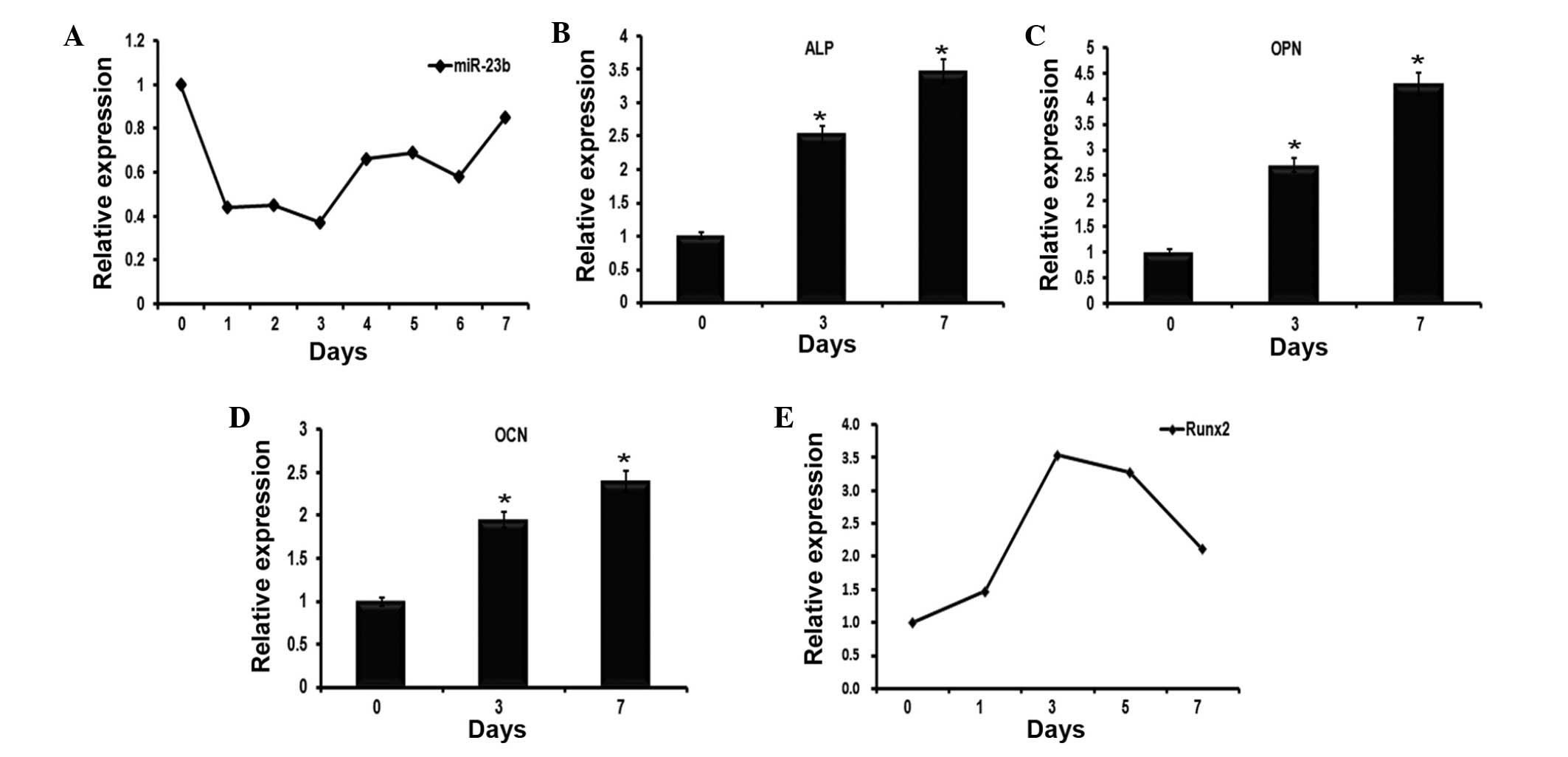 | Figure 1Expression profiles of miR-23b and
osteogenesis-associated genes during the early stages of
BMP9-induced osteogenic differentiation of C2C12 myoblasts. (A)
C2C12 myoblast cell lines were stimulated to undergo osteoblast
differentiation using BMP9. RT-qPCR was used to examine the
expression levels of miR-23b, which were normalized to U6 as a
control. The data demonstrated that miR-23b was reduced over seven
days. (B–D) Cells underwent the same treatment as in (A), then were
collected at 0, 3 and 7 days. The expression levels of (B) ALP, (C)
OCN and (D) OPN were evaluated by RT-qPCR. n=3,
*P<0.05, vs. 0 day. (E) RT-qPCR detected the
expression of Runx2 in BMP9-induced C2C12 myoblasts over 7 days.
miR-23b, microRNA-23b; BMP9, bone morphogenetic protein 9; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; ALP,
alkaline phosphatase; OCN, osteocalcin; OPN, osteopontin; Runx2,
runt-related transcription factor 2. |
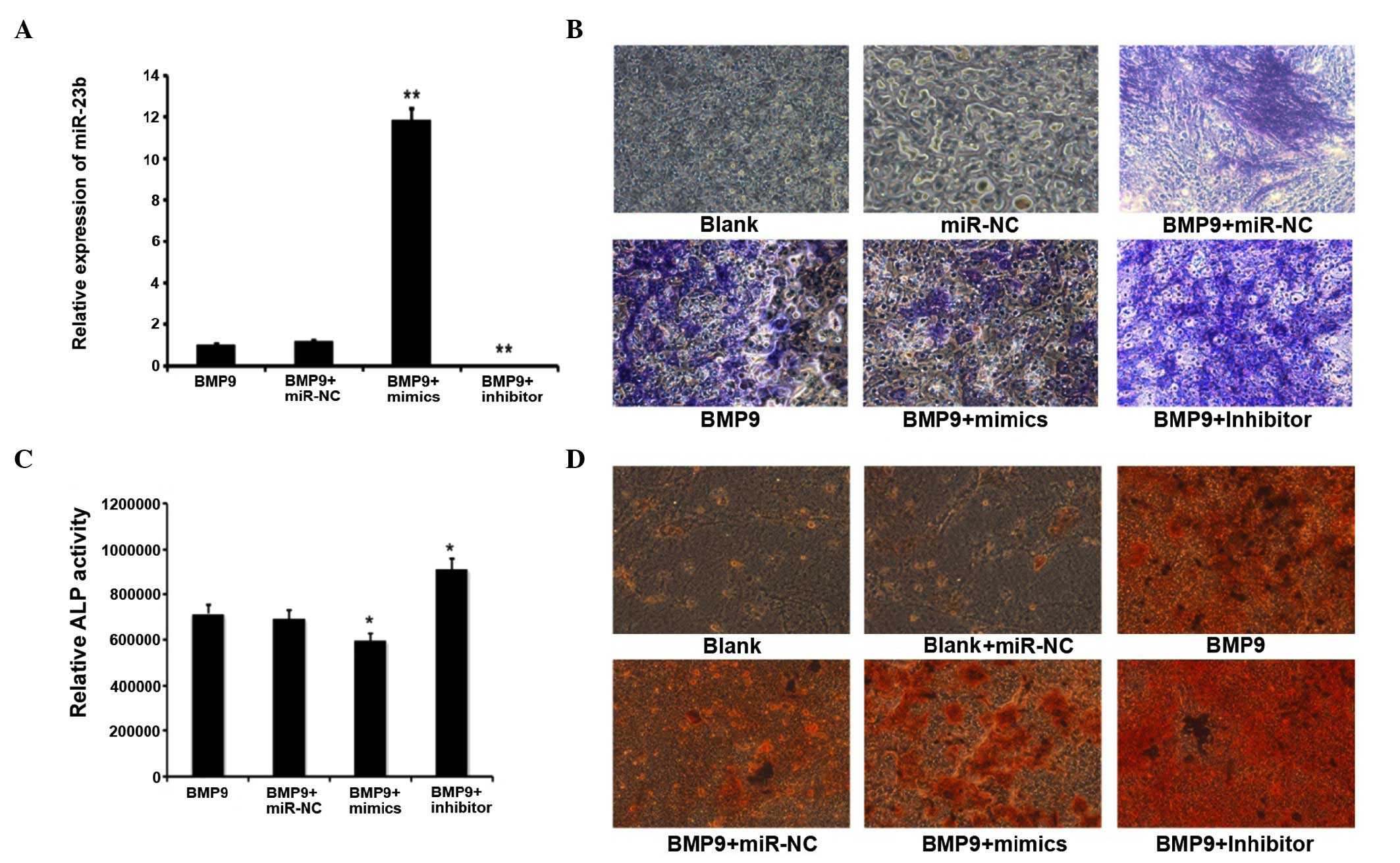
Downregulation of miR-23b could promote
osteoblast differentiation of C2C12 myoblasts induced by BMP9
RT-qPCR analyzed the intracellular miR-23b content
subsequent to transfection of exogenous synthetic molecules
expressing or inhibiting miR-23b, and the control, termed miR-23b
mimics, miR-23b inhibitor and miR-C, respectively. Subsequent to
induction of C2C12 by BMP9 for 7 days, the intracellular miR-23b
level was identified to be significantly elevated by transfection
with the miR-23b mimics, whereas the miR-23b inhibitor led to a
reduction in miR-23b content, miR-NC had a non-significant effect
(Fig. 2A). ALP (Fig. 2B) and ARS (Fig. 2D) staining demonstrated that the
ALP level and calcium deposition in miR-23b-transfected C2C12 cells
induced by BMP9 were significantly lower than those in the negative
control group. On the contrary, these levels were much higher in
the miR-23b inhibitor-transfected group. The quantitative ALP
assay, which was performed using the same method as for ALP
staining at the same time points subsequent to transfection with
miR-23b, the miR-23b inhibitor and miR-NC, implied that ALP
activity was inhibited by 27% compared with the control group on
day 7 following miR-23b transfection. The miR-23b inhibitor
enhanced ALP levels by 2.4-fold when compared with the miR-NC group
(Fig. 2C). In conclusion, the
above results indicated that the overexpression of miR-23b was able
to repress BMP9-induction of C2C12 osteogenesis, however
downregulation of miR-23b promoted the osteoblast differentiation
of C2C12 myoblasts induced by BMP9.
Runx2 is a direct target of miR-23b
To investigate the mechanism of miR-23b regulating
BMP9-induced osteogenesis, biological information analysis was used
to search the potential targets of miR-23b. According to the
bioinformatics databases used, Runx2 was identified as a potential
target of miR-23b in the C2C12 osteogenesis induced by BMP9
(Fig. 3A). The predicted binding
site of mmu-miR-23b is located at position 1002–1008 of the 3′UTR
of the Runx2 mRNA. The miR-23b mimics and inhibitors were
individually transfected into BMP9-induced C2C12 cells. The RT-qPCR
analysis indicated that Runx2 mRNA levels were not significantly
altered in different transfection groups (Fig. 3B). However, the Runx2 protein level
was repressed by miR-23b mimics, and promoted by the miR-23b
inhibitor when compared with the control group (Fig. 2C). To verify the predicted binding
sites, the firefly lucif-erase reporter system containing the wild
type 3′-UTR binding site (Runx2-3′-UTR-WT) and the mutant 3′-UTR
binding site (Runx2-3′-UTR-mt) was constructed and co-transfected
with the miR-23b mimics. As presented in Fig. 2D, the luciferase activity assay
detected that co-transfection of miR-23b mimics and the
Runx2-3′-UTR-WT plasmid reduced the luciferase expression level
compared with the control group. Furthermore, the co-transfection
of miR-23b and Runx2-3′-UTR-mt plasmid had no clear effect on
luciferase levels. In summary, the results suggested that Runx2
mRNA is a direct target of miR-23b.
 | Figure 3miR-23b directly binds to the 3′UTR of
Runx2. (A) Schematic diagram illustrating the the binding sites of
mmu-miR-23b and Runx2 as predicted by the target prediction
databases. (B) Reverse transcription-quantitative polymerase chain
reaction demonstrated that neither the miR-23b mimic nor the
miR-23b inhibitor had significant effects on Runx2 mRNA levels.
n=3, P>0.05 (C) Western blot analysis indicated that the Runx2
expression level was promoted by the downregulation of miR-23b,
however the overexpression of miR-23b via the transfection of the
miR-23b mimics inhibited its expression. (D) All data demonstrated
co-transfection of miR-23b and its WT 3′-UTR binding site, termed
Runx2-3′UTR-WT, significantly reduced the luciferase activity,
whereas miR-23b had no effect on the Mut 3′-UTR binding region.
n=3, **P<0.01. miR-23b, microRNA-23b; UTR,
untranslated region; Runx2, runt-related transcription factor 2;
BMP9, bone morphogenetic protein 9; WT, wild type; Mut, mutant. |
Runx2 knockdown reduces the effect of
miR-23b
To verify the association between miR-23b and Runx2
further, Ad-sim-Runx2 was transfected into the C2C12 cells induced
by BMP9 (Fig. 4A), the role of
Runx2 in osteogenic differentiation induced by BMP9 was identified
by the mRNA expression of ALP (Fig.
4B), OCN (Fig. 4C) and OPN
(Fig. 4D). The expression of these
osteoblast-specific genes was significantly downregulated following
the knockdown of Runx2. Subsequently, the miR-23b mimics and
inhibitors were transfected into the BMP9-induced C2C12 cells with
Runx2 knockdown. ALP staining and the quantitative ALP assay were
then subsequently conducted, in order to detect the effects of
miR-23b in Runx2-knockdown C2C12 cells. As indicated, transfection
of the miR-23b inhibitor in Runx2-knockdown C2C12 cells had no
significant impact on the promotion of osteogenesis when compared
with the group only transfected with the miR-23b inhibitor. In the
Runx2-knockdown C2C12 cells, the miR-23b inhibitor promoted C2C12
osteogenesis by greater than 1.5-fold when compared with the
control. ALP staining and the quantification results indicated that
the osteogenesis of Runx2-knockdown C2C12 cells was likely to be
enhanced by the miR-23b inhibitor, as presented in Fig. 4E. Taken together, these results
indicate that the knockdown of Runx2 inhibits the effects of
miR-23b on the osteogenesis of BMP9-induced C2C12 cells.
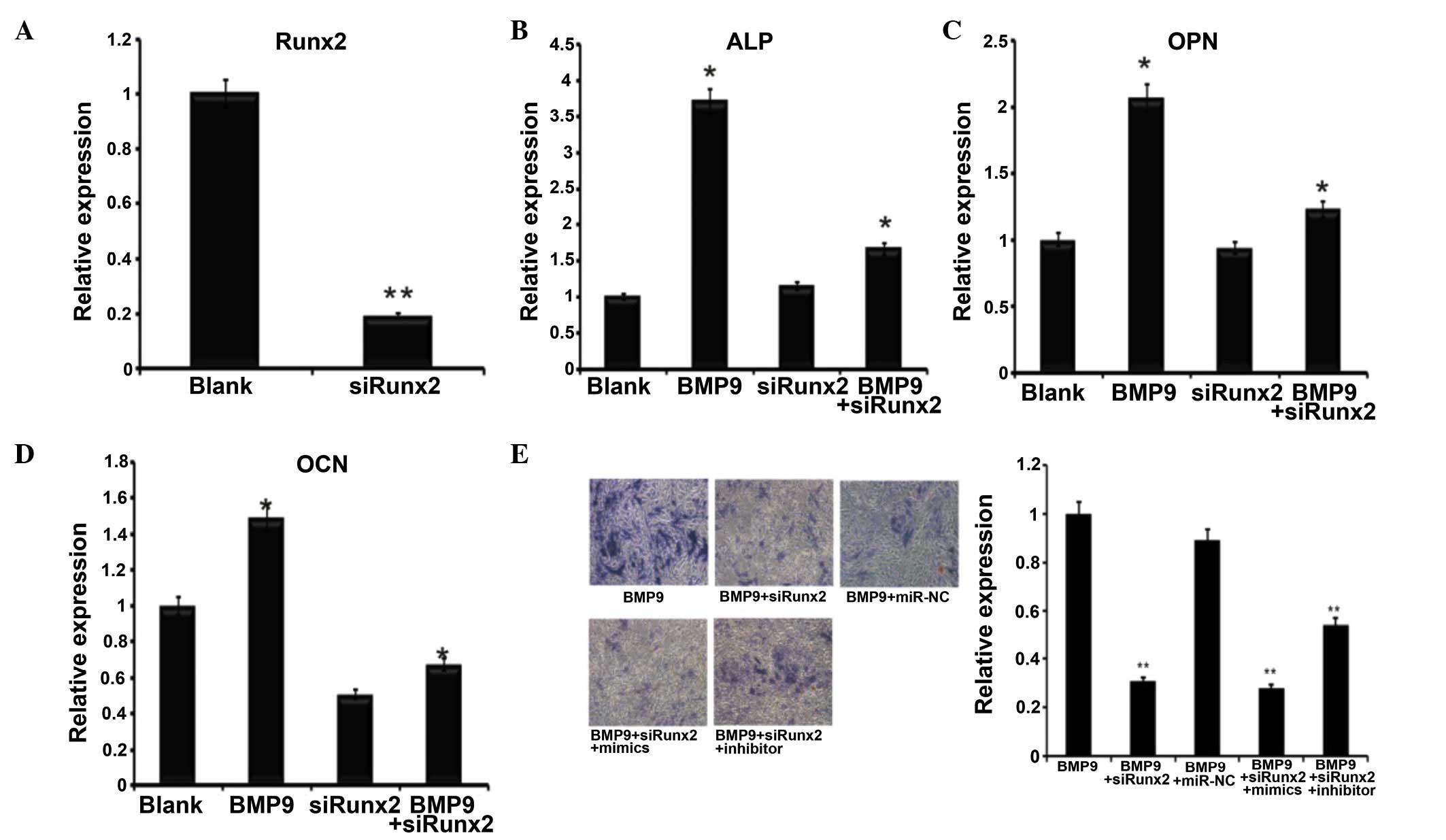 | Figure 4Runx2 affected BMP9-induced
differentiation of C2C12 cells. (A) Ad-siRunx2 was used to
knockdown the expression of Runx2. n=3, **P<0.01.
(B-D) Co-infected Ad-siRunx2 and Ad-BMP9 cells were collected at 3
days. (B) ALP, (C) OCN and (D) OPN expression levels were detected
by reverse transcription-quantitative polymerase chain reaction,
n=3, *P<0.05 vs. the blank group. (E) ALP staining of
BMP9-induced C2C12 myoblasts at day 7 of osteogenic differentiation
treated with Ad-siRunx2 followed by transfection with mimics and
inhibitors (magnification, ×200). **P<0.01 vs. BMP9
group. Runx2, runt-related transcription factor 2; BMP9, bone
morphogenetic protein 9; Ad, adenovirus; si, short interfering;
ALP, alkaline phosphatase; OCN, osteocalcin; OPN, osteopontin. |
Discussion
While BMP9 has been demonstrated to exert potent
osteogenic activity (21,22), the detailed molecular mechanisms
underlying BMP9 action remain to be fully elucidated. The current
study aimed to investigate the possible effects of miRNA inhibition
on BMP9-induced osteogenic differentiation due to the fact that
epigenetic regulation serves an important role in regulating
osteogenesis (23).
The present study investigating the molecular
mechanisms of BMP9 predominantly focused on transcriptional
regulation, while the post-transcriptional mechanism has not been
thoroughly studied (24). miRNAs
are endogenous, non-coding RNAs that are negative regulators of
their target genes at the post-transcriptional level (12). miR-23b belongs to the
miR-23a/24/27a cluster which is located on chromosome 19p13.12 and
the miR-23b/27b/24-1 cluster which is located on chromosome 9q22.32
(25,26). The clusters have been previously
reported to be enhanced in acute lymphoblastic leukemia, acute
myeloid leukemia, glioblastoma, hepatocellular carcinoma, gastric
cancer, pancreatic cancer and uterine leiomyoma (27–29).
The majority of these studies have focused on the function of
miR-23b as an oncogene in non-small cell lung cancer while ignoring
its role in bone formation (26).
In the current study, it was demonstrated that
miR-23b reduced activity of the early osteogenic marker ALP in
BMP9-induced C2C12 cells using miR-23b mimics, in addition to
reducing late osteogenic marker matrix mineralization. The
dual-luciferase reporter assays demonstrated that miR-23b reduced
BMP9-induced bone formation possibly via suppression of Runx2
translation. Runx2 is considered as the most important early
osteogenic transcriptional factor (30). Ad-siRunx2 was employed to evaluate
its important role in the course miR-23b restrained BMP9-inducing
osteogenesis.
In conclusion, the observations suggest that miR-23b
serves an important role in BMP9-mediated osteogenic signaling
through negative regulation. Thus, miR-23b inhibitors may be used
as a novel therapeutic strategy in bone fracture healing.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (grant no. 31200971) and the
Program of the Ministry of Science and Technology of Yu-Zhong
District (grant no. 20130136). The authors would like to thank Dr
Tong-Chuan He (University of Chicago, Chicago, IL, USA) for
donating the Ad-BMP9 and Ad-siRunx2.
References
|
1
|
Urist MR: Bone: Formation by
autoinduction. Science. 150:893–899. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wozney JM, Rosen V, Celeste AJ, Mitsock
LM, Whitters MJ, Kriz RW, Hewick RM and Wang EA: Novel regulators
of bone formation: Molecular clones and activities. Science.
242:1528–1534. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okla M, Ha JH, Temel RE and Chung S: BMP7
drives human adipogenic stem cells into metabolically active beige
adipocytes. Lipids. 50:111–120. 2015. View Article : Google Scholar :
|
|
4
|
Wei Y, Wu Y, Zeng B and Zhang H: Effects
of sodium fluoride treatment in vitro on cell proliferation, BMP-2
and BMP-3 expression in human osteosarcoma MG-63 cells. Biol Trace
Elem Res. 162:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiqing C, Yaqin L, Yingyin L, Fei C, Huili
Z, Yuling Z, Juan Y, Shanwei F and Cheng Z: BMP4 inhibits myogenic
differentiation of bone marrow-derived mesenchymal stromal cells in
mdx mice. Cytotherapy. 17:1213–1219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown MA, Zhao Q, Baker KA, Naik C, Chen
C, Pukac L, Singh M, Tsareva T, Parice Y, Mahoney A, et al: Crystal
structure of BMP-9 and functional interactions with pro-region and
receptors. J Biol Chem. 280:25111–25118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang Q, Sun MH, Cheng H, Peng Y, Montag
AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, et al:
Characterization of the distinct orthotopic bone-forming activity
of 14 BMPs using recombinant adenovirus-mediated gene delivery.
Gene Ther. 11:1312–1320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuda H, Wada T, Yamashita T and Hamada H:
Enhanced osteoinduction by mesenchymal stem cells transfected with
a fiber-mutant adenoviral BMP2 gene. J Gene Med. 7:1322–1334. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen P, Vukicevic S, Sampath TK and Luyten
FP: Osteogenic protein-1 promotes growth and maturation of chick
sternal chondrocytes in serum-free cultures. J Cell Sci.
108:105–114. 1995.PubMed/NCBI
|
|
10
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Papaioannou G, Mirzamohammadi F and
Kobayashi T: MicroRNAs involved in bone formation. Cell Mol Life
Sci. 71:4747–4761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data expression data using real-time
quantitative PCR and the 2(−Delta Delta C (T)) method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
14
|
Mo RH, Zaro JL, Ou JH and Shen WC: Effects
of Lipofectamine 2000/siRNA complexes on autophagy in hepatoma
cells. Mol Biotechnol. 51:1–8. 2012. View Article : Google Scholar
|
|
15
|
He TC, Zhou S, Da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu DJ, Zhao Y-Z, Wang J, He JW, Weng YG
and Luo JY: Smads, p38 and ERK1/2 are involved in BMP9-induced
osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB
Rep. 45:247–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv Z, Yang D, Li J, Hu M, Luo M, Zhan X,
Song P, Liu C, Bai H, Li B, et al: Bone morphogenetic protein 9
overexpression reduces osteosarcoma cell migration and invasion.
Mol Cells. 36:119–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song T, Wang W, Xu J, Zhao D, Dong Q, Li
L, Yang X, Duan X, Liang Y, Xiao Y, et al: Fibroblast growth factor
2 inhibits bone morphogenetic protein 9-induced osteogenic
differentiation of mesenchymal stem cells by repressing Smads
signaling and subsequently reducing Smads dependent up-regulation
of ALK1 and ALK2. Int J Biochem Cell Biol. 45:1639–1646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo J, Tang M, Huang J, He BC, Gao JL,
Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, et al: TGFbeta/BMP type I
receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic
signaling in mesenchymal stem cells. J Biol Chem. 285:29588–29598.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Li L, Dong Q, Wang Y, Feng Q, Ou
X, Zhou P, He T and Luo J: Activation of PKA/CREB signaling is
involved in BMP9-induced osteogenic differentiation of mesenchymal
stem cells. Cell Physiol Biochem. 37:548–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luu HH, Song WX, Luo X, Manning D, Luo J,
Deng ZL, Sharff KA, Montag AG, Haydon RC and He TC: Distinct roles
of bone morphogenetic proteins in osteogenic differentiation of
mesenchymal stem cells. J Orthop Res. 25:665–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye G, Li C, Xiang X, Chen C, Zhang R, Yang
X, Yu X, Wang J, Wang L, Shi Q and Weng Y: Bone morphogenetic
protein-9 induces PDLSCs osteogenic differentiation through the ERK
and p38 signal pathways. Int J Med Sci. 11:1065–1072. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng L and Zhong X: Epigenetic regulation
of drug metabolism and transport. Acta Pharm Sin B. 5:106–112.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Weng Y, Yuan T, Zhang H, Bai H, Li
B, Yang D, Zhang R, He F, Yan S, et al: CXCL12/CXCR4 signal axis
plays an important role in mediating bone morphogenetic protein
9-induced osteogenic differentiation of mesenchymal stem cells. Int
J Med Sci. 10:1181–1192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jahid S, Sun J, Edwards RA, Dizon D,
Panarelli NC, Milsom JW, Sikandar SS, Gümüs ZH and Lipkin SM:
miR-23a promotes the transition from indolent to invasive
colorectal cancer. Cancer Discov. 2:540–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donadelli M, Dando I, Fiorini C and
Palmieri M: Regulation of miR-23b expression and its dual role on
ROS production and tumour development. Cancer Lett. 349:107–113.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma G, Dai W, Sang A, Yang X and Gao C:
Upregulation of microRNA-23a/b promotes tumor progression and
confers poor prognosis in patients with gastric cancer. Int J Clin
Exp Pathol. 7:8833–8840. 2014.
|
|
28
|
Aghaee-Bakhtiari SH, Arefian E, Naderi M,
Noorbakhsh F, Nodouzi V, Asgari M, Fard-Esfahani P, Mahdian R and
Soleimani M: MAPK and JAK/STAT pathways targeted by miR-23a and
miR-23b in prostate cancer: Computational and in vitro approaches.
Tumour Biol. 36:4203–4212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiyomaru T, Seki N, Inoguchi S, Ishihara
T, Mataki H, Matsushita R, Goto Y, Nishikawa R, Tatarano S, Itesako
T, et al: Dual regulation of receptor tyrosine kinase genes EGFR
and c-Met by the tumor-suppressive microRNA-23b/27b cluster in
bladder cancer. Int J Oncol. 46:487–496. 2015.
|
|
30
|
Liu TM and Lee EH: Transcriptional
regulatory cascades in Runx2-dependent bone development. Tissue Eng
Part B Rev. 19:254–263. 2013. View Article : Google Scholar :
|