Introduction
Chronic periodontitis is characterized by persistent
inflammation of the periodontium, which is initiated by infection
with oral bacteria and results in damage to cells and the matrices
of the periodontal connective tissues (1). According to the latest
epidemiological analysis, periodontitis affects up to 50% of adults
in the US (2); it is the leading
cause of tooth loss in adults and is shown to positively correlate
with life-threatening systemic diseases (3). Although periodontal damage is known
to result from the secretion of toxins and generation of reactive
oxygen species by periodontal pathogens, the principal clinical
feature of periodontitis is the activation of the host
immunoinflammatory response (4,5).
The immune response against oral pathogenic bacteria
in the periodontium acts as a double-edged sword (6–9).
Bacteria can elicit innate and adaptive immune responses, however,
the responding inflammatory cytokines and activated inflammatory
cells can mediate destruction of the periodontal tissues (5,10–12).
The shift in balance between protecting the periodontal tissue and
inducing periodontal destruction is caused by the persistent
chronic inflammatory response at the periodontal ligament (PDL), a
connective tissue located between the cementum and the alveolar
bone (13). PDL cells (PDLCs) are
an important cell type in the periodontium, and are key in
maintaining homeostasis and in the remodeling of periodontal
tissues (14). In addition, PDLCs
exhibit stem cell properties by inducing chondrogenesis through the
growth factors, transforming growth factor (TGF)-β3 and bone
morphogenetic protein (BMP)-6 (15). PDLCs remodel extracellular matrices
through phagocytosis and the release of matrix metalloproteinases,
and pathological changes in resident PDLCs are positively
correlated with periodontitis-associated destructive processes
(16).
Programmed cell death 1 ligand 1 (PD-L1) is a
transmembrane protein of the B7 family (17). The expression of PD-L1 has been
shown to suppress the immune responses elicited by chronic
infections in cancer (18). PDL-1
receptors (programmed death-1; PD-1) are constitutively expressed
on macrophages, antigen-presenting cells (APCs) and dendritic
cells, and are inducibly expressed on activated T cells, B cells,
endothelial cells and epithelial cells (19). The binding of PD-L1 with PD-1 can
inhibit the activation and proliferation of immune cells, and the
secretion of cytokines, which leads to apoptosis of the immune
cells. The PDL-1/PD-1 pathway has been demonstrated to be important
in the immune evasion and immune tolerance of tumors (20,21).
PD-L1 also regulates the development, maintenance and function of
induced regulatory T cells (22).
The expression of PD-L1 has been investigated
extensively in the majority of types of human cancer, and PD-L1 has
been shown to suppress the antitumor immune responses of the host
(21,23). Although the expression of PD-L1 in
periodontal tissues has been reported by Konermann et al
(24), its function in periodontal
tissue damage remains to be elucidated. In the present study, PDLCs
were used as representatives of periodontal tissue cells to examine
the expression of PD-L1 on PDLCs stimulated with inflammatory
cytokines and periodontal pathogens in vitro. Furthermore,
the association between the expression of PD-L1 and periodontal
tissue destruction was investigated in mouse model of experimental
periodontitis. The present study aimed to investigate whether PD-L1
was negatively associated with peridontal tissue destruction in
vivo. Thus, PD-L1 expression may have potential to be utilized
to regulate peridontal tissue destruction.
Materials and methods
Animals
Twenty male BALB/c mice (6-week-old; 23–25 g) were
purchased from the Animal Center of Chinese Academy of Sciences
(Shanghai, China) and housed in a specific-pathogen free laminar
flow room under constant temperature (25–27°C), a 12-h light/dark
cycle and humidity (40–50%) with access to food and water ad
libitum. All experiments and animal care procedures were
approved by the Animal Center of Sichuan University (Sichuan,
China). All experimental procedures were approved by the
Experimental Animal Committee of the State Key Laboratory of Oral
Diseases, West China College of Stomatology, Sichuan University
(Chengdu, China).
Isolation and culture of PDLCs
The PDL tissues were obtained from the premolar
teeth of three donors, which were extracted for orthodontic
purposes at the West China Hospital of Stomatology of Sichuan
University. The protocols regarding the use and manipulation of PDL
tissues were approved by the Institutional Review Board of West
China Hospital of Stomatology, Sichuan University (Chengdu, China)
and written informed consent was obtained from each donor. The
extracted teeth were rinsed and placed in Dulbecco's modified
Eagle's medium (DMEM; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 1,000 U/ml penicillin and 1,000 µg/ml
streptomycin (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA). The remaining procedures were performed, as described by
Arnold et al (25). PDLs
attached to the middle third of the root were removed with a
curette to avoid contamination with the gingival and apical
tissues. The PDL tissues were cut into ~1 mm2 pieces and
placed in 25 mm2 culture flasks for cell culture in DMEM
medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml of
penicillin and 100 µg/ml of streptomycin at 37°C, under 5%
CO2 and 95% humidity. After reaching confluence of ~75%
(in ~7 days), the cells were treated with 0.25% trypsin-0.1% EDTA
(Hyclone; GE Healthcare Life Sciences) for cell passage. PDLCs at
the 5th or 6th passage were used for the present studies.
Preparation of pathogens and peripheral
blood mononuclear cells (PBMCs)
The periodontal pathogens Porphyromonas
gingivalis (P.g; ATTC33277), Prevotella
intermedia (P.i; ATTC25611) and Fusobacterium
nucleatum (F.n; ATTC25586) were obtained from State Key
Laboratory of Oral Diseases (Chengdu, China) and cultured in brain
heart infusion broth (Oxoid Ltd, Basingstoke, UK) under anaerobic
conditions at 37°C for 48 h. The supernatants of the P.g
culture were collected by centrifugation for 20 min at 9,600 × g,
and stored at −80°C. Blood samples (50 ml) were taken from six
healthy donors and the PBMCs were isolated using human lymphocyte
separation tubes (DAKEWE, China), according to the manufacturer's
protocols. The supernatants of the PBMCs were collected by
centrifugation at 1,000 × g for 15 min and stored at −80°C. The
full details of the procedure were as described by Lu et al
(23). The protocols regarding the
use and manipulation of PBMCs were approved by the Institutional
Review Board of West China Hospital of Stomatology, Sichuan
University (approval no. WCHSIRB-D-2013-039) and written informed
consent was obtained from the donors.
Stimulation of PDLCs with inflammatory
cytokines
The PDLCs were seeded in a 24-well plate at a
density of 8×104 cells/well and were incubated overnight
at 37°C, under 5% CO2 and 95% humidity. The cells were
then stimulated with 10 ng/ml interleukin (IL)-1, IL-6, TNF-α or
interferon (IFN)-γ, 50 ng/ml lipopolysaccharide (LPS), a
combination of IL-1, IL-6, TNF-α and IFN-γ mixed at the ratio of
1:1:1:1 to a final concentration of 10 ng/ml of cytokines, or
periodontal pathogenic bacteria at a PDLC:bacteria ratio of 1:50.
The surface expression of PD-L1 on the stimulated PDLCs was
measured using flow cytometric analysis. Recombinant human IFN-γ,
IL-1, IL-2, IL-6 and TNF-α were purchased from R&D Systems
(Minneapolis, MN, USA).
Flow cytometry
The pretreated PDLCs were harvested, washed twice
with FCM buffer, comprising phosphate-buffered saline (PBS;
ZSGB-BIO, Beijing, China) with 5% FBS (Hyclone; GE Healthcare Life
Sciences) and 0.1% NaN3 (Sigma-Aldrich), and incubated
with either phycoerythrin (PE) mouse anti-human PD-L1 monoclonal
antibody (3 µl per sample; dilution, 1:40; BioLegend, Inc.,
San Diego, CA, USA; cat. no. 329706) or isotype control antibody (3
µl per sample; dilution, 1:40; BioLegend, Inc.; cat. no.
400320) for 30 min at 4°C. The stained cells were washed twice with
FCM buffer and analyzed using flow cytometry (Beckman Coulter
FC500; Beckman Coulter, Miami, FL, USA) with Submit 5.2 software
(Beckman Coulter).
T cell apoptosis assay
The PDLCs were pretreated with 10 ng/ml of TNF-α or
IFN-γ for 48 h to induce the surface expression of PD-L1. The
isolated PBMCs were seeded at a density of 1×107
cells/well in a six-well plate and incubated with 10 µg/ml
phytohemagglutinin (PHA; Roche Diagnostics GmbH, Mannheim, Germany)
for 72 h at 37°C to activate T lymphocytes (26). The surviving TNF-α or IFN-γ
pre-treated, PDLCs were collected and then co-cultured with
PHA-activated PBMCs at a ratio of 1:50 for 48 h for 37°C; untreated
PDLCs were used as a negative control. To inhibit the binding of
PD-L1 to PD-1, 10 µg/ml of purified mouse anti-human
monoclonal CD274 (PD-L1, B7-H1) antibody (dilution, 1:40;
eBioscience, San Diego, CA, USA; cat. no. 14-5983-82) was also
added to the TNF-α-pretreated PDLC and PHA-activated PBMC
co-culture. Following 18 h of incubation at 37°C, the cells were
collected and labeled with propidium iodide (PI)/Annexin
V-fluorescein isothiocyanate (FITC; Annexin V-FITC Apoptosis
Detection kit; Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China)/APC-mouse anti-human CD4 monoclonal antibody (dilution,
1:40; BioLegend, Inc.; cat. no. 317416) or PI/annexin
V-FITC/APC-mouse anti-human monoclonal CD8 antibody (dilution,
1:40; BioLegend, Inc.; cat. no. 300911) at 4°C for 30 min. The
labeled cells were subjected to flow cytometric analysis, and the
resulting APC+/FITC+ cells were gated to
identify apoptotic T lymphocyte populations.
PDLC survival assay
The PDLCs (1×106) pretreated with 10
ng/ml TNF-α were stained with carboxyfluorescein diacetate
succinimidyl ester (CFSE; Invitrogen; Thermo Fisher Scientific,
Inc.) to label the live cells. Briefly, these PDLCs were incubated
with CFSE (final concentration 10 µM) at 37°C for 10 min
with gentle agitation. The cells were then washed twice with DMEM
supplemented with 10% FBS and resuspended in DMEM. The CFSE-labeled
cells were co-cultured with PHA-treated PBMCs at a ratio of 1:50
for 48 h. PBMCs and PDLCs alone were cultured as CFSE-negative and
CFSE-positive controls, respectively. To inhibit the binding of
PD-1 to PD-L1, 10 µg/ml of purified anti-human CD274 (PD-L1,
B7-H1) antibody was added to the co-culture system at the time of
mixing of the two cell types. Following 18 h of incubation, the
cells were collected and stained with PI (final concentration 10
µg/ml) for 10 min to label the dead cells. The cells were
then subjected to flow cytometric analysis, and signals gated as
CFSE+/PI− were considered live cells.
Establishment of a mouse model of
experimental periodontitis, and measurement of the expression
levels of PD-L1 and PD-1 in the periodontitis tissues
To establish an experimental model or peridontitis,
10 of the mice were randomly selected and were injected with
P.g (P.g-injected group); and another 10 mice were
injected with PBS as healthy controls (27). All surgery was performed under 200
mg/kg chloral hydrate [Meryer (Shanghai) Chemical Technology Co.,
Ltd., Shanghai, China] anesthesia and efforts were made to minimize
suffering. For analysis of the outcomes, the severity of
periodontitis in the model was categorized into two case types:
Case type I was termed mild periodontitis and was defined by the
presence of bleeding on probing, furcation cul-de-sac involvement,
and facial-lingual tooth movement, without movements in a vertical
or mesial direction. Case type II was termed severe periodontitis
and was defined by the presence of bleeding on probing, furcation
through-and-through involvement, and tooth movement in
facial-lingual, vertical and mesial directions.
The mice were sacrificed by cervical dislocation
following anesthesia at 10 weeks following the injection, and the
inflammatory tissues of the mice were removed. Half of the tissues
were embedded in paraffin (Hualin Kangfu, Shanghai, China) for
sectioning, followed by immunohistochemical staining of the
sections to visualize the expression levels of PD-L1 in the
tissues. Single periodontal tissue cells of the inflammatory
periodontium were isolated from the other half of the tissues by
enzymatic digestion, and the expression levels of PD-L1 and PD-1 on
the surface of the cells were analyzed using flow cytometry.
Briefly, the tissues were minced with scalpels into sizes <1
mm3. The minced tissues were then digested with 2 mg/ml
type I collagenase (Sigma-Aldrich) and 100 mg/ml DNase
(Sigma-Aldrich) in PBS at 4°C overnight. Finally, the digested
tissues were passed through a 200-mesh filter (Yangin Biological,
Shanghai, China) to obtain single cells. The spleens of the mice
were also removed and passed through the 200-mesh filter to obtain
single cells. The cells were stained with rat PE-anti-mouse PD-L1
(3 µl per sample; dilution, 1;40; BioLegend, Inc.; cat. no.
124304) or APC-rat anti-mouse PD-1 antibodies (3 µl per
sample; dilution, 1:40; BioLegend, Inc.; cat. no. 135209), and the
expression levels of PD-L1 and PD-1 were determined using flow
cytometric analysis.
Immunohistochemistry
Immunohistochemical analyses were performed, as
described by Karlsson et al (28). The paraffin-embedded specimens were
sliced into 4–5 µm-thick sections. The tissue sections were
deparaffinized in xylene (Shanghai Macklin Biochemical Co., Ltd.,
Shanghai, China) for 3–10 min and dehydrated through a series of
graded alcohols (100, 100, 95 and 80%) to displace the water. All
sections were treated with 100% methanol containing 0.3%
H2O2 for 15 min to block any endogenous
peroxidase activity. The tissue sections were immersed in 50 ml 10
mM citrate buffer (pH 6.0) and placed in a microwave oven. Antigens
were retrieved by microwaving in citrate buffer (pH 6.0) for three
6-min cycles. Bovine serum albumin (ZSGB-BIO) was used to block
nonspecific IgG bindings. The sections were incubated with rabbit
anti-mouse PD-L1 polyclonal antibodies (dilution, 1:200; Abcam,
Cambridge, MA, USA; cat. no. ab58810) overnight at 4°C, followed by
incubation with biotinylated goat anti-rabbit secondary antibodies
(1:5,000; ZSGB-BIO, Beijing, China) for 30 min at room temperature,
and with streptavidin-peroxidase complex solution for another 30
min at room temperature. The slides were stained with 3,
3′-diaminobenzidine (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China). Finally, the sections were counterstained
with hematoxylin and observed under an optical microscope (BX51TR;
Olympus Corporation, Tokyo, Japan). Image-Pro Plus version 6.0
(Media Cybernetics, Inc., USA) was used for image analysis.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, USA). All values are presented as
the mean ± standard error of the mean. Data were analyzed by
one-way analysis of variance, followed by Bonferroni's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Surface expression of PD-L1 on PDLCs is
induced by inflammatory cytokines
In the present study, PDLCs were stimulated with
five inflammatory cytokines that are present in the inflammatory
microenvironment of the body, including IL-1, IL-6, TNF-α, IFN-γ
and LPS, to examine the inducible expression of PD-L1. As shown in
Fig. 1, the expression of PD-L1
was upregulated by IL-1, IL-6, TNF-α, IFN-γ and LPS, and by the
combination of IL-1, IL-6, TNF-α and IFN-γ, compared with the
unstimulated control.
Surface expression of PD-L1 on PDLCs is
induced by periodontal pathogens
The inflammatory cytokines secreted by immune and
non-immune cells are known to be involved in the destruction of
periodontal tissues, and are inducible upon bacterial infection
(29). Therefore, the present
study examined whether infection with periodontal bacteria induces
the expression of PD-L1 on PDLCs. For this assessment, three
strains of extensively investigated periodontal pathogens,
including P.g, F.n and P.I, were selected for
co-culture with the PDLCs for 18 h, followed by flow cytometric
analyses of the surface expression of PD-L1. As shown in Fig. 2, the expression of PD-L1 was
upregulated by P.g, F.n, and P.i, compared
with the unstimulated control.
Expression of PD-L1 on PDLCs causes
apoptosis of activated T cells and improves survival of PDLCs
The present study further investigated the molecular
function of PD-L1 on PDLCs by co-culturing PHA-activated PBMCs with
PDLCs pretreated with TNF-α or IFN-γ, followed by two-color flow
cytometric analysis. The TNF-α- and IFN-γ-induced expression of
PD-L1 were shown to cause significant apoptosis of the activated
CD4+ and CD8+ T lymphocytes. Pretreatment of
the PDLCs with TNF-α led to increases in the percentages of
apoptotic CD4+ and apoptotic CD8+ T cells,
and the addition of IFN-γ resulted in increases in the percentages
of apoptotic CD4+ and CD8+ T cells (Fig. 3A–D). The PI− cells were
gated to exclude necrotic cells (Fig.
3E). In addition, the TNF-α-pretreated PDLCs induced higher
levels of apoptosis of the CD4+ and CD8+ T
lymphocytes, compared with the IFN-γ-pretreated PDLCs. This was
consistent with the more marked inducibility of TNF-α, compared
with IFN-γ on the expression of PD-L1, as shown in Fig. 1A. To clarify the cause of the
apoptosis, anti-PD-L1 antibodies were added to the cell co-culture
at the time of mixing of the PHA-activated PBMCs with
TNF-α-pretreated PDLCs. As shown in Fig. 3C and D, the percentages of
apoptosis of the CD4+ and CD8+ T lymphocytes
were reduced significantly, suggesting that the apoptosis of
lymphocytes was correlated with the induced expression of PD-L1 on
the PDLCs, and that PD-L1 may have negatively regulated the
inflammatory responses. The increasing apoptosis of lymphocytes
resulting from the upregulation of PD-L1 on the PDLCs inhibited the
progression of excessive inflammatory immune responses, and thus
reduced the destruction of the PDLCs.
 | Figure 3TNF-α- and IFN-γ-induced expression
of PD-L1 on PDLCs caused apoptosis of activated T cells. (A)
Two-color flow cytometry histograms of activated PBMCs co-cultured
with untreated PDLCs. PDLCs pretreated with (B) IFN-γ or (C) TNF-α,
or incubated with (D) TNF-α-pretreated PDLCs and anti-PD-L1
antibody. (E) PI− cells were gated in R1 to exclude
necrotic cells. Comparison of (F) CD8+ and (G)
CD4+ T cell apoptosis induced by TNF-α and IFN-γ, and in
the presence of anti-PDL1 antibodies. Data are presented as the
mean ± standard error of the mean of three independent experiments.
The increases in T cell apoptosis caused by pretreatment with TNF-α
or IFN-γ were statistically significant (*P<0.05),
and the presence of anti-PD-L1 antibodies caused a considerable
reduction in the fraction of apoptotic T cells
(†P<0.05). PD-L1, programmed death 1 ligand 1; PDLCs,
periodontal ligament cells; IFN-γ, ibterferon-γ; TNF-α, tumor
necrosis factor-α; APC, antigen-presenting cell; PI, propidium
iodide; FITC, fluorescein isothiocyanate. |
To validate these initial observations of the
present study, the cellular effect of the induced expression of
PD-L1 on PDLCs was examined by tracking the viability of the PDLCs
with CFSE and PI staining. The CFSE-stained PDLCs were co-cultured
with PHA-activated PBMCs for 48 h, followed by staining with PI,
resulting in a decrease in viable (CFSE+/PI−)
PDLCs (Fig. 4A–C). However, the
percentage of CFSE+PDLCs increased by 20%, compared with
the untreated control when the PDLCs were pretreated with TNF-α to
induce the expression of PD-L1 prior to co-culturing with the
PHA-activated PBMCs (Fig. 4D),. In
addition, anti-PD-L1 antibodies were added to the TNF-α-pretreated
PDLCs and activated PBMCs co-culture at the time of mixing of the
two cell cultures, to confirm the cause of cell survival. The
percentage of viable PDLCs was reduced to 33.9%, which was not
statistically different with that of the untreated control
(Figs. 4E and F). These findings
further confirmed the protective role of PD-L1 on PDLCs against
inflammatory damage.
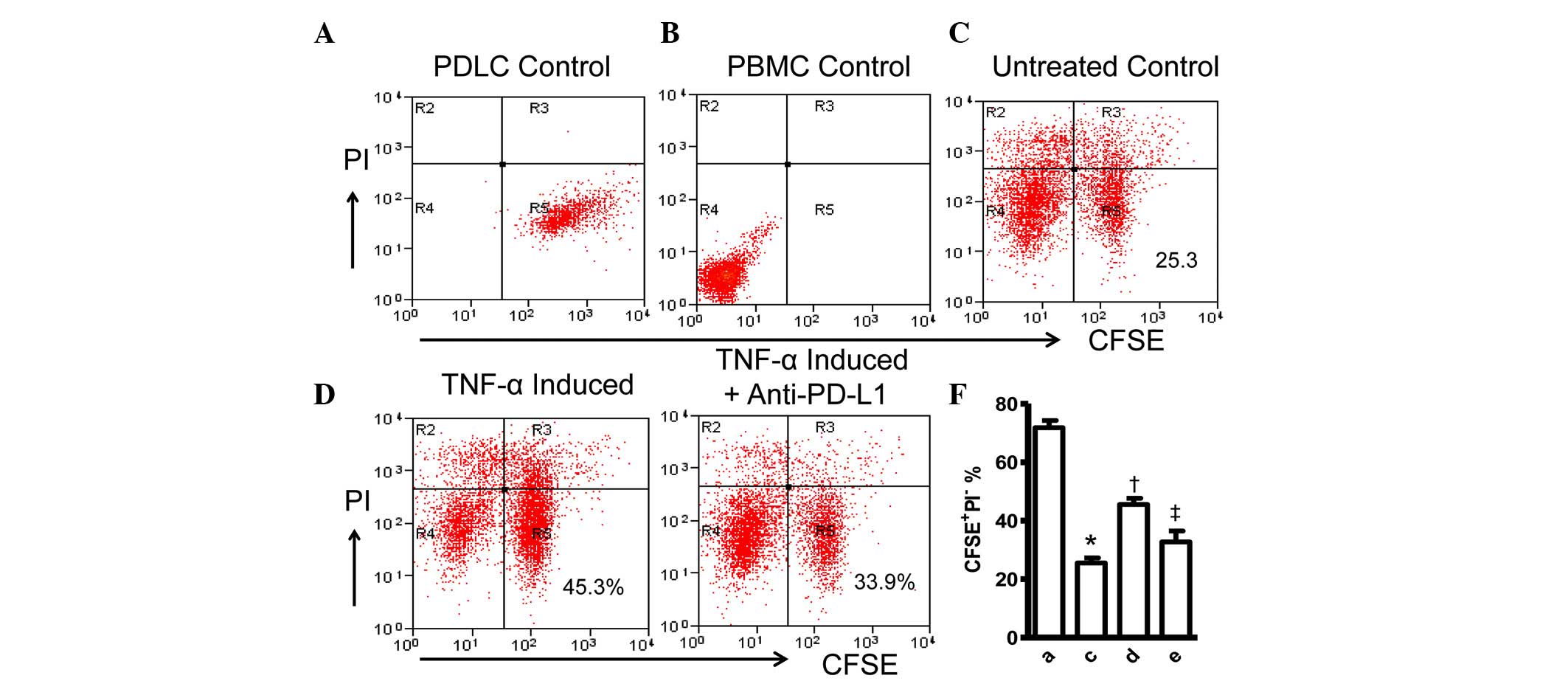 | Figure 4Expression of PD-L1 on PDLCs improves
survival of PDLCs. Flow cytometry histrograms of (A) PDLCs, (B)
PHA-activated PBMCs, (C) PDLCs co-cultured with activated PBMCs,
(D) PDLCs pretreated with TNF-α and co-cultured with activated
PBMCs, and (E) PDLCs pretreated with TNF-α, and incubated with
activated PBMCs and anti-PD-L1 antibodies. (F) Comparison of PDLC
survival, according to the percentages of
CFSE+/PI− cells. a, c, d and e represent the
PDLC control, untreated control, TNF-α induced and TNF-α
induced+anti-PD-L1 groups, respectively. Data are expressed as the
mean ± standard error of the mean of three independent experiments.
Co-culturing the activated PMBCs with untreated PDLCs resulted in a
significant decrease in viable PDLCs (*P<0.05).
†P<0.05, significant increase in the viability of the
PDLCs following pretreatment with TNF-α. The addition of anti-PD-L1
antibody caused a significant decrease in the viability of the
PDLCs pretreated with TNF-α (‡P<0.05). PD-L1,
programmed death 1 ligand 1; PDLCs, periodontal ligament cells;
PMBCs, peripheral blood mononuclear cells; PI, propidium iodide;
CFSE, carboxyfluorescein diacetate succinimidyl ester; TNF-α, tumor
necrosis factor-α. |
Expression of PD-L1 is correlated with
the severity of periodontitis in the mouse model of experimental
periodontitis
A mouse model of experimental periodontitis,
exhibiting alveolar bone loss at the maxillary first molar was
established in the present study. The expression of PD-L1 in the
inflamed periodontium of the mice was analyzed using flow cytometry
and immunohistochemistry. As shown in Fig. 5A, the mice with mild periodontitis
expressed ~19% more PD-L1, compared with those with severe
periodontitis, indicating that the presence of overexpressed PD-L1
may have ameliorated inflammation in the periodontal tissues, which
resulted in periodontitis with less alveolar bone loss. The
immunohistochemical staining of the corresponding tissue sections
also indicated a considerable increase in the expression of PD-L1
in the periodontal tissues of the mice with mild periodontitis only
(Fig. 5B and C). The animal
experiments revealed a negative correlation between the expression
of PD-L1 and the severity of destruction of the periodontal tissues
(Fig. 5D). This is in accordance
with the suggested protective role of PD-L1 in immunological
damage.
As the PD-L1/PD-1 pathway is known to inhibit T
cell-mediated immune responses (30), the present study also examined the
expression levels of PD-1 in the periodontal tissues, peripheral
blood and spleen of the periodontitis mouse model. Notably, no
significant correlation was found between the expression levels of
PD-L1 and PD-1 in the periodontal tissues (Fig. 5E and F) and other tissues (data not
shown). This suggested the involvement of an alternative
mechanism.
Discussion
Periodontitis is an inflammatory disease caused by
pathogenic oral microbiota. The majority of periodontal
microorganisms can destroy tissues either directly through tissue
invasion and production of harmful substances, which induce cell
death and tissue necrosis, or indirectly through the activation of
inflammatory cells, which secrete mediators that act on effectors
to destruct periodontal tissues (1,31–34).
The infection of periodontal tissues by periodontal pathogens
triggers host immune and inflammatory responses to defend the oral
tissues against the bacteria (5,12,31,35).
The immune and inflammatory responses initiated by
periodontal pathogens can act as a double-edged sword, which may
either protect or damage the periodontal tissues. However, in
several cases, aberrant host immune responses, rather than
pathogen-specific toxins or by-products, are the real pathogenic
factors that cause chronic inflammatory diseases or function as
risk factors for diseases (18). T
cells can modulate bacterium-induced periodontal inflammation
and/or alveolar bone destruction (36).
PD-L1 has a crucial immunoregulatory role in the
chronicity of inflammatory disorders. The present study
demonstrated that PD-L1 was inducibly expressed on PDLCs by
inflammatory cytokines and periodontal pathogens. Although
Konermann et al (24)
reported that the expression of PD-L1 in PDLCs is involved in
periodontal immunoinflammatory processes, the function of PD-L1 in
periodontitis has received little investigation. Investigating
PD-L1 contributes to providing insight into periodontal disease.
The dual functions of PD-L1 in regulating T cell responses have
been reported. PD-L1-mediated signals are able to co-stimulate
early T cell priming and differentiation in vivo and in
vitro (37), and PD-L1
negatively regulates the function and survival of activated T cells
(38). The results of in
vitro experiments were consistent with the latter. The
PD-L1/PD-1 pathway are involved in the negative regulation of T
cell responses (39). By contrast,
there was no significant difference in the expression of PD-1
between the experimental group and the healthy controls. These data
suggested that PD-L1 may inhibit the destruction of periodontal
tissues through an alternative way. Further investigations are
required to resolve this issue.
The present study analyzed the expression levels of
PD-L1 and PD-1 on the surface of cells, which were separated from
periodontium. Only the cells of the mild periodontitis group showed
increased expression of PD-L1 in the inflamed periodontium. This
suggested that the mice assessed had different sensitivities
against the same periodontal infection, and expressed different
levels of PD-L1. Those with high expression levels of PD-L1
following P.g infection, exhibited a downregulated
inflammatory response, avoiding damage to the periodontal tissues,
and exhibiting mild periodontitis. By contrast, those expressing
lower levels of PD-L1 resulted in severe periodontitis. Although
the present study did not identify a direct association between
PD-L1 and periodontal tissue destruction, evidence suggests that
inflammatory tissue destruction can be inhibited by PD-L1 (40).
In conclusion, the present study provided direct
in vitro evidence supporting the role of PDLC-expressed
PD-L1 in the inflammatory response against infection with
periodontal pathogens. In addition, the results demonstrated a
negative correlation between the expression of PD-L1 and the
severity of destruction of the periodontal tissues. The results of
the present study provides further understanding on the
pathogenesis of periodontitis and the protective mechanism against
bacteria-induced inflammatory damage, and has potential implication
on the prevention and treatment of periodontitis.
Abbreviations:
|
PDLCs
|
periodontal ligament cells
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
P.g
|
Porphyromonas gingivalis
|
|
F.n
|
Fusobacterium nucleatum
|
|
P.i
|
Prevotella intermedia
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81372892).
References
|
1
|
Lindhe J, Ranney R, Lamster I, et al:
Consensus report: Chronic periodontitis. Annals of Periodontology.
4:38. 1999. View Article : Google Scholar
|
|
2
|
Eke PI, Dye BA, Wei L, Thornton-Evans GO
and Genco RJ; CDC Periodontal Disease Surveillance workgroup: James
Beck(University of North Carolina, Chapel Hill, USA), Gordon
Douglass (Past President, American Academy of Periodontology), Roy
Page (University of Washin): Prevalence of periodontitis in adults
in the United States: 2009 and 2010. J Dent Res. 91:914–920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bascones-Martínez A, Arias-Herrera S,
Criado-Cámara E, Bascones-Ilundáin J and Bascones-Ilundáin C:
Periodontal disease and diabetes. Adv Exp Med Biol. 771:76–87.
2012.
|
|
4
|
Bascones-Martínez A, Muñoz-Corcuera M,
Noronha S, Mota P, Bascones-Ilundain C and Campo-Trapero J: Host
defence mechanisms against bacterial aggression in periodontal
disease: Basic mechanisms. Med Oral Patol Oral Cir Bucal.
14:e680–e685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Genco RJ and Slots J: Host responses in
periodontal diseases. J Dent Res. 63:441–451. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Brito LC, Teles FR, Teles RP, Totola
AH, Vieira LQ and Sobrinho AP: T-lymphocyte and cytokine expression
in human inflammatory periapical lesions. J Endod. 38:481–485.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hitzig C and Ciosi P: Periodontitis and
the immune system. Actual Odontostomatol (Paris). 33:573–579.
1979.In French.
|
|
8
|
Kilian M: Role of the immune system in
chronic periodontitis. Tandlaegebladet. 83:123–127. 1979.In
Swedish. PubMed/NCBI
|
|
9
|
Wang LY, Jin Y and Lin XP: The role of
adaptive immune response in periodontitis. Zhonghua Kou Qiang Yi
Xue Za Zhi. 48:115–118. 2013.In Chinese. PubMed/NCBI
|
|
10
|
Benakanakere M and Kinane DF: Innate
cellular responses to the periodontal biofilm. Front Oral Biol.
15:41–55. 2012. View Article : Google Scholar
|
|
11
|
Dixon DR, Bainbridge BW and Darveau RP:
Modulation of the innate immune response within the periodontium.
Periodontol 2000. 35:53–74. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gemmell E and Seymour GJ: Modulation of
immune responses to periodontal bacteria. Curr Opin Periodontol.
28–38. 1994.PubMed/NCBI
|
|
13
|
El-Awady AR, Messer RL, Gamal AY, Sharawy
MM, Wenger KH and Lapp CA: Periodontal ligament fibroblasts sustain
destructive immune modulators of chronic periodontitis. J
Periodontol. 81:1324–1335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukushima H, Kajiya H, Takada K, Okamoto F
and Okabe K: Expression and role of RANKL in periodontal ligament
cells during physiological root-resorption in human deciduous
teeth. Eur J Oral Sci. 111:346–352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi S, Cho TJ, Kwon SK, Lee G and Cho J:
Chondrogenesis of periodontal ligament stem cells by transforming
growth factor-β3 and bone morphogenetic protein-6 in a normal
healthy impacted third molar. Int J Oral Sci. 5:7–13. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujita T, Shiba H, Sakata M, Uchida Y,
Nakamura S and Kurihara H: SPARC stimulates the synthesis of
OPG/OCIF, MMP-2 and DNA in human periodontal ligament cells. J Oral
Pathol Med. 31:345–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Freeman GJ, Long AJ, Iwai Y, Borque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong H and Chen X: Immunoregulatory role
of B7-H1 in chronicity of inflammatory responses. Cell Mol Immunol.
3:179–187. 2006.PubMed/NCBI
|
|
19
|
Groeger S, Domann E, Gonzales JR,
Chakraborty T and Meyle J: B7-H1 and B7-DC receptors of oral
squamous carcinoma cells are upregulated by Porphyromonas
gingivalis. Immunobiology. 216:1302–1310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zitvogel L and Kroemer G: Targeting
PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology.
1:1223–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y
and Zhang P: Interferon-γ-induced PD-L1 surface expression on human
oral squamous carcinoma via PKD2 signal pathway. Immunobiology.
217:385–393. 2012. View Article : Google Scholar
|
|
22
|
Francisco LM, Salinas VH, Brown KE,
Vanguri VK, Freeman GJ, Kuchroo VK and Sharpe AH: PD-L1 regulates
the development, maintenance, and function of induced regulatory T
cells. J Exp Med. 206:3015–3029. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu W, Lu L, Feng Y, Chen J, Li Y, Kong X,
Chen S, Li X, Chen Q and Zhang P: Inflammation promotes oral
squamous carcinoma immune evasion via induced programmed death
ligand-1 surface expression. Oncol Lett. 5:1519–1526.
2013.PubMed/NCBI
|
|
24
|
Konermann A, Beyer M, Deschner J, Allam
JP, Novak N, Winter J, Jepsen S and Jäger A: Human periodontal
ligament cells facilitate leukocyte recruitment and are influenced
in their immunomodulatory function by Th17 cytokine release. Cell
Immunol. 272:137–143. 2012. View Article : Google Scholar
|
|
25
|
Arnold LF and Baram P: In vitro culture of
periodontal ligament cells. J Dent Res. 51:953–959. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Binns RM, Licence ST, Wooding FB and
Duffus WP: Active lymphocyte traffic induced in the periphery by
cytokines and phytohemagglutinin: Three different mechanisms? Eur J
Immunol. 22:2195–2203. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Polak D, Wilensky A, Shapira L, Halabi A,
Goldstein D, Weiss EI and Houri-Haddad Y: Mouse model of
experimental periodontitis induced by Porphyromonas
gingivalis/Fusobacterium nucleatum infection: Bone loss and host
response. J Clin Periodontol. 36:406–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karlsson L, Bondjers C and Betsholtz C:
Roles for PDGF-A and sonic hedgehog in development of mesenchymal
components of the hair follicle. Development. 126:2611–2621.
1999.PubMed/NCBI
|
|
29
|
Graves DT: The potential role of
chemokines and inflammatory cytokines in periodontal disease
progression. Clin Infect Dis. 28:482–490. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carter L, Fouser LA, Jussif J, Fitz L,
Deng B, Wood CR, Collins M, Honjo T, Freeman GJ and Carreno BM:
PD-1: PD-L inhibitory pathway affects both CD4(+) and CD8(+) T
cells and is overcome by IL-2. Eur J Immunol. 32:634–643. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kinane DF and Lappin DF: Immune processes
in periodontal disease: A review. Ann Periodontol. 7:62–71. 2002.
View Article : Google Scholar
|
|
32
|
Alexander MB and Damoulis PD: The role of
cytokines in the pathogenesis of periodontal disease. Curr Opin
Periodontol. 39–53. 1994.PubMed/NCBI
|
|
33
|
Birkedal-Hansen H: Role of cytokines and
inflammatory mediators in tissue destruction. J Periodontal Res.
28:500–510. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cochran DL: Inflammation and bone loss in
periodontal disease. J Periodontol. 79(Suppl 8): S1569–S1576. 2008.
View Article : Google Scholar
|
|
35
|
Hashim JR, Ruben MP and Kramer GM:
Cellular and immune mechanisms in juvenile periodontitis. J West
Soc Periodontol Periodontal Abstr. 27:40–47. 1979.PubMed/NCBI
|
|
36
|
Teng YT, Nguyen H, Gao X, Kong YY,
Gorczynski RM, Singh B, Ellen RP and Penninger JM: Functional human
T-cell immunity and osteoprotegerin ligand control alveolar bone
destruction in periodontal infection. J Clin Invest. 106:R59–R67.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Subudhi SK, Zhou P, Yerian LM, Chin RK, Lo
JC, Anders RA, Sun Y, Chen L, Wang Y, Alegre ML and Fu YX: Local
expression of B7-H1 promotes organ-specific autoimmunity and
transplant rejection. J Clin Invest. 113:694–700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blank C, Kuball J, Voelkl S, Wiendl H,
Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R
and Mackensen A: Blockade of PD-L1 (B7-H1) augments human
tumor-specific T cell responses in vitro. Int J Cancer.
119:317–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liapatas S, Nakou M and Rontogianni D:
Inflammatory infiltrate of chronic periradicular lesions: An
immunohistochemical study. Int Endod J. 36:464–471. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scandiuzzi L, Ghosh K, Hofmeyer KA, Abadi
YM, Lázár-Molnár E, Lin EY, Liu Q, Jeon H, Almo SC, Chen L, et al:
Tissue-expressed B7-H1 critically controls intestinal inflammation.
Cell Rep. 6:625–632. 2014. View Article : Google Scholar : PubMed/NCBI
|



















