Introduction
Inflammation is a complex biological response,
acting as a self-defense mechanism against harmful environmental
insults, which involves a network of cellular responses, cytokines,
including interleukin (IL)-1β, IL-6, tumor necrosis factor-α
(TNF-α), and humoral factors (1,2).
However, their persistence may lead to chronic inflammation, which
may be associated with several diseases, including cancer (1,3),
neoplasia, inflammatory bowel disease, ulcerative colitis (4,5),
arthritis (6), asthma and
Alzheimer's disease (7). The
detrimental effects of chronic inflammation can be controlled by
conventional treatments, however, resistance to the drugs used and
their side-effects necessitate the development of novel
anti-inflammatory drugs (8).
Previous studies have investigated the use of adjunct
immunotherapies to improve treatment success for tuberculosis (TB).
Among these, the immunosuppression-mediated reduction of TNF-α
levels by current antibiotic therapies may eventually be effective
in treating highly contagious TB (9).
In general, when M. tuberculosis enters the
lung, it interacts with macrophages. Macrophages have been the most
commonly examined cells in investigations of TB, although
epithelial cells are being increasingly examined in TB, as they are
essential in the immune response during pulmonary tuberculosis
(10–13). Nitric oxide (NO) is an important
mediator in cell signaling, neurotransmission and in the host
defense mechanism (14,15). M. tuberculosis infection
induces the activity of inducible nitric oxide synthase (iNOS) in
A549 cells, which leads to the production of a significant level of
NO (13).
A natural triterpenoid carboxylic acid,
3-β-3-hydroxy-urs-12-ene-28-oic-acid (uracil; UA), is present in a
wide variety of foods (16). Its
biochemical and pharmacological effects include anti-inflammatory,
antioxidant, antiproliferative, anticancer, antimutagenic,
antihypertensive, and antiviral properties (17,18).
UA can also inhibit the immunoregulatory transcription factor,
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB),
in response to a wide variety of carcinogens and inflammatory
agents (19). There is also
evidence supporting the anti-inflammatory effects of UA (2).
In the present study, M. tuberculosis-induced
RAW 264.7 mouse monocyte macrophages, A549 type II alveolar cells
and mitogen, concanavalin A-induced rat splenocytes were used to
examine the effect of UA on immune regulation. The aim of the
present study was to investigate the anti-inflammatory potential of
UA, a candidate drug for controlling inflammation-associated
diseases.
Materials and methods
Cell culture
RAW 264.7 mouse monocyte macrophages and A549 type
II alveolar cells, purchased from American Type Culture Collection
(ATCC; Manassas, VA, USA) were maintained in Dulbecco's Modified
Eagle's Medium (DMEM) supplemented with 10% heat-inactivated fetal
bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C in a humidified incubator with 5% CO2
and sub-cultured every 2–3 days.
Reagents, treatment conditions and
durations
The UA, NG-monomethyl-L-arginine
(L-NMMA), and concanavalin A (Con A) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Throughout the present study,
the cells (1×105 cells/ml) were treated with 10
µg/ml UA and 5 mM L-NMMA for 6 h following M.
tuberculosis infection, following which they were incubated for
the specified durations. Immediately prior to infection or
treatment, the medium were replaced with serum-free DMEM. In the
case of the splenocytes, the cells (1×106 cells/ml) were
co-treated with 5 µg/ml Con A and 10 µg/ml UA for the
specified durations.
Animals and primary splenocyte
collection
Female Wistar rats (190–220 g; 8-weeks old; n=8)
were purchased from Nara Biotech, Ltd. (Pyeongtaek-si, Korea). The
animals were housed in a solid-bottomed cage at 23±2°C, maintained
under a 12 h light-dark cycle and fed standard rodent chow (Purina
Rodent Chow; Purina Co., Ltd., Seoul, Korea) and water ad
libitum. The present study was performed according to the
guidelines of the United States National Institutes of Health, and
was approved by the ethics committee of Soonchunhyang University
(Asan, Korea; SCH14-0031).
Primary splenocytes were collected from the rats
following sacrifice by cervical dislocation, and aseptic collection
of the spleen was performed. Each spleen was immersed in RPMI 1640
culture medium supplemented with 10% (v/v) heat-inactivated FBS and
a 1% (v/v) antibiotic/antimycotic cocktail, comprising 100 U/ml
penicillin, 100 µg/ml streptomycin and 0.25 µg/ml
amphotericin B (Invitrogen; Thermo Fisher Scientific, Inc.). The
cells were passed through a cell strainer (BD Biosciences, Durham,
NC, USA) to form a single cell suspension. Erythrocytes were
excluded from the resulting cell suspension using lysis buffer
(Life Technologies, Seoul, Korea), containing 0.15 M
NH4Cl, 10 mM KHCO3 and 0.1 mM
Na2EDTA (pH 7.3). The cells were washed once with
phosphate-buffered saline (PBS) and cultured at a density of
106 cells/ml. Cell viability was determined according to
the exclusion of Trypan blue (Sigma-Aldrich). The cells were
maintained under standard culture conditions at 37°C.
Mycobacterium infection/invasion
The M. tuberculosis H37Rv strain was
purchased from ATCC and was grown in Middlebrook 7H11 agar (Becton
Dickinson, Sparks, MD, USA) or Ogawa medium for ~3 weeks. Isolated
colonies were inoculated in Middlebrook 7H9 broth (Becton
Dickinson) in am incubator with agitation for 15 days. To avoid
clumping, the bacterial suspension was vortexed vigorously with
glass beads, and then passed through an 8-µm filter to form
a single cell suspension. The suspension was allowed to stand for
several minutes to settle and two-thirds of the clear upper portion
of suspension was used for quantification at 600 nm using the
UVmini-1240 spectrophotometer (Shimadzu, Kyoto, Japan) adjusted
with McFarland standards. A 0.5 McFarland standard was prepared by
mixing 0.05 ml of 1.175% barium chloride dihydrate with 9.95 ml of
1% sulfuric acid. Subsequently, 10 µl of the suspension was
inoculated in Middlebrook 7H11 agar or Ogawa medium at 37°C in an
atmosphere of 5% CO2 for 3–4 weeks to quantify the
bacterial number in colony forming units (cfu). Following count
determination, the bacterial suspension was aliquoted and stored at
−76°C as a single volume
For in vitro infection, the RAW 264.7
(2×105 cells) and A549 (2×105 cells) cells
were grown in six-well plates overnight and infected with M.
tuberculosis H37Rv at a 1:10 ratio for 3 h in the
aforementioned cell culture conditions. The cells were washed with
warm PBS three to five times to remove extracellular bacteria. To
confirm successful invasion of the bacteria, the final wash medium
and cell extract (100 µl), following cell dissolution with
0.1% Triton X-100 (Sigma-Aldrich), were inoculated in Middlebrook
7H11 agar for colony counting, Middlebrook 7H9 broth for a
resazurin assay (20) and in a
Bactec MGIT system (Becton Dickinson) for the determination of time
to detection (TTD) (21), as
described previously (22). Prior
to proceeding, it was confirmed that the bacteria present in the
final wash medium were absent or few in number, compared with the
high numbers of viable bacteria in the extracted cell suspension,
which confirmed successful bacterial invasion.
Cell viability assay
The cell viability assay performed in the present
study was based on the conversion of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) to formazan crystals by the mitochondrial
dehydrogenase enzyme (23). In
brief, the cells (1×105 cells/ml) were seeded overnight
to achieve ~80% confluence and were treated with 10 µg/ml UA
for 0, 6, 12, 24, 48 and 72 h. At the determined time, 20 µl
of 5 mg/ml MTT reagent was added. Following 4 h incubation at 37°C,
the medium were aspirated, and 100 µl of dimethylsulfoxide
(Samchun Pure Chemical Co., Ltd., Pyeongtaek, Korea) was added to
dissolve the formazan crystals. The absorbance was measured at 570
nm using a Victor™ X3 multilabel reader (Perkin Elmer, Inc.,
Waltham, MA, USA).
Splenocyte viability was determined using an
MTS-based assay (24). In brief,
the cells were treated for the indicated times periods, and the
detection reagent was prepared using MTS and phenazine methosulfate
(Sigma-Aldrich) at a ratio of 20:1, which was added at a 1:5 ratio
of reagent mixture to cell culture. The absorbance was detected at
492 nm using the Victor™ X3 multilabel reader.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The cells were infected and/or treated for the
indicated time periods, following which the total RNA was extracted
using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) and quantified
using an ND-1000 spectrophotometer (NanoDrop Technologies,
Wilmington, NC, USA) at 260 nm. The assay used RNA at a 260:280 nm
ratio of 1.8–2.0, indicating high purity. Subsequently, cDNA was
prepared using 1,000 ng of the total RNA using Oligo
dT15 Primer (Maxime™ RT PreMix kit; Intron
Biotechnology, Inc., Seongnam, Korea) in a Veriti®
96-Well Thermal Cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Subsequently, qPCR was performed to amplify the
cDNA using an iQ SYBR Green Supermix kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), according to the manufacturer's protocol,
in a CFX96TM real-time PCR detection system (Bio-Rad Laboratories,
Inc.). The temperature cycle followed for qPCR was as follows: 95°C
for 5 min, followed by 40 cycles of 95°C for 10 sec, 42°C for 10
sec and 72°C for 20 sec. A dissociation curve was acquired to
ensure the specificity of the PCR product in every PCR assay. The
assay results were normalized to the endogenous control gene,
glyceraldehyde 3-phosphate dehydrogenase. The primers used were
purchased from Bioneer Corporation (Seoul, Korea) and are listed in
Table I, with the exception of
human interleukin 1-β (IL-1β), and interleukin 6
(IL-6), which were also purchased from Bioneer Corporation
(Seoul, Korea; cat. nos. N-1058 and N-1063, respectively). Relative
quantification was obtained using the comparative threshold cycle
(∆∆Cq) method. A CFX96™ real-time PCR detection system (Bio-Rad
Laboratories, Inc.) with a default calculation system was used.
 | Table IList of primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
List of primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| Mouse | | |
| TNF-α |
TGTCTCAGCCTCTTCTCATT |
AGATGATCTGAGTGTGAGGG |
| IL-6 |
TTGCCTTCTTGGGACTGATG |
CCACGATTTCCCAGAGAACA |
| IL-1β |
GGGCTGCTTCCAAACCTTTG |
TGATACTGCCTGCCTGAAGCTC |
| iNOS |
CCCTTCCGAAGTTTCTGGCAGCAGC |
GGCTGTCAGAGCCTCGTGGCTTTGG |
| COX-2 |
CCAGCACTTCACCCATCAGTT |
ACCCAGGTCCTCGCTTATGA |
| L32 |
GCCAGGAGACGACAAAAAT |
AATCCTCTTGCCCTGATCC |
| Human | | |
| TNF-α |
TCTTCTCGAACCCCGAGTGA |
CCTCTGATGGCACCACCAG |
| iNOS |
CCTCTGATGGCACCACCAG |
ACCCTGCCAACGTGGAATTCACTCAG |
| COX-2 |
TTCAAATGAGATTGTGGGAAAATTGCT |
AGATCATCTCTGCCTGAGTATCTT |
| GAPDH |
TCCCATCACCATCTTCCA |
CATCACGCCACAGTTTCC |
| Rat | | |
| TNF-α |
GACCCTCACACTCAGATCATCTTCT |
TGCTACGACGTGGGCTACG |
| IL-6 |
CGAGCCCACCAGGAACGAAAGTC |
CTGGCTGGAAGTCTCTTGCGGAG |
| IL-1β |
CCCTGCAGCTGGAGAGTGTGG |
TGTGCTCTGCTTGAGAGGTGCT |
| iNOS |
GTGCTAATGCGGAAGGTCATG |
GCTTCCGACTTTCCTGTCTCAGTA |
| COX-2 |
GTGTCCCTTTGCCTCTTTCAAT |
GAGGCACTTGCGTTGATGGT |
| GAPDH |
ATGATTCTACCCACGGCAAG |
CTGGAAGATGGTGATGGGTT |
NO release assay
NO release was measured by detecting the
concentration of nitrite produced, using the Griess reagent system
(Promega Corporation, Madison, WI, USA). Briefly, nitrite standards
were prepared, ranging between 100 and 1.56 µM (100, 50, 25,
12.5, 6.25, 3.13 and 1.56 µM) by serial 2-fold dilutions.
The cell-free supernatants were obtained by centrifugation at 2,000
× g for 1 min at room temperature. The nitrite standards and
cell-free supernatants from the infected and/or treated samples (50
µl) were added to a 96-well tissue culture plate in
triplicate. Subsequently, 50 µl sulphanilamide solution was
added to each well, and incubated for 10 min at room temperature in
the dark, followed by the addition of 50 µl
N-1-napthylethylenediamine dihydrochloride. The absorbance was
measured at 540 nm using the Victor™ X3 multilabel reader.
Statistical analysis
Data are presented as the mean ± standard deviation.
At least three individual experiments were performed. Statistical
analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA), Differences between groups were analyzed using one-way
analysis of variance, followed by the Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
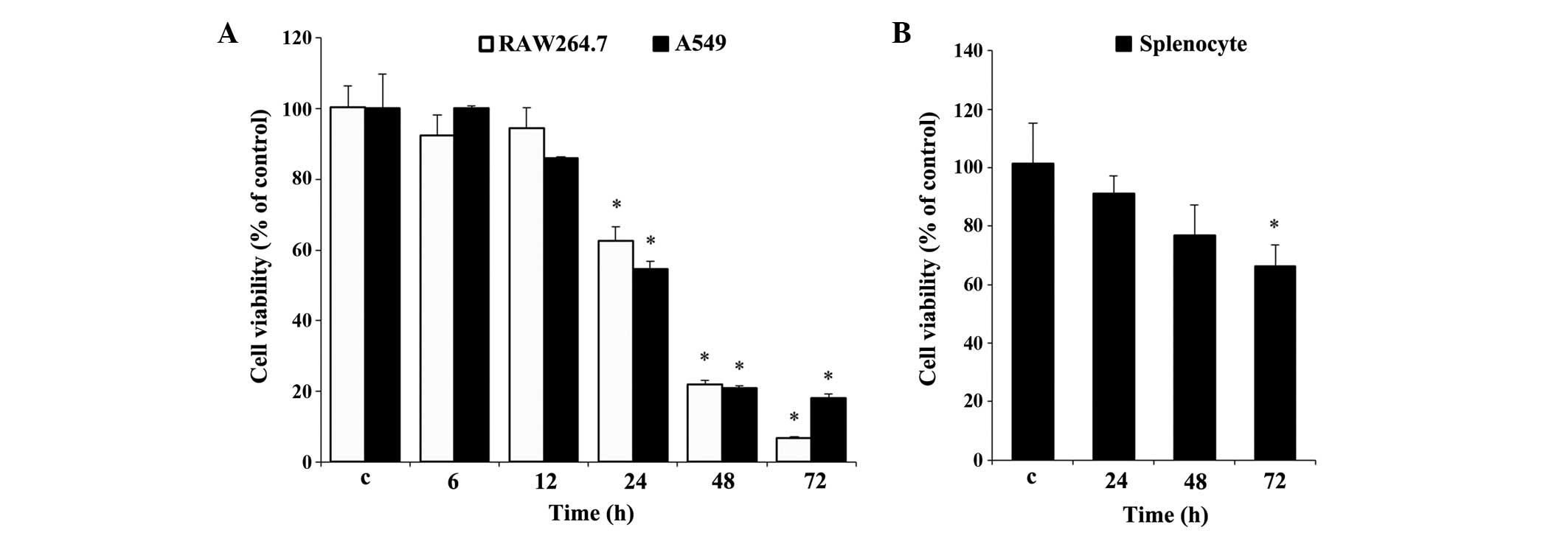
Effect of UA on cell viability
Prior to the experiments, it was necessary to
evaluate the effect of UA on cell viability. Cell viability was
determined using an MTT assay for the RAW 264.7 and A549 cells
(Fig. 1A), and using an MTS assay
for the rat splenocytes (Fig. 1B).
Measurements over time established that significant cytotoxicity
was evident, beginning at 24 h, in the two cell lines. At 72 h, 10
µg/ml UA resulted in cytotoxicity towards the RAW 264.7
cells, which was almost 11% higher than that in the A549 cells
(Fig. 1A). By contrast, the
splenocytes showed less cytotoxicity under similar treatment
conditions between 24 and 72 h, compared with the RAW 264.7 and
A549 cells. No significant difference in splenocyte viability was
found at any time point, with the exception of 72 h (Fig. 1B), at which the cell viability was
decreased by almost 35%, which was marginal, compared with the
other two cell types at 72 h (Fig.
1B).
UA suppresses infection- and Con
A-induced cytokine expression
Cytokines are critical in the development and
regulation of immune responses. Several phytochemicals are involved
in the immunomodulatory activities of the immune system and, thus,
are key in immunosuppression. The expression levels of genes
encoding TNF-α, IL-6 and IL1-β in different
cells was evaluated following infection and/or treatment. The M.
tuberculosis-infected RAW 264.7 cells showed a significant
induction of the mRNA expression of TNF-α at 12 and 24 h
(Fig. 2A). This induction was
significantly reduced by treatment of the RAW 264.7 cells with UA.
In the A549 cells, the induction of TNF-α by infection, and
the reduction by UA were not significant (Fig. 2B). UA treatment alone reduced the
mNRA levels of TNF-α in the RAW 264.7 cells at 12 h,
however, the expression returned to a basal level at 24 h. In the
A549 cells, UA did not induce significant changes at any time point
(Fig. 2A and B). In the
splenocytes, the Con A mitogen was used to investigate the
immunoregulatory activity of UA. Con A increased the mRNA
expression of TNF-α by ~2-fold at 12 h, which increased to
3-fold at 24 h. At both time points, UA significantly reduced the
mRNA expression levels of TNF-α, compared with the control
(Fig. 2C).
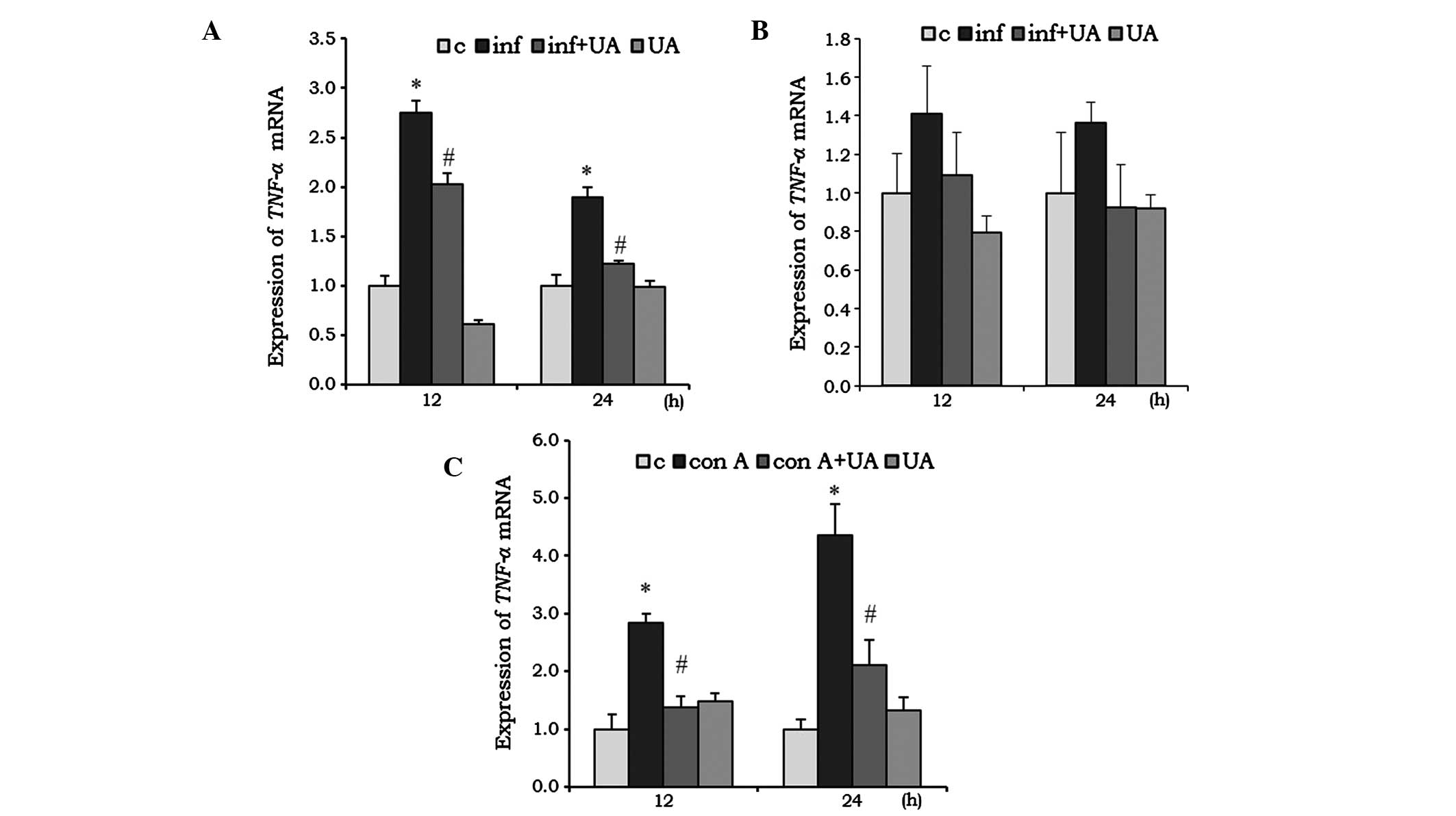 | Figure 2UA has an inhibitory effect on the
expression of TNF-α. (A) RAW 264.7 cells and (B) A549 cells
were infected with M. tuberculosis H37Rv (1:10) for 3 h
and/or treated with 10 µg/ml UA for 6 h. (C) Rat splenocytes
were co-treated with 5 µg/ml Con A and/or 10 µg/ml
UA. Following 12 and 24 h treatment, the cells were harvested for
total RNA collection, cDNA preparation and reverse
transcription-quantitative polymerase chain reaction analysis. Data
is presented as the mean ± standard deviation of three independent
experiments. *P<0.05, vs. control;
#P<0.05, inf+UA, vs. inf or Con A. TNF-α, tumor
necrosis factor-α; c, control; inf, infection; UA, ursolic acid;
Con A, concanavalin A. |
IL-6 acts as pro-inflammatory, as well as an
anti-inflammatory, cytokine. In the RAW 264.7 cells, infection
induced the mRNA expression of IL-6 at 24 h, and was
significantly reduced by UA treatment. Of note, the reduction was
even lower than the basal level. Treatment with UA alone had no
significant effect on the mRNA expression of IL-6 (Fig. 3A). In the A549 cells, infection
and/or treatment showed no significant change at 12 h, however, at
24 h the mRNA expression of IL-6 increased almost 5-fold. UA
significantly reduced this induced expression (Fig. 3B). No significant changes in the
mRNA expression of IL-6 mRNA were observed in the Con A
and/or UA-treated splenocytes at 12 h. However, at 24 h, the
expression of IL-6 was significantly induced when treated
with Con A, and UA successfully reduced this induced expression
(Fig. 3C).
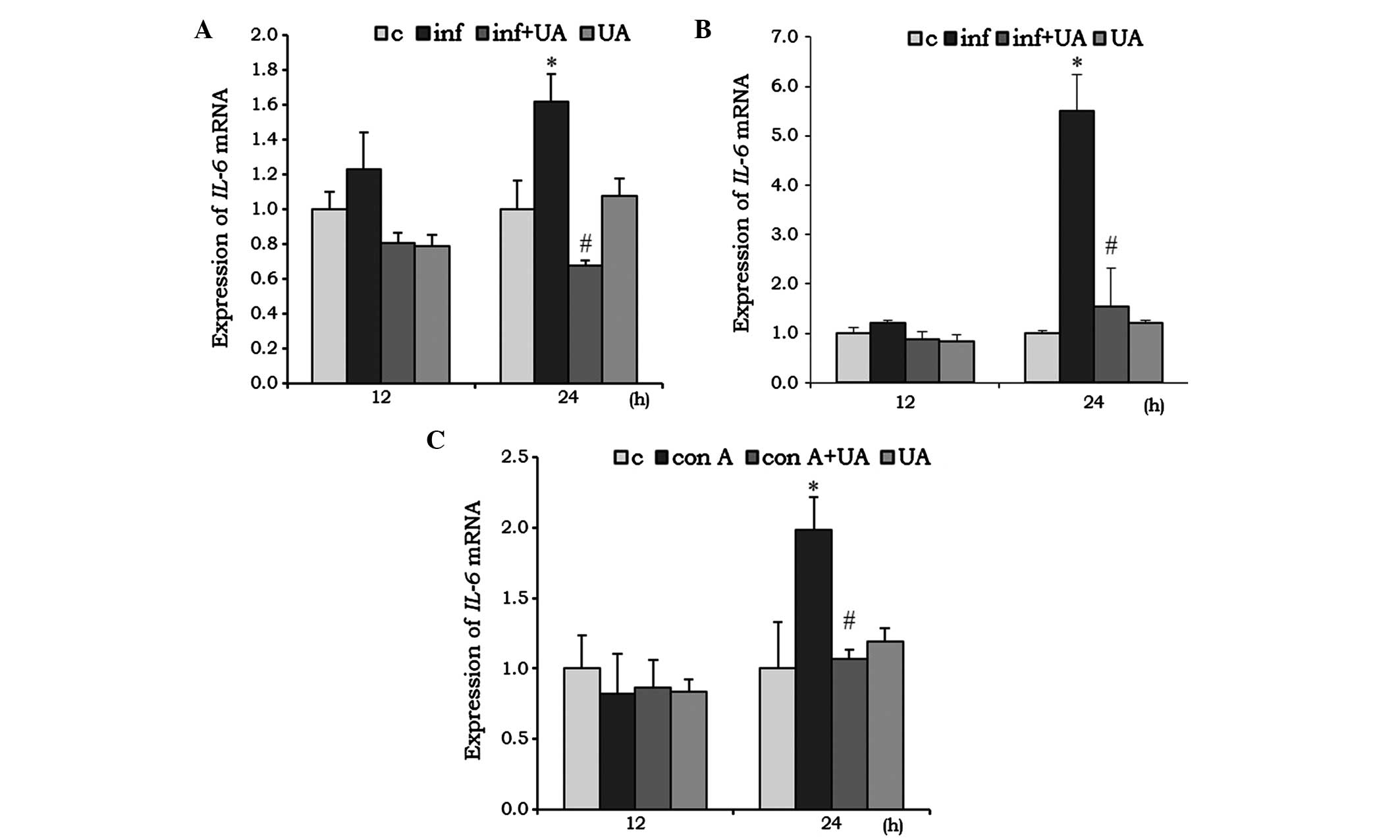 | Figure 3UA has an inhibitory effect on the
expression of IL-6 in RAW 264.7 cells, A549 cells and rat
splenocytes. (A) RAW 264.7 cells and (B) A549 cells were infected
with M. tuberculosis H37Rv for 3 h and/or treated with 10
µg/ml UA for 6 h. (C) Rat splenocytes were treated with 5
µg/ml Con A and/or 10 µg/ml UA. Following 12 and 24 h
of incubation, the cells were harvested for total RNA collection,
cDNA preparation and reverse transcription-quantitative polymerase
chain reaction analysis of IL-6 mRNA. Data is presented as
the mean ± standard deviation of three independent experiment.
*P<0.05, vs, control; #P<0.05, inf+UA,
vs. inf or Con A. IL, interleukin; c, control; inf, infection; UA,
ursolic acid; Con A, concanavalin A. |
IL-1β is a pro-inflammatory cytokine, which was
found to be significantly induced by M. tuberculosis H37Rv
infection in RAW 264.7 cells (Fig.
4A) and A549 cells (Fig. 4B)
at 12 and 24 h. Their induction was effectively reduced by UA
treatment, however, in the RAW 264.7 cells, the reduction was below
the basal level at 12 h, compared with the control (Fig. 4A and B). Compared with the RAW
264.7 and A549 cells, no significant changes were observed in the
splenocytes treated with Con A and/or UA at 12 h. However, at 24 h,
Con A induced the expression of IL-1β, whereas UA had no
significant effect (Fig. 4C).
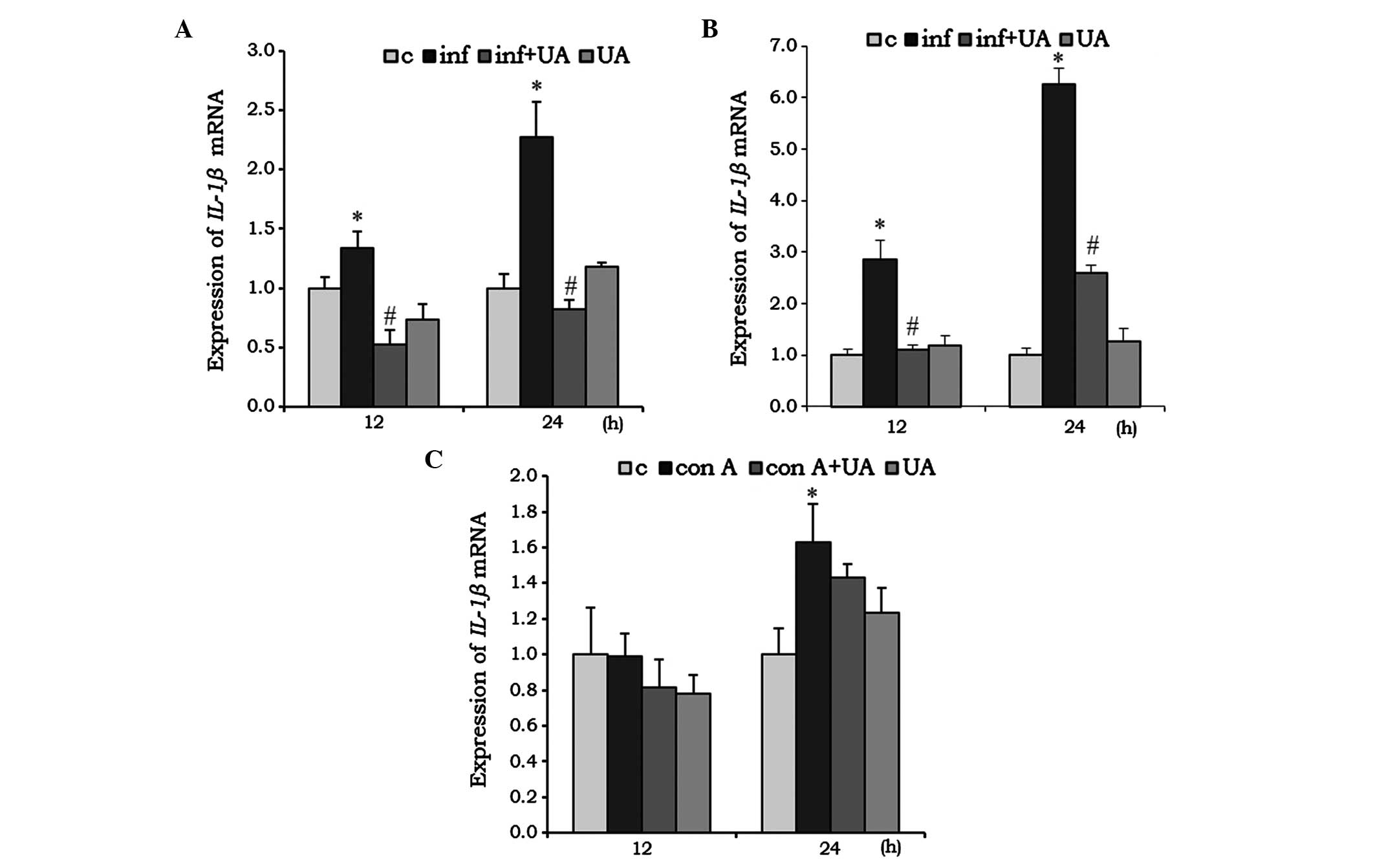 | Figure 4Effect of UA on the mRNA expression
of IL-1β. (A) RAW 264.7 cells, (B) A549 cells were infected
and/or treated with UA, and (C) rat splenocytes were infected
and/or treated with Con A. Following indicated period of
incubation, the cells were harvested for total RNA collection, cDNA
preparation and reverse transcription-quantitative polymerase chain
reaction analysis for IL-1β. Data is presented as the mean ±
standard deviation of three independent experiments
*P<0.05, vs. control; #P<0.05 vs. Inf
or Con A. IL, interleukin; c, control; inf, infection; UA, ursolic
acid; Con A, concanavalin A. |
Infection- and Con A-induced expression
of iNOS and COX-2 expression is regulated by UA
The iNOS and COX-2 genes are usually
induced by inflammation. To evaluate the effect of UA on their
expression levels, the present study infected RAW 264.7 cells with
M. tuberculosis H37Rv and/or treated the cells with UA.
Infection induced the expression of the two genes significantly at
12 and 24 h. These levels of expression were significantly
suppressed by UA at both time points. The gene expression of
iNOS and COX-2 were suppressed below the basal level
at 24 h. No significant changes in the gene expression of
iNOS or COX-2 were observed in the cells treated with
UA only (Fig. 5A and B).
 | Figure 5UA suppresses the expression of the
iNOS and COX-2 inflammatory mediators. (A and B) RAW
264.7 cells were infected and/or treated with UA. (C and D) rat
splenocytes were treated with con A and/or UA. Following
incubation, the cells were harvested, total RNA was collected, cDNA
was prepared and reverse transcription-quantitative polymerase
chain reaction analysis was performed for iNOS and
COX-2. Data is presented as the mean ± standard deviation of
three independent experiments *P<0.05, vs. control;
#P<0.05, vs. inf or Con A. iNOS, inducible nitric
oxide synthase; COX-2, cyclooxygenase-2; c, control; inf,
infection; UA, ursolic acid; Con A, concanavalin A. |
The inflammatory response was induced in the
splenocytes by treatment with the Con A mitogen. At 24 h, the
expression of the iNOS gene was induced almost 5-fold,
compared with the control, however, UA suppressed the expression by
~2-fold. No significant changes in the expression of iNOS
were evident at 12 h in the samples treated with Con A and/or UA
(Fig. 5C). However, at 12 and 24
h, the expression of COX-2 was induced significantly by Con
A treatment. UA treatment reduced this induced expression
significantly at 24 h, but not at 12 h (Fig. 5D). Treatment of the splenocytes
with UA alone did not significantly alter the gene expression
levels of iNOS or COX-2 from the basal level
(Fig. 5C and D).
UA suppresses NO release in RAW 264.7 and
A549 cells
NO is a signaling molecule, which is important in
modulating the release of various inflammatory mediators from a
wide range of cells (25).
Infection of RAW 264.7 (Fig. 6A)
and A549 (Fig. 6B) cells with
M. tuberculosis H37Rv produced a significant release of NO
into the culture medium at 24 and 48 h, respectively. The alveolar
epithelial A549 cells did not appear to release NO prior to 48 h.
In addition, treatment with UA or the NO synthase inhibitor
significantly reduced the release of NO in the two cell types at
these time points. Of note, UA and the NO synthase inhibitor also
induced NO release in the two cell type, although without
statistical significance (Fig. 6A and
B).
 | Figure 6UA inhibits NO release. M.
tuberculosis H37Rv infected (A) RAW 264.7 cells and (B) A549
(B) cells were treated with UA or L-NMMA for 6 h, or left
untreated, and incubated for 24 and 48 h, respectively. Data is
presented as the mean ± standard deviation of three independent
experiments. *P<0.05, vs. control;
#P<0.05, vs. inf. NO, nitric oxide; L-NMMA,
NG-monomethyl-L-arginine; c, control; inf, infection;
UA, ursolic acid; Con A, concanavalin A. |
Discussion
Inflammation is a healing process in the body,
however, when it is prolonged, various diseases can result
(26). Immunomodulatory therapy of
TB reduces excessive inflammation and restricts pathology. Pro- and
anti-inflammatory drugs may have significant potential in tailoring
TB treatment, when administered with routine anti-TB drugs
(27–29). In the present study, M.
tuberculosis H37Rv was selected to infect mouse monocyte
macrophages, RAW 264.7 and A549 type II alveolar cells. In the
majority of previous studies, alveolar macrophages were considered
for TB-associated studies. Alveolar epithelial cells are now also
considered to be involved in pathogenesis and in the innate immune
response (30,31). Natural compounds are a valuable
source for a wide array of prospective biomedical uses, due to
their limited side effects. UA shows anti-inflammatory potential in
RAW 264.7 cells by attenuating the expression of iNOS and
COX-2 (32,33). The anti-inflammatory potential of
UA has also been reported in activated T cells, B cells and
macrophages (34). The mechanism
underlying these effects has been attributed to the inhibition of
mitogen-induced phosphorylation of extracellular signal-regulated
kinase and c-Jun, N-terminal kinase, and the suppression of
immunoregulatory transcription factors, NF-κB and nuclear factor of
activated T cells and activator protein-1. Notably, UA mitigates
lipopoly-saccharide-induced expression of TNF-α, IL-1β and IL-6 in
splenic adherent macrophages (34). In the present study, UA was
investigated for its potential anti-inflammatory activity in
differentially stimulated cells. A comparative investigation was
performed to determine the gene expression levels of the
TNF-α, IL-1β and IL-6 cytokines, the
iNOS and COX-2 inflammatory mediators, and the
important signal transducer, NO. As a model, M. tuberculosis
H37Rv was used to sensitize RAW 264.7 cells and A549 cells, and Con
A-stimulated rat splenocytes.
The present study focused on the expression of
COX-2 and iNOS, as they are usually induced during
inflammatory conditions, and are reduced by the effect of
flavonoids (35,36). Abnormal upregulation of COX-2
and/or iNOS may be associated with certain types of cancer or
inflammatory disorders (37,38).
In the present study, infection by M. tuberculosis H37Rv or
treatment with Con A significantly increased the mRNA levels of
COX-2 and/or iNOS, which were downregulated by UA.
NO, the product of iNOS, is crucial during inflammation. In
addition to downregulating the expression levels of COX-2
and iNOS, UA decreased the release of NO, which was induced
following mycobacterial infection, in the cell culture medium. The
infected A549 cells exhibited delayed NO release, compared with the
RAW 264.7 cells. It has been reported that mycobacterial antigenic
components produce significantly higher levels of NO at 48 h in
A549 cells (39).
To detect anti-inflammatory activity, the present
study examined the ability of UA to reduce the production of
TNF-α in the mycobacteria-infected RAW 267.4 and A549 cells,
and Con A-stimulated rat splenocytes. TNF-α is pivotal in
inflammation, and its effect on the reduction of IL-1β and
IL-6 were examined in similar treatment conditions. All
three cytokines were upregulated when stimulated by M.
tuberculosis H37Rv in the RAW 267.4 cells, A549 cells and Con
A-stimulated rat splenocytes. The three cytokines were induced by
different stimuli in different cells, however, UA significantly
reduced their expression levels at the transcriptional level in the
present study. Upon stimulation, the expression levels of various
cytokines, inflammatory mediators, including iNOS and
COX-2, and NO release were induced at varying magnitudes and
at different times.
UA induced marked inhibitory effects on the
expression levels of cytokines and immunomodulatory mediators, and
on NO release. These findings, and the results of previous reports
suggest that UA has significant potential in mitigating the induced
inflammatory response. Further detailed investigations of UA are
required, to determine its potential as a functional remedy to
control inflammation in various diseases.
Acknowledgments
This study was supported by a Grant from the
Ministry of Health & Welfare R&D Project, Republic of Korea
(grant no. HI13C0828).
References
|
1
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Checker R, Sandur SK, Sharma D, Patwardhan
RS, Jayakumar S, Kohli V, Sethi G, Aggarwal BB and Sainis KB:
Potent anti-inflammatory activity of ursolic acid, a triterpenoid
antioxidant, is mediated through suppression of NF-kB, AP-1 and
NF-AT. PLoS One. 7:e313182012. View Article : Google Scholar
|
|
3
|
Dalgleish AG and O'Byrne KJ: Chronic
immune activation and inflammation in the pathogenesis of AIDS and
cancer. Adv Cancer Res. 84:231–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaser A, Zeissig S and Blumberg RS:
Inflammatory bowel disease. Annu Rev Immunol. 28:573–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishiguro Y: Mucosal proinflammatory
cytokine production correlates with endoscopic activity of
ulcerative colitis. J Gastroenterol. 34:66–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scott DL, Wolfe F and Huizinga TW:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee YJ, Han SB, Nam SY, Oh KW and Hong JT:
Inflammation and alzheimer's disease. Arch Pharm Res. 33:1539–1556.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Achoui M, Appleton D, Abdulla MA, Awang K,
Mohd MA and Mustafa MR: In vitro and in vivo anti-inflammatory
activity of 17-O acetylacuminolide through the inhibition of
cytokines, NF-kB translocation and IKKβ activity. PLoS One.
5:e151052010. View Article : Google Scholar
|
|
9
|
Zumla A, Rao M, Parida SK, Keshavjee S,
Cassell G, Wallis R, Axelsson-Robertsson R, Doherty M, ersson J and
Maeurer M: Inflammation and tuberculosis: Host-directed therapies.
J Intern Med. 277:373–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ellner JJ: Review: The immune response in
tuberculosis implication for tuberculosis control. J Infect Dis.
176:1351–1359. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fenton MJ and Vermeulen MW:
Immunopathology of tuberculosis: Roles of macrophages and
monocytes. Infect Immun. 64:683–690. 1996.PubMed/NCBI
|
|
12
|
Rich EA, Torres M, Sada E, Finegan CK,
Hamilton BD and Toossi Z: Mycobacterium tuberculosis
(MTB)-stimulated production of nitric oxide by human alveolar
macrophages and relationship of nitric oxide production to growth
inhibition of MTB. Tubercle Lung Dis. 78:247–255. 1997. View Article : Google Scholar
|
|
13
|
Roy S, Sharma S, Sharma M, Aggarwal R and
Bose M: Induction of nitric oxide release from the human alveolar
epithelial cell line A549: An in vitro correlate of innate immune
response to mycobacterium tuberculosis. Immunology. 112:471–480.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Celada A and Nathan C: Macrophage
activation revisited. Immunol Today. 15:100–102. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nathan CF and Hibbs JB Jr: Role of nitric
oxide synthesis in macrophage antimicrobial activity. Curr Opin
Immunol. 3:65–70. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda Y, Murakami A and Ohigashi H:
Ursolic acid: An anti- and proinflammatory triterpenoid. Mol Nutr
Food Res. 52:26–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsai SJ and Yin MC: Antioxidative and
anti-inflammatory protection of oleanolic acid and ursolic acid in
PC12 cells. J Food Sci. 73:H174–H178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shishodia S, Majumdar S, Banerjee S and
Aggarwal BB: Ursolic acid inhibits nuclear factor-kappaB activation
induced by carcinogenic agents through suppression of IkappaBalpha
kinase and p65 phosphorylation: Correlation with down-regulation of
cyclooxygenase 2, matrix metalloproteinase 9 and cyclin D1. Cancer
Res. 63:4375–4383. 2003.PubMed/NCBI
|
|
20
|
Martin A, Camacho M, Portaels F and
Palomino JC: Resazurin microtiter assay plate testing of
mycobacterium tuberculosis susceptibilities to second-line drugs:
Rapid, simple and inexpensive method. Antimicrob Agents Chemother.
47:3616–3619. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diacon AH, Maritz JS, Venter A, van Helden
PD, Andries K, McNeeley DF and Donald PR: Time to detection of the
growth of mycobacterium tuberculosis in MGIT 960 for determining
the early bactericidal activity of antituberculosis agents. Eur J
Clin Microbiol Infect Dis. 29:1561–1565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zerin T, Lee M, Jang WS, Nam KW and Song
HY: Ursolic acid reduces Mycobacterium tuberculosis-induced nitric
oxide release in human alveolar A549 cells. Mol Cells. 38:610–615.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zerin T, Kim YS, Hong SY and Song HY:
Protective effect of methylprednisolone on paraquat-induced A549
cell cytotoxicity via induction of efflux transporter,
P-glycoprotein expression. Toxicol Lett. 208:101–107. 2012.
View Article : Google Scholar
|
|
24
|
Lee HJ, Zerin T, Kim YH, Lee BE and Song
HY: Immunomodulation potential of Artemisia capillaris extract in
rat splenocytes. Intern J Phytomed. 5:356–361. 2013.
|
|
25
|
Wallace JL: Nitric oxide as a regulator of
inflammatory processes. Mem Inst Oswaldo Cruz. 100(Suppl 1): S5–S9.
2005. View Article : Google Scholar
|
|
26
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Subbian S, Tsenova L, O'Brien P, Yang G,
Koo MS, Peixoto B, Fallows D, Dartois V, Muller G and Kaplan G:
Phosphodiesterase-4 inhibition alters gene expression and improves
isoniazid-mediated clearance of mycobacterium tuberculosis in
rabbit lungs. PLoS Pathog. 7:e10022622011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tobin DM, Roca FJ, Oh SF, McFarland R,
Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, et al: Host
genotype-specific therapies can optimize the inflammatory response
to mycobacterial infections. Cell. 148:434–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Skerry C, Harper J, Klunk M, Bishai WR and
Jain SK: Adjunctive TNF inhibition with standard treatment enhances
bacterial clearance in a murine model of necrotic TB granulomas.
PLoS One. 7:e396802012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bermudez LE and Goodman J: Mycobacterium
tuberculosis invades and replicates within type II alveolar cells.
Infect Immun. 64:1400–1406. 1996.PubMed/NCBI
|
|
31
|
Garcia-Perez BE, Mondragon-Flores R and
Luna-Herrera J: Internalization of mycobacterium tuberculosis by
macropinocytosis in non-phagocytic cells. Microb Pathog. 35:49–55.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suh N, Honda T, Finlay HJ, Barchowsky A,
Williams C, Benoit NE, Xie QW, Nathan C, Gribble GW and Sporn MB:
Novel triterpenoids suppress inducible nitric oxide synthase (iNOS)
and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer
Res. 58:717–723. 1998.PubMed/NCBI
|
|
33
|
Ryu SY, Oak MH, Yoon SK, Cho DI, Yoo GS,
Kim TS and Kim KM: Anti-allergic and anti-inflammatory triterpenes
from the herb of Prunella vulgaris. Planta Med. 66:358–360. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Checker R, Sandur SK, Sharma D, Patwardhan
RS, Jayakumar S, Kohli V, Sethi G, Aggarwal BB and Sainis KB:
Potent anti-inflammatory activity of ursolic acid, a triterpenoid
antioxidant, is mediated through suppression of NF-κB, AP-1 and
NF-AT. PLoS One. 7:e313182012. View Article : Google Scholar
|
|
35
|
Moita E, Gil-Izquierdo A, Sousa C,
Ferreres F, Silva LR, Valentão P, Domínguez-Perles R, Baenas N and
Andrade PB: Integrated analysis of COX-2 and iNOS derived
inflammatory mediators in LPS-stimulated RAW macrophages
pre-exposed to Echium plantagineum L. bee pollen extract. PLoS One.
8:e591312013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JB, Han AR, Park EY, Kim JY, Cho W,
Lee J, Seo EK and Lee KT: Inhibition of LPS-induced iNOS, COX-2 and
cytokines expression by poncirin through the NF-kappaB inactivation
in RAW 264.7 macrophage cells. Biol Pharm Bull. 30:2345–2351. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weinberg JB: Nitric oxide synthase 2 and
cyclooxygenase 2 interactions in inflammation. Immunol Res.
22:319–341. 2000. View Article : Google Scholar
|
|
38
|
Camuesco D, Comalada M, Rodríguez-Cabezas
ME, Nieto A, Lorente MD, Concha A, Zarzuelo A and Gálvez J: The
intestinal anti-inflammatory effect of quercitrin is associated
with an inhibition in iNOS expression. Br J Pharmacol. 143:908–918.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roy S, Sharma S, Sharma M, Aggarwal R and
Bose M: Induction of nitric oxide release from the human alveolar
epithelial cell line A549: An in vitro correlate of innate immune
response to mycobacterium tuberculosis. Immunology. 112:471–480.
2004. View Article : Google Scholar : PubMed/NCBI
|