Introduction
CML is a malignant clonal disorder of pluripotent
hematopoietic stem cells. Patients with CML have a t (9;22)
(q34;q11) translocation that results in a breakpoint cluster region
(BCR)-ABL fusion gene. In general, three breakpoint cluster regions
in the BCR gene have been described: Major (M-bcr), minor (m-bcr)
and micro (u-bcr). The M-bcr region consists of BCR introns
downstream of either exon 13 (e13, previously known as b2) or 14
(e14, previously known as b3) and introns upstream of ABL exon 2
(a2). These BCR-ABL e13a2 and e14a2 fusions result in a 210-kDa
fusion protein. m-bcr and u-bcr are two less common breakpoints in
the intronic region between the alternative BCR exon 2 and exons 19
and 20, which encode a 190-kDa (e1a2) and 230-kDa fusion protein
(e19a2), respectively.
However, a number of 'atypical' BCR-ABL transcripts
(e1a3, e13a3, e14a3, e19a3, e6a2 and e8a2) resulting from
chromosomal breakpoints outside the ABL intron 1 or BCR intron 1,
13 or 14, have been reported (1).
These atypical transcripts may escape detection when using methods
that are optimized to detect only the typical ones (1).
In the present study, a case of CML, which tested
positive for the BCR-ABL translocation by fluorescence in
situ hybridization (FISH) and cytogenetic analysis, but tested
negative by reverse transcription quantitative-polymerase chain
reaction (RT-qPCR) molecular analysis at the time of diagnosis was
reported. Further RT-qPCR analysis with alternative primer sets
demonstrated the presence of an e13a3 BCR-ABL fusion gene (2), in which ABL exon 3 rather than exon 2
was fused to BCR, which is extremely rare (3). BCR-ABL with the e13a3 transcript in
CML patients, however, usually predicts improved treatment response
and a longer survival time (4).
This patient responded immediately to Nilotinib with the
achievement of a complete cytogenetic remission.
Case report
A 32-year-old male was admitted to the Department of
General Surgery at The First Affiliated Hospital of Lanzhou
University (Lanzhou, China) in June 2014 with a history of weight
loss and splenomegaly. Routine peripheral blood analysis
demonstrated white blood cell (WBC) counts of
310.10×109/l (2% blasts, 2% promyelocytes, 25%
myelocytes, 9% metamyelocytes, 19% band neutrophils, 26% segmented
neutrophils, 5.0% basophils, 9% eosinophils and 3% lymphocytes), a
hemoglobin count of 8.7 g/dl and a platelet count of
130×109/l. In conclusion, the patient was diagnosed with
leukemia and admitted to the Department of Hematology. Following
this, the patient underwent a bone marrow aspirate, which
demonstrated hypercellularity with a marked myeloid predominance.
Bone marrow mononuclear cells were cultured according to standard
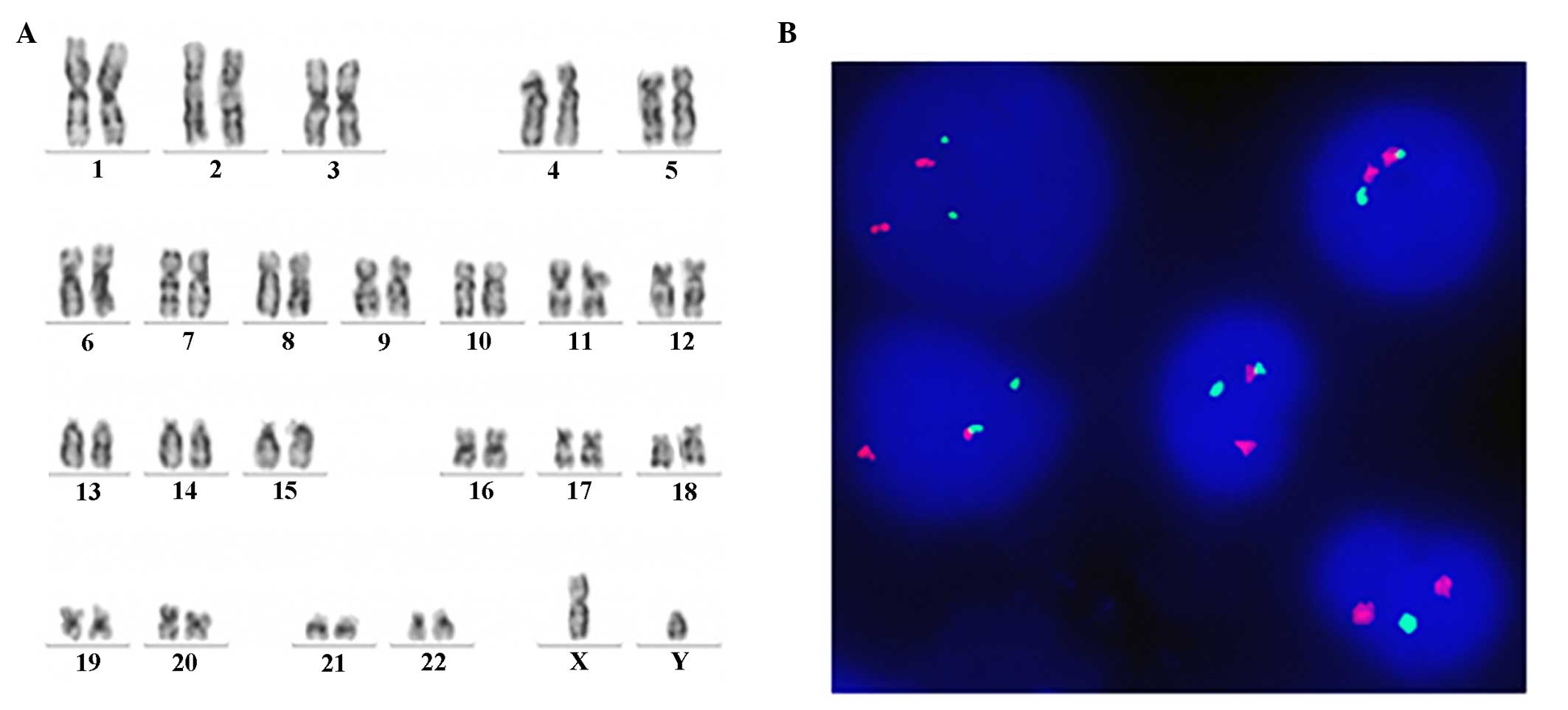
methods and the karyotypes were analyzed by G-banding, which
demonstrated 46,XY, t (9,22) in 20/20 metaphases (Fig. 1). The positive rate of BCR-ABL
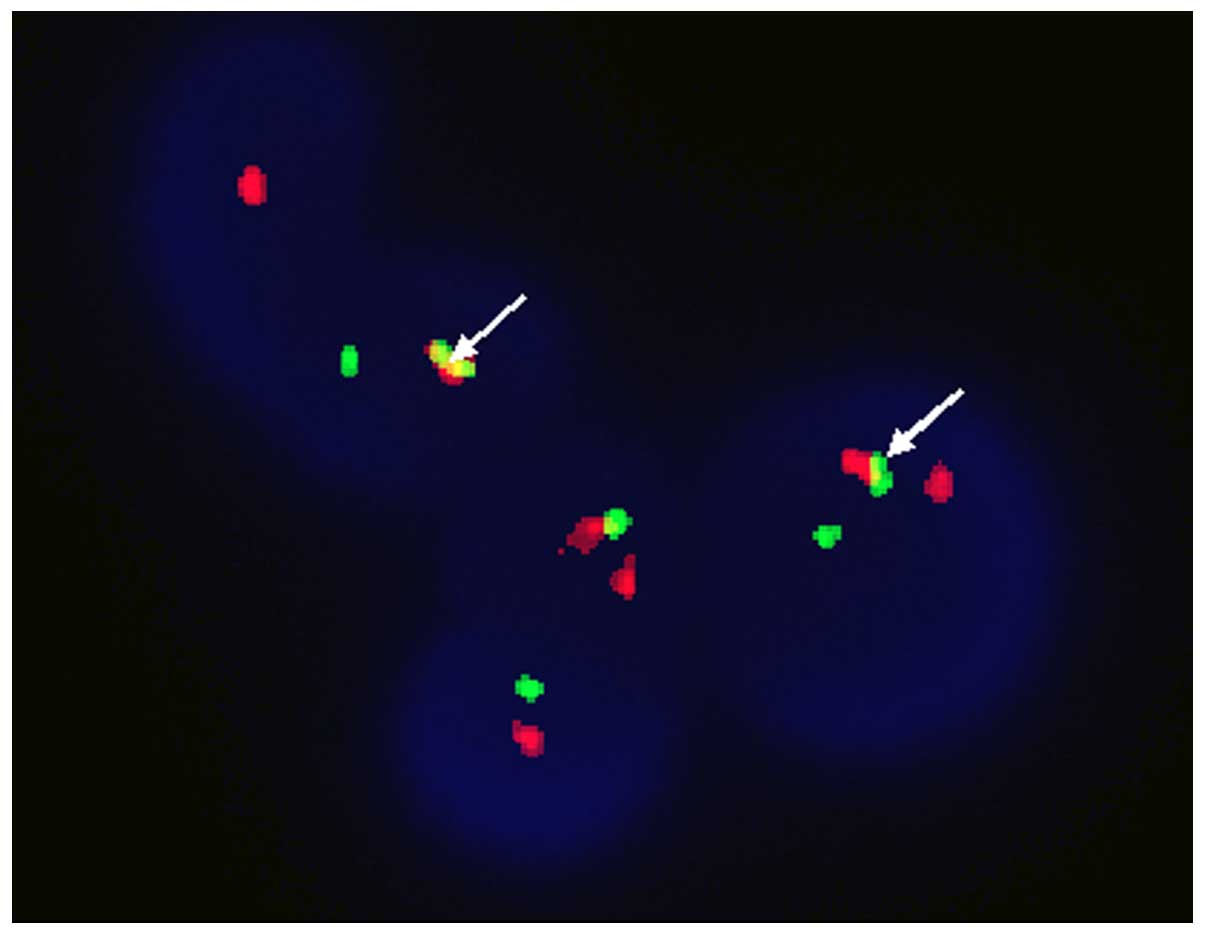
fusion was 100% as determined by FISH (results were considered
clonal when the percentage of cells containing a reciprocal t(9;22)
exceeded 4.0%; Fig. 2). No BCR-ABL
fusion gene (e13a2, e14a2, e1a2 and e19a2) was detected by RT-qPCR.
However, further detection by RT-PCR demonstrated the presence of
the e13a3 fusion gene (Fig. 3).
Subsequently, the patient was treated initially with hydroxyurea
(3,000 mg/day) with reasonably good control of WBCs. After 3 weeks,
the WBC count was decreased (9.8×109/l) and the previous
relevant symptoms disappeared. Following this, the patient was
administered 800 mg of Nilotinib daily. No side effects or
hematologic toxicity were observed. The karyotype was normalized
[46,XY (20/20)] and FISH for BCR-ABL decreased to 0% after 3 months
on Nilotinib, indicating a complete cytogenetic response (Fig. 4). Written informed consent was
obtained from the patient and the study was approved by the Ethics
Committee of The First Hospital of Lanzhou University.
Discussion
BCR-ABL transcripts with intronic breakpoints
downstream of ABL a2, lacking ABL exon 2, are rare. The present
study described a chronic myeloid leukemia (CML) case with an e13a3
BCR-ABL fusion transcript. To date, only 16 cases of CML with e13a3
BCR-ABL transcripts have been reported (3,5–7). Ito
et al reported the frequency and distribution of BCR-ABL
transcript types among the Japanese. Overall, the percentage of
patients with the e14a2, e13a2 and e13a3 transcript types was 67.50
(85/126), 30.20 (38/126) and 0.80% (1/126), respectively (8). Goh et al reported that the
majority of patients (538/548, 98.18%) were found to have e14a2 or
e13a2 in Korea, and the frequency of occurrence of e13a3 was 0.18%
(1/548) (9). In a previous study
by Todoric-Zivanovic et al, the e14a2 form of BCR-ABL was
detected in 100 patients (73.5%) and the e13a2 form was detected in
34 patients (25%). One (0.75%) patient had the e1a1 transcript of
BCR-ABL, however, no BCR-a3 case was detected (10).
The number of reported BCR-a3 cases is small
compared with the theoretical frequency of BCR-a3 cases. An
explanation for this mismatch may be due to the methodology of
RT-qPCR. Initially, the e13a3 fusion transcript was missed by
RT-qPCR using the primer corresponding to ABL a2 sequences despite
the existence of the t (9;22) (q34;q11) translocation by G-banding.
In addition, FISH also detected this translocation due to the large
size of the probes used. Following this, RT-qPCR analysis with the
ABL primer, which was located in ABL exon 3 to enable the detection
of fusions with either ABL exon 2 (a2) or exon 3 (a3) demonstrated
a 169 bp band in the present case, in comparison with an
e13a2-positive control band (343 bp), suggesting that the ABL a2
region (174 bp) was completely deficient. There may be more cases
that present BCR-a3 fusion transcripts if a proper primer is used
routinely in RT-qPCR. The patient was administered 800 mg of
Nilotinib daily, and using classic cytogenetics, the Ph+
metaphases decreased from 100% prior to Nilotinib treatment to 0%
by 3 months. The patient did respond quickly and completely to
Nilotinib, with rapid achievement of complete hematologic and
cytogenetic remission.
The ABL a2 region encodes a part of the Src homology
(SH)3 domain. The SH3 domain is considered to have a negative
regulatory role in the kinase domain (SH1). Therefore, the lack of
a SH3 domain may result in a more aggressive form of Ph-positive
leukemia. By contrast, the SH3 domain is required for activation of
signal transducer and activator of transcription 5 by the BCR-ABL
protein, leading to full leukemogenesis. Thus, deletion of the SH3
domain may induce a less progressive clinical course (5,11).
The BCR-ABL a3 break-point does not alter the sequence coding for
the ATP/imatinib binding domain, but alterations in tertiary
structure compared with a typical a2 fusion could affect drug
response. The clinical outcomes specific to CML patients with
BCR-ABL a3 fusions are difficult to define due to the limited
number of cases reported (2,4).
In conclusion, CML with a BCR-ABL a3 fusion gene is
a rare and challenging disease, which could lead to negative
RT-qPCR results and be erroneously interpreted. According to
National Comprehensive Cancer Network (NCCN) practice guidelines,
cytogenetics, FISH and RT-qPCR are recommended as the initial
workup for chronic phase adult CML, as each essay can provide
unique information. As mentioned, FISH and standard cytogenetics
can identify uncommon BCR-ABL translocations that may be missed by
RT-qPCR. Karyotyping identifies other cytogenetic abnormalities
that may have prognostic significance. Once a complete cytogenetic
remission has been obtained, the NCCN guidelines recommend RT-qPCR
every 3 to 6 months, which may not be effective for patients with
rare breakpoints. Further studies are required to interpret natural
frequency and unique clinical manifestations of this rare BCR-ABL
fusion in patients with CML.
Acknowledgments
The authors would like to thank High Trust
Diagnostics Inc. and Dr Ren Li and Dr Luo Xiu Feng for their
assistance with the experiment.
References
|
1
|
Burmeister T and Reinhardt R: A multiplex
PCR for improved detection of typical and atypical BCR-ABL fusion
transcripts. Leuk Res. 32:579–585. 2008. View Article : Google Scholar
|
|
2
|
Jinawath N, Norris-Kirby A, Smith BD,
Gocke CD, Batista DA, Griffin CA and Murphy KM: A rare e14a3 (b3a3)
BCR-ABL fusion transcript in chronic myeloid leukemia: Diagnostic
challenges in clinical laboratory practice. J Mol Diagn.
11:359–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Achkar WA, Wafa A, Ali BY, Manvelyan M and
Liehr T: A rare chronic myeloid leukemia case with Philadelphia
chromosome, BCR-ABL e13a3 transcript and complex translocation
involving four different chromosomes. Oncol Lett. 1:797–800.
2010.PubMed/NCBI
|
|
4
|
Pienkowska-Grela B, Woroniecka R, Solarska
I, Kos K, Pastwińska A, Konopka L and Majewski M: Complete
cytogenetic and molecular response after imatinib treatment for
chronic myeloid leukemia in a patient with atypical karyotype and
BCR-ABL b2a3 transcript. Cancer Genet Cytogenet. 174:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujisawa S, Nakamura S, Naito K, Kobayashi
M and Ohnishi K: A variant transcript, e1a3, of the minor BCR-ABL
fusion gene in acute lymphoblastic leukemia: Case report and review
of the literature. Int J Hematol. 87:184–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masuko M, Furukawa T, Abe T, Wada R,
Maruyama S, Kitajima T, Shibasaki Y, Toba K, Okada M and Aizawa Y:
A chronic myeloid leukemia patient with atypical karyotype and
BCR-ABL e13a3 transcript caused by complex chromosome
rearrangement. Int J Hematol. 90:230–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Braekeleer E, Douet-Guilbert N, Rowe D,
Bown N, Morel F, Berthou C, Férec C and De Braekeleer M: ABL1
fusion genes in hematological malignancies: A review. Eur J
Haematol. 86:361–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito T, Tanaka H, Tanaka K, Ito K, Kyo T,
Dohy H, Kamada N and Kimura A: Insertion of a genomic fragment of
chromosome 19 between BCR intron 19 and ABL intron 1a in a chronic
myeloid leukaemia patient with micro-BCR-ABL (e19a2) transcript. Br
J Haematol. 126:752–753. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goh HG, Hwang JY, Kim SH, Lee YH, Kim YL
and Kim DW: Comprehensive analysis of BCR-ABL transcript types in
Korean CML patients using a newly developed multiplex RT-PCR.
Transl Res. 148:249–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Todoric-Zivanovic B, Strnad M, Stamatovic
D, Tukic L, Krtolica K, Tatomirovic Z, Djordjevic V, Bogdanovic A,
Jankovic G and Magic Z: Frequency of BCR-ABL fusion transcripts in
Serbian patients with chronic myeloid leukemia. J BUON. 16:104–107.
2011.PubMed/NCBI
|
|
11
|
Snyder DS, McMahon R, Cohen SR and Slovak
ML: Chronic myeloid leukemia with an e13a3 BCR-ABL fusion: Benign
course responsive to imatinib with an RT-PCR Advisory. Am J
Hematol. 75:92–95. 2004. View Article : Google Scholar : PubMed/NCBI
|