Introduction
Breast cancer is one of most common causes of
cancer-associated mortality worldwide (1). In general, the development stage of
breast cancer includes the benign proliferation, precancer and
cancer like other types of cancer (1). During the early stages of breast
cancer, intervention of the progression of the precancer can
eradicate the cancer. Once organisms have entered into the cancer
stages, breast cancer has a poor survival rate (3). Thus, accurate and effective specific
biomarkers for breast cancer are required for monitoring the
progression of breast cancer. At present, the commonly used
therapeutic strategies for breast cancer are surgical resection,
chemotherapy and radiotherapy (3).
However, these treatments have a low long-term survival rate
(1). The pathogenesis of cancer is
complex, involving genetic, epigenetic and environmental factors
(4,5). Thus, improvement in the understanding
of the developmental mechanisms would aid in the diagnosis of
patients and the development of effective treatment strategies.
Studies investigating the epigenetics of cancer may provide a
strategy for the identification of diagnostic biomarkers and for
novel therapeutic strategies.
In cancer cells, the genomes are often unstable,
exhibiting a duplication and/or deletion of genes and deviant
epigenetic alterations (5).
Previously, epigenetic alterations including mRNA silencing, genome
methylation and histone modification during the progression of
cancer have been thoroughly investigated (5,6).
Genome hypomethylation influences cancer progression by affecting
transcription and transposable elements (TEs) (7). Several previous studies have
indicated that the methylation levels of oncogenes and tumor
suppressor genes are associated with abnormal expression of these
genes, and result in increased cancer progression (5–9). In
addition, the unstable TEs in genome transfer in the chromosomes
have been reported to lead to deleterious mutations and aberrant
expression of oncogenes and tumor suppressor genes (4,10).
These active TEs contribute to the progression of cancer. Thus,
epigenetics may potentially be used for diagnosis and monitoring of
prognosis. However, the association between epigenetics and cancer
progression remains unclear.
In previous studies, a broad pathway, the PIWI-piRNA
pathway, was identified in germline cells from mouse testes
(11,12). Subsequent studies demonstrated that
this pathway was highly conserved in different organisms, from
invertebrates to mammals (13–15).
PIWI homologs were initially suggested to be specifically expressed
in germline cells. Low and high expression levels of PIWI have been
reported to result in reproductive disorders in different animals,
including those in flies (14),
fish (15), frogs (16) and mammals (17). Previously, studies have identified
aberrant expression levels of PIWI in multiple types of human
cancer, including ovarian, lung and pancreatic cancer (18,19).
In breast, endometrial and gastrointestinal tract cancer, PIWI
expression has been detected, while in normal tissues, the
expression of PIWI was not observed (20). Thus, it is suggested that PIWI
participates in the progression of cancer. In humans, four
different homologs, including piwi-like RNA-mediated gene silencing
1 (PIWIL1/HIWI), piwi-like RNA-mediated gene silencing 2
(PIWIL2/HILI), piwi-like RNA-mediated gene silencing 3 (PIWIL3) and
piwi-like RNA-mediated gene silencing 4 (PIWIL4/HIWI2) have been
identified (13). The function of
these different homologs remains to be fully elucidated. HIWI has
been regarded as an inducer for tumor growth and mortality
(21). The expression of HILI has
been identified in different types of cancer cells, including those
of breast and cervical cancer (19). PIWIL3 and HIWI2 have been
identified in colon cancer alone (22). Thus, the expression profiles and
its association with cancer progression require further
investigation.
The present study focused upon the expression
profile of PIWI homologs during the progression of breast cancer,
and in association with the patient prognosis. In addition, by
transfection of cells, the effects of overexpression, interference,
overexpression reversal and interference reversal of the key PIWI
homologs were assayed in the breast cancer cell line MCF-7.
Furthermore, the role of HIWI in regulating the expression levels
of TβR and cyclin-dependent kinases (CDKs) was investigated in the
breast cancer cell line MCF-7.
Materials and methods
Materials
The human tissue samples including normal (n=25),
benign (n=19) and malignant (n=8) tissues were obtained from 27
female patients (age, 30–45 years) during tumor resection at the
First Hospital Affiliated to Soochow University (Suzhou, China).
The tissue type was confirmed pathologically. All patients provided
written informed consent and approval was provided by the Ethics
Committee of the First Hospital Affiliated to Soochow University.
Informed consent was obtained from all patients.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The gene expression in tissues was assayed by
RT-qPCR. The total RNAs of the tissue samples were extracted by
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer's instructions. Subsequently, 1
µg RNA was transcribed to the first-strand cDNA using
SuperScript II reverse transcriptase (Invitrogen Life
Technologies). The primers used are presented in Table I. β-actin was used as an internal
control. RT-qPCR was performed with the Applied Biosystems 7500
Real-Time PCR System (Applied Biosystems Life Technologies, Foster
City, CA, USA). The PCR conditions were as follows: 94°C for 2 min
followed by 40 cycles of 94°C for 15 sec and 60°C for 45 sec. A
final extension step was conducted at 72°C for 30 sec. Subsequent
to the amplification, melting curve analysis was conducted to
confirm the specificity of the amplification products using AB 7500
software, version 2.0.6 (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). For each sample, RT-qPCR
reactions were performed in triplicate. The results were analyzed
with the 2−ΔΔCt method (23).
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
5′→3′ |
|---|
| MAEL | F:
CTGATGATAGAACCAGAGTC |
| MAEL | R:
GAATCCAAGTCTTAGAGGGC |
| HIWI | F:
GAAGCAGCCTGTCTTGGTCAGCCAGCCTG |
| HIWI | R:
GAATCAAAGCTCAAACCCCAGTCTC |
| HILI | F:
ATCTATATCTGGCTGCTCCTC |
| HILI | R:
GATGCAAGATGTGTCCTGAC |
| PIWIL3 | F:
GGTGATTTGTATCCTGCCCA |
| PIWIL3 | R:
TGACCATCTCCCACTCCATC |
| HIWI2 | F:
AATGCTCGCTTTGAACTAGAGAC |
| HIWI2 | R:
ATTTTGGGGTAGTCCACATTAAATC |
| TβRI | F:
TGGCGGGGAGAAGAAGTTG |
| TβRI | R:
CTGCTGCTATAAATCCCAGGATG |
| TβRII | F:
AGAAGTCGGATGTGGAAATGGA |
| TβRII | R:
CTGCACCGTTGTTGTCAGTG |
| CDK4 | F:
CAGGACCTAAGGACATATCTGGA |
| CDK4 | R:
CTCGGTACCAGAGTGTAACAACC |
| CDK6 | F:
TGATCAACTAGGAAAAATCTTGGAC |
| CDK6 | R:
GGCAACATCTCTAGGCCAGT |
| CDK8 | F:
ATCAGTCGGGCTGGTGCTG |
| CDK8 | R:
CTTTATCATCCTTCCCATCTTTCC |
| β-actin | F:
CACCATGAAGATCAAGATCATTGC |
| β-actin | R:
GGCCGGACTCATCGTACTCCTGC |
Western blot analysis
The tissues were lysed in radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
on ice and centrifuged at 12,000 × g for 30 min at 4°C to obtain
the protein. The extracted proteins were then separated by 15%
polyacrylamide gels and transferred onto polyvinylidene difluoride
membranes (GE Healthcare Life Sciences, New Orleans, LA, USA).
Subsequent to blocking in milk for 1 h, the membranes were
incubated with the dilution of primary antibodies overnight at 4°C.
The primary antibodies were: Rabbit polyclonal anti-human MAEL
(1:500; Abcam, Cambridge, MA, USA; cat. no. ab106713), rabbit
polyclonal anti-human HIWI (1:500; Abcam; cat. no. ab12337), rabbit
polyclonal anti-human HILI (1:500; Abcam; cat. no. ab181340),
rabbit polyclonal anti-human PIWIL3 (1:500; Abcam; cat. no.
ab93709), rabbit polyclonal anti-human HIWI2 (1:500; Abcam; cat.
no. 180867) and rabbit polyclonal anti-human GAPDH (1:2,000; Abcam;
cat. no. ab9485). The samples were washed with phosphate-buffered
saline (PBS; Beyotime Institute of Biotechnology) three times and
then probed with the goat anti-rabbit horseradish peroxidase
(HRP)-conjugated IgG (H+L) secondary antibody (1:2,000; Thermo
Fisher Scientific, Inc.; cat. no. 31460) for 1 h at room
temperature. The signals were detected using the Bio-Rad ChemiDoc
Touch System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Three
independent experiments were repeated, with each sample protein
normalized to GAPDH.
Cell culture
The MCF-7 cell line was provided by the American
Type Culture Collection (Manassas, VA, USA). These cells were
incubated in Dulbecco's modified Eagle's medium (DMEM; Gibco Life
Technologies, Grand Island, NY, USA), which was supplemented with
10% fetal bovine serum (Invitrogen Life Technologies), at 37°C in
an incubator with 5% CO2. The viability of the cells was
analyzed using trypan blue exclusion (Sangon Biotech Co., Ltd.,
Shanghai, China) subsequent to cell culture for 24 h. For the
detection of cellular morphology, the cells were grown in glass
cover slips for 24 h at 37°C and observed using an inverted
microscope (PM-10AD; Olympus Corporation, Tokyo, Japan).
Cell transfection
The interference and overexpression vectors were
constructed in order to examine the biophysical properties of the
cell line subsequent to knockdown and overexpression of HIWI. The
transfection vector was constructed using a plasmid of pcDNA3.1(t)
(pc3.1) (Invitrogen Life Technologies) and HIWI. The open reading
frame fragment of HIWI was obtained and cloned into pc3.1 between
the BamHI and EcoRI sites in order to construct the
recombinant plasmids. Subsequent to construction of the plasmids,
transfection was conducted using Lipofectamine™ 2000 (Invitrogen
Life Technologies) according to the manufacturer's instructions.
The cells were cultured in DMEM at 37°C in an incubator and
collected at 24 h for the following analysis.
The cells were divided into five groups (5 parallel
treatments for each group), including the control (non-treated
group), HIWI interference group (1 µg HIWI shRNA plasmid
transfection), HIWI overexpression group (1 µg
pcDNA3.1(t)-HIWI plasmid transfection), HIWI interference reversal
group (1 µg HIWI shRNA plasmid transfection for 12 h
followed by 1 µg pcDNA3.1(t)-HIWI plasmid transfection for
12 h) and HIWI overexpression reversal group (1 µg
pcDNA3.1(t)-HIWI plasmid transfection for 12 h followed by 1
µg HIWI shRNA plasmid transfection for 12 h).
Flow cytometry
Flow cytometric analysis was used to investigate the
apoptosis of the cells. All fluorescence signals of labeled cells
were detected using the FACScan flow cytometer (BD Biosciences, San
Jose, CA, USA). Using the Annexin V-Fluorescein Isothiocyanate
(FITC) Apoptosis Detection kit (BD Biosciences), the
phosphatidylserine redistribution in the plasma membranes was
measured according to the manufacturer's instructions.
Statistical analysis
All data are presented as the mean ± standard error.
The statistical analyses were conducted using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). The significant
differences among the groups were determined by one-way analysis of
variance. The Cox's regression analysis based on gene expression
and prognostic outcome was performed. The follow-up information for
187 patients for a period of 100 months was collected. The patients
were divided into two groups: higher HIWI expression group (mRNA
relative expression, >5) and the lower HIWI expression group
(mRNA relative expression, >2 but <4). The result was
calculated by fitting the predictive prognosis model using SPSS. In
addition, expression of HIWI and HILI were analyzed using a
Kruskal-Wallis test, followed by Dunn's multiple comparison tests
with SPSS. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of HIWI and HILI are
increased in malignant breast cancer tissues
In order to investigate the important role of PIWI
in breast cancer, RT-qPCR and western blotting were conducted in
order to analyze the expression of HIWI, HILI, PIWIL3, HIWI2 and
maelstrom spermatogenic transposon silencer (MAEL) in normal breast
tissue (n=25), benign breast tumors (n=19) and malignant breast
cancer tumors (n=8). The results demonstrated that the relative
expression levels of MAEL, HIWI and HILI were significantly higher
in malignant breast cancer tissues compared with normal and benign
breast tissues (Fig. 1A–C).
However, the relative expression levels of PIWIL3 and HIWI2 in
malignant breast tissues were not significantly different to the
normal and benign breast tissues (Fig.
1D and E). Furthermore, the results of western blotting of
these genes were consistent with the results of RT-qPCR (Fig. 1F). The protein expression levels of
MAEL, HIWI and HILI in malignant breast cancer were significantly
higher than those of normal and benign breast tissues, while no
significant differences in PIWIL3 and HIWI2 expression levels were
observed between the three tissue groups.
 | Figure 1Box plots representing the expression
of HIWI pathway genes in normal breast, benign breast cancer and
malignant breast cancer tissues. Reverse transcription-quantitative
polymerase chain reaction analysis of mRNA expression of (A) MAEL,
(B) HIWI, (C) HILI, (D) PIWIL3 and (E) HIWI2. (F) The protein
expression levels of HIWI pathway genes in normal breast, benign
breast cancer and malignant breast cancer tissues. The
Kruskal-Wallis test was performed to compare the gene expression
levels between the two groups. Filled dots indicate the mean of
each group, *P<0.05. HIWI, piwi-like RNA-mediated
gene silencing 1; MAEL, maelstrom spermatogenic transposon
silencer; HILI, piwi-like RNA-mediated gene silencing 2; PIWIL3,
piwi-like RNA-mediated gene silencing 3; HIWI2, piwi-like
RNA-mediated gene silencing 4. |
HIWI is associated with a reduced
prognosis in breast cancer
The follow-up information for 187 patients for a
period of 100 months was collected (Table II). The patients were first
divided into two groups: The higher HIWI expression group (mRNA
relative expression >5) and the lower HIWI expression group
(mRNA relative expression >2). The tumor specific survival rates
with HIWI and HILI are presented in Fig. 2A. For HIWI, a significant
correlation between HIWI and survival rate was identified
(P<0.05). The higher HIWI expression group exhibited
significantly higher survival curves than the lower HIWI expression
group. However, no significant differences were observed for HILI
(Fig. 2B).
 | Table IICharacteristics for the 187 patients
at the beginning of the experiment. |
Table II
Characteristics for the 187 patients
at the beginning of the experiment.
| Characteristic | Expression
| P-value |
|---|
| Low (n =98) | High (n =89) |
|---|
| Age | 47.98±5.62 | 49.52±7.84 | 0.58 |
| Stage I | 32 | 41 | 0.45 |
| Stage II | 48 | 37 | 0.78 |
| Stage III + IV | 18 | 11 | 0.74 |
| Tumor size
(mm3) | 5.42±2.61 | 23.54±1.62 | 0.012 |
Regulation of breast cancer cell
progression by HIWI
In order to detect the effect of HIWI on breast
cancer cell lines, the interference and overexpression HIWI vectors
were constructed and transfected into cells by Lipofectamine™ 2000.
The mRNA expression and protein expression was analyzed by RT-qPCR
and western blotting, respectively.
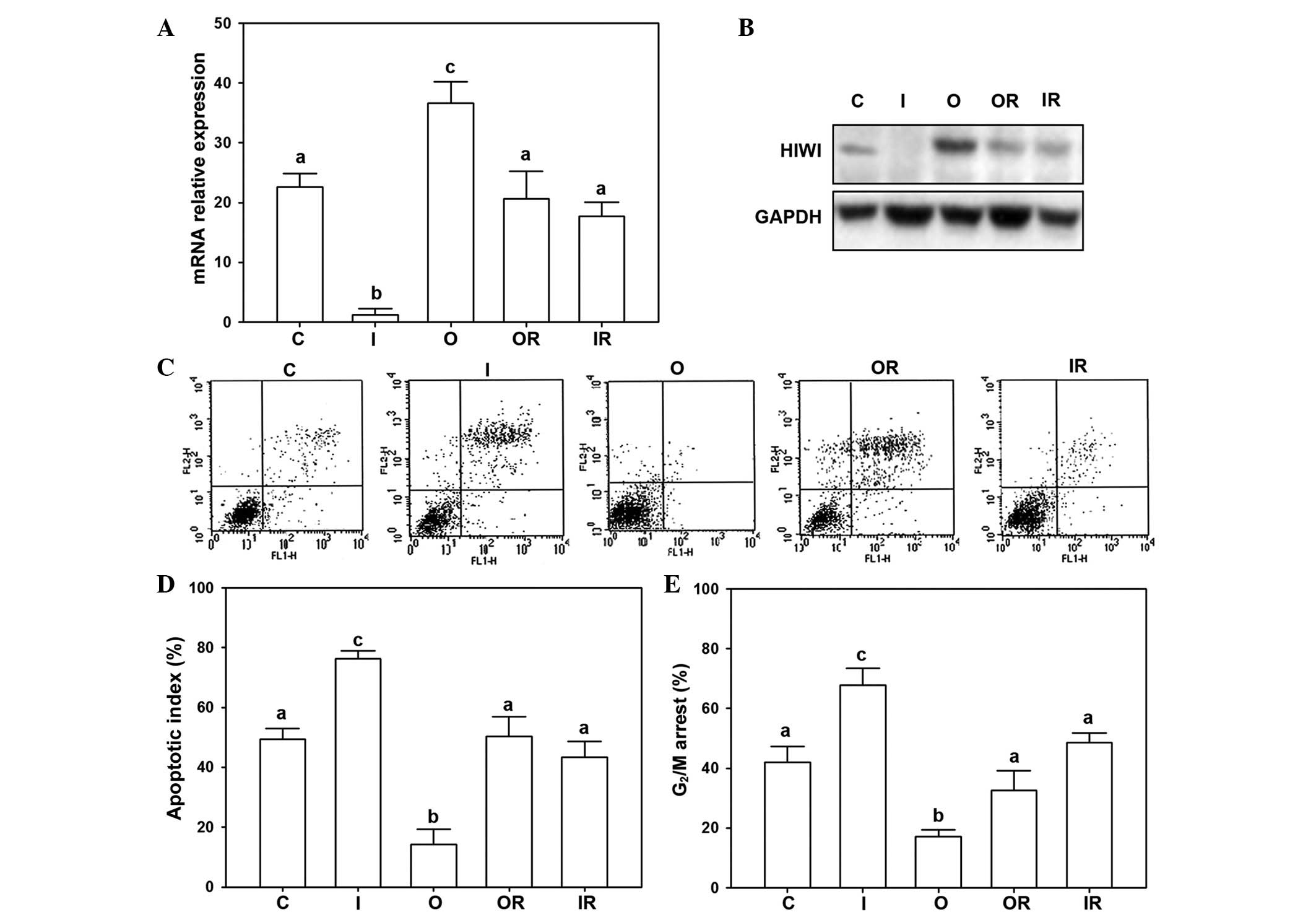
The results indicated that the highest expression of
HIWI was present in the overexpression group and the lowest mRNA
expression was observed in the interference group. While the
control group, overexpression reversal group and interference
reversal group exhibited intermediate expression (Fig. 3A). Accordingly, the protein
expression results were similar to that of the mRNA expression. The
overexpression group had the strongest signals compared with the
other groups. The interference group had the lowest expression of
HIWI protein among all of the groups (Fig. 3B).
To elucidate the effects of HIWI on cellular
regulation, the cell cycle progression and cellular apoptosis were
detected by flow cytometry. The results demonstrated that the
lowest apoptotic rate in was in the overexpression group, whereas
the highest apoptotic rate was in the interference group (Fig. 3C and D). In addition, the
interference group exhibited a significantly greater rate of
G2/M arrest compared with other groups (Fig. 3E).
HIWI regulates TβR and CDK expression
levels in breast cancer cells
In order to demonstrate the mechanism of HIWI
regulation in the TβR and CDK pathway, the mRNA and protein
expression levels of TβR and CDK were assayed. The mRNA and protein
expression of TβRI and TβRII were observed to be downregulated by
overexpression of HIWI, while the other groups had no significant
differences (Fig. 4A, B and F).
However, the mRNA and protein expression levels of CDK4 were
upregulated by overexpression of HIWI (Fig. 4C). The mRNA expression levels of
CDK4 were the lowest in the control, interference and
overexpression reversal groups, with no significant differences
observed (Fig. 4C). While the
expression in the interference reversal group exhibited no
significant differences from the other groups. By contrast, the
levels of CDK6 and CDK8 in the overexpression group exhibited the
highest expression, while no significant differences were observed
in the remaining four groups (Fig. 4D
and E). In addition, CDK4, CDK6 and CDK8 exhibited a
significant increase in the overexpression group, while other
groups exhibited no significant differences (Fig. 4F).
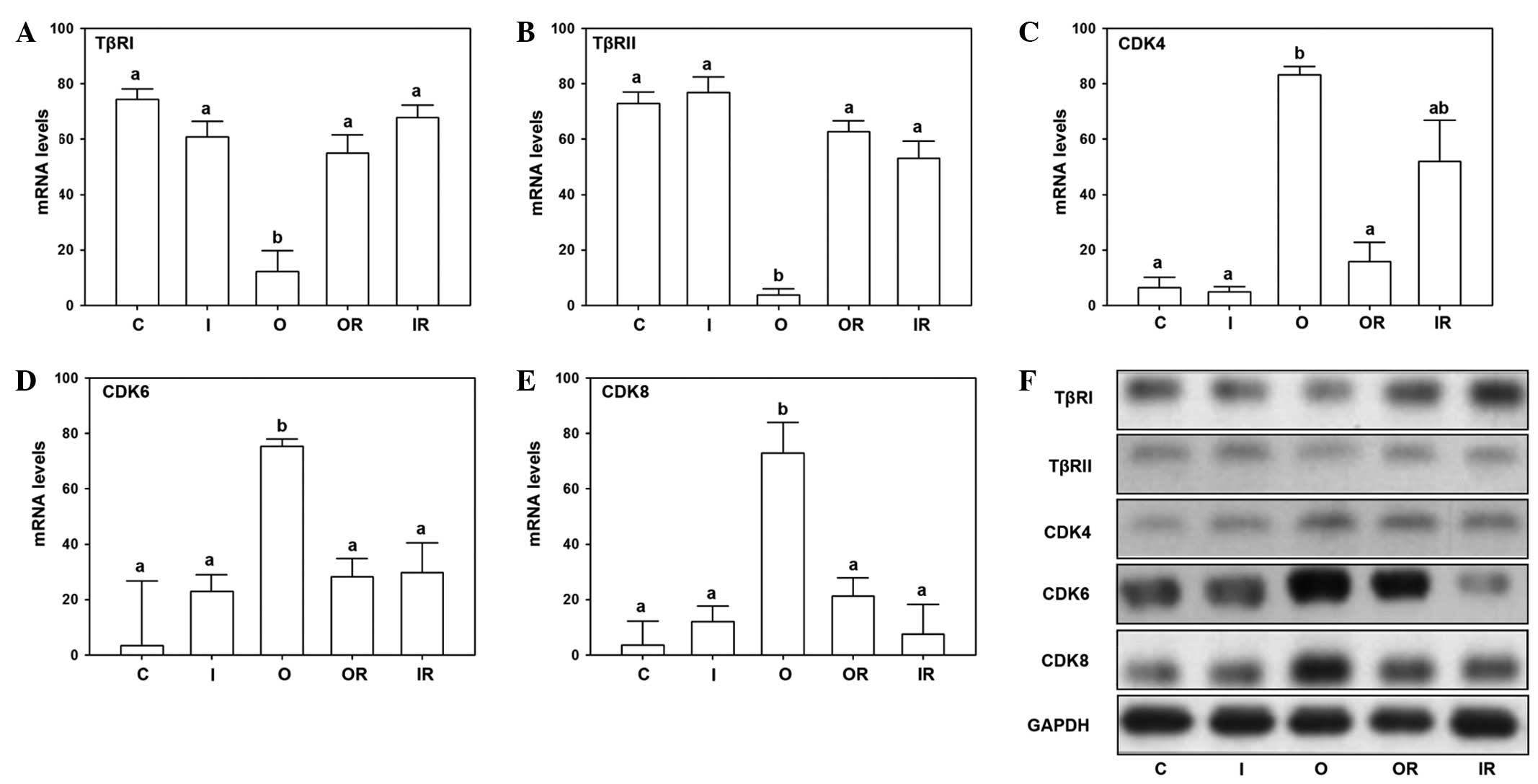 | Figure 4HIWI regulates TβR and CDKs in the
breast cancer cell line. mRNA expression of (A) TβRI, (B) TβRII,
(C) CDK4, (D) CDK6 and (E) CDK8 analyzed by reverse
transcription-quantitative polymerase chain reaction (characters
indicate a significant difference; P<0.05). (F) Protein
expression levels of TβRI, TβRII, CDK4, CDK6 and CDK8 analyzed by
western blotting. HIWI, piwi-like RNA-mediated gene silencing 1;
CDK, cyclin-dependent kinase; TβRI, transforming growth factor-β
type I receptor; C, control; I, interference; O, overexpression;
OR, overexpression reversal; IR, interference reversal. |
Discussion
In the present study, it was demonstrated for the
first time, to the best of our knowledge, that overexpression of
PIWI promotes progression of breast cancer in vivo and in
vitro. By comparing the expression levels of PIWI genes among
normal, benign and malignant tissues, it was demonstrated that HIWI
and HILI exhibited high levels of expression in malignant breast
cancer. The results indicated that these two overexpressed genes
may mediate the progression of breast cancer. Furthermore, it was
identified that high expression levels of HIWI are associated with
an impaired prognosis, while HILI did not exhibit such effects in
breast cancer. Previous studies have demonstrated similar results,
observing high expression levels of PIWI genes in colorectal and
endometrial cancer (13,24,25).
In these types of cancer, the overexpression of PIWI was observed
to be associated with the initiation and progression of cancer. In
addition, PIWI homologs have been observed to exhibit varied
alterations in different types of cancer studied. For example, HILI
is highly expressed in precancerous stem cells during tumorigenesis
(19) while in colon cancer, only
PIWIL3 and HIWI2 have been observed (22,26).
In breast cancer, Liu et al (20) demonstrated that HILI was expressed
in various stages of cancer, and they proposed that HILI may be a
potential biomarker for diagnosis. The results of the current study
additionally suggested that high expression of HILI is present in
malignant breast cancer, however the association of patient
prognosis with HILI was poor. By contrast, high expression of HIWI
was additionally observed in malignant breast cancer, and a
significant correlation between HIWI expression and prognosis was
identified. Thus, based on the observations of the current study,
HIWI is suggested as a more suitable biomarker for breast cancer
than HILI.
Subsequent to identification of the abnormally high
expression of HIWI during the development of breast cancer, the
question of how this factor influences the progression of cancer
was addressed. Thus, transfection was used to detect the effect of
HIWI on progression of breast cancer cells. Subsequent to
interference and overexpression of HIWI in cells, the expression
levels were altered accordingly. The flow cytometry results
suggested that overexpression of HIWI promoted cell activity and
inhibited the apoptosis of cells compared with the control group.
Subsequent to interference of HIWI, the apoptotic rate increased
significantly. Activities of tumor cells, including metastasis and
invasion, are regulated by the cell cycle (27). Previous studies have demonstrated
that HIWI participates in the stability of the genome and regulates
genes expression via binding with piRNAs (7,28).
The PIWI-piRNA pathway was only identified in 2006, thus the
understanding of this pathway in cancer remains limited (29). Although HIWI is aberrantly
expressed in human cancer and its correlation with prognosis has
been reported in previous studies (21,30,31),
the effects of HIWI on the activities of cells remain unclear. In
addition, interference of HIWI may inhibit the progression of
breast cancer cells, which provides a novel potential therapy for
use in cancer treatment.
HILI may inhibit transforming growth factor β
(TGF-β) signaling by regulating Hsp90 and promoting TβR degradation
(32). In the present study, the
results suggested that HIWI promoted cell activities. Thus, the
current study additionally investigated gene regulation by HIWI.
The results suggested that inhibition of HIWI arrested cells in the
G2/M stage. Due to the fact that HIWI may regulate cell
progression of breast cancer via controlling the cell cycle, the
cell cycles were affected by HIWI. The results suggest that TGF-β
signaling and/or CDK genes may be responsible for cell cycle
regulation via HIWI. The results of the current study demonstrated
that overexpression of HIWI reduced the levels of TβRI and TβRII,
which is similar to the result of HILI overexpression. However, it
remains unclear whether this effect is mediated by HILI. In
addition, the expression of HIWI, which is suggested to regulate
TβR degradation, may serve a crucial role in the progression of
cancer. In order to understand the molecular mechanism of HIWI on
the cell cycle, the expression levels of factors associated with
the cell cycle, including CDK4, CDK6 and CDK8 were detected
(33). The results demonstrated
that overexpression of HIWI promotes mRNA in addition to protein
expression levels of these genes. The members of the CDK family
serve an important role in the cell cycle (34). In the CDK family, CDK4, CDK6 and
CDK8 are key genes which control DNA synthesis and the transition
from G1-S stage (33).
Under or overexpression of these genes leads to dysfunction of cell
progression (34). Thus, it is
proposed that the overexpression of HIWI in breast cancer
contributed to the promotion of CDK gene expression and the
induction of cancer progression.
In conclusion, the present study demonstrated that
high expression levels of HIWI are associated with a reduction in
the patient prognosis in breast cancer. Suppression of HIWI may
arrest the cells at the G2/M stage. Furthermore, the
expression levels of TβRI, TβRII, CDK4, CDK6 and CDK8 were
regulated by HIWI in breast cancer cells. These observations
indicate that HIWI participates in the progression of breast cancer
via regulating the gene expression of TβRs and CDKs.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Montagna E, Cancello G, Dellapasqua S,
Munzone E and Colleoni M: Metronomic therapy and breast cancer: A
systematic review. Cancer Treat Rev. 40:942–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Snee M: Follow-up of women treated for
breast cancer. Clin Oncol (R Coll Radiol). 8:85–89. 1996.
View Article : Google Scholar
|
|
4
|
Nowsheen S, Aziz K, Tran PT, Gorgoulis VG,
Yang ES and Georgakilas AG: Epigenetic inactivation of DNA repair
in breast cancer. Cancer Lett. 342:213–222. 2014. View Article : Google Scholar
|
|
5
|
Fucito A, Lucchetti C, Giordano A and
Romano G: Genetic and epigenetic alterations in breast cancer: What
are the perspectives for clinical practice? Int J Biochem Cell
Biol. 40:565–575. 2008. View Article : Google Scholar
|
|
6
|
Sandoval J and Esteller M: Cancer
epigenomics: Beyond genomics. Curr Opin Genet Dev. 22:50–55. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chénais B: Transposable elements and human
cancer: A causal relationship? Biochim Biophys Acta. 1835:28–35.
2013.
|
|
8
|
Hansen KD, Timp W, Bravo HC, Sabunciyan S,
Langmead B, McDonald OG, Wen B, Wu H, Liu Y, Diep D, et al:
Increased methylation variation in epigenetic domains across cancer
types. Nat Genet. 43:768–775. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez-Serra P and Esteller M: DNA
methylation-associated silencing of tumor-suppressor microRNAs in
cancer. Oncogene. 31:1609–1622. 2012. View Article : Google Scholar :
|
|
10
|
Levin HL and Moran JV: Dynamic
interactions between trans-posable elements and their hosts. Nat
Rev Genet. 12:615–627. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Girard A, Sachidanandam R, Hannon GJ and
Carmell MA: A germline specific class of small RNAs binds mammalian
Piwi proteins. Nature. 442:199–202. 2006.PubMed/NCBI
|
|
12
|
Grivna ST, Beyret E, Wang Z and Lin H: A
novel class of small RNAs in mouse spermatogenic cells. Genes Dev.
20:1709–1714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seto AG, Kingston RE and Lau NC: The
coming of age for Piwi proteins. Mol Cell. 26:603–609. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Senti KA and Brennecke J: The piRNA
pathway: A fly's perspective on the guardian of the genome. Trend
Genet. 26:499–509. 2010. View Article : Google Scholar
|
|
15
|
Zhou Y, Zhong H, Liu S, Yu F, Hu J, Zhang
C, Tao M and Liu Y: Elevated expression of Piwi and piRNAs in
ovaries of triploid crucian carp. Mol Cell Endocrinol. 383:1–9.
2014. View Article : Google Scholar
|
|
16
|
Zhang D, Duarte-Guterman P, Langlois VS
and Trudeau VL: Temporal expression and steroidal regulation of
piRNA pathway genes (mael, piwi, vasa) during Silurana (Xenopus)
tropicalis embryogenesis and early larval development. Comp Biochem
Physiol C Toxicol Pharmacol. 152:202–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu M, You Y, Hunsicker P, Hori T, Small C,
Griswold MD and Hecht NB: Mice deficient for a small cluster of
Piwi-interacting RNAs implicate Piwi-interacting RNAs in transposon
control. Biol Reprod. 79:51–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye Y, Yin DT, Chen L, Zhou Q, Shen R, He
G, Yan Q, Tong Z, Issekutz AC, Shapiro CL, et al: Identification of
Piwil2-like (PL2L) proteins that promote tumorigenesis. PLoS One.
5:e134062010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nikpour P, Forouzandeh-Moghaddam M, Ziaee
SA, Dokun OY, Schulz WA and Mowla SJ: Absence of PIWIL2 (HILI)
expression in human bladder cancer cell lines and tissues. Cancer
Epidemiol. 33:271–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JJ, Shen R, Chen L, Ye Y, He G, Hua K,
Jarjoura D, Nakano T, Ramesh GK, Shapiro CL, et al: Piwil2 is
expressed in various stages of breast cancers and has the potential
to be used as a novel biomarker. Int J Clin Exp Pathol. 3:328–337.
2010.
|
|
21
|
Taubert H, Greither T, Kaushal D, Würl P,
Bache M, Bartel F, Kehlen A, Lautenschläger C, Harris L, Kraemer K,
et al: Expression of the stem cell self-renewal gene Hiwi and risk
of tumour-related death in patients with soft-tissue sarcoma.
Oncogene. 26:1098–1100. 2007. View Article : Google Scholar
|
|
22
|
Li L, Yu C, Gao H and Li Y: Argonaute
proteins: Potential biomarkers for human colon cancer. BMC Cancer.
10:382010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Liu W, Jiang X and Zhang Z: Expression of
PSCA, PIWIL1, and TBX2 in endometrial adenocarcinoma. Oncol Res
Treat. 33:241–245. 2010.
|
|
25
|
Oh SJ, Kim SM, Kim YO and Chang HK:
Clinicopathologic implications of PIWIL2 expression in colorectal
cancer. Korean J Pathol. 46:318–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Samowitz WS: Genetic and epigenetic
changes in colon cancer. Exp Mol Pathol. 85:64–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang D, Fang Z, Dong M, Liang C, Xing C,
Zhao J and Yang Y: Effect of RNA interference-related HiWi gene
expression on the proliferation and apoptosis of lung cancer stem
cells. Oncol Lett. 4:146–150. 2012.PubMed/NCBI
|
|
28
|
O'Donnell KA and Boeke JD: Mighty Piwis
defend the germline against genome intruders. Cell. 129:37–44.
2007. View Article : Google Scholar
|
|
29
|
Siddiqi S and Matushansky I: Piwis and
piwi-interacting RNAs in the epigenetics of cancer. J Cell Biochem.
113:373–380. 2012. View Article : Google Scholar
|
|
30
|
Grochola LF, Greither T, Taubert H, Möller
P, Knippschild U, Udelnow A, Henne-Bruns D and Würl P: The stem
cell-associated Hiwi gene in human adenocarcinoma of the pancreas:
Expression and risk of tumour-related death. Br J Cancer.
99:1083–1088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Sun Y, Guo J, Ma H, Li J, Dong B,
Jin G, Zhang J, Wu J, Meng L, et al: Expression of hiwi gene in
human gastric cancer was associated with proliferation of cancer
cells. Int J Cancer. 118:1922–1929. 2006. View Article : Google Scholar
|
|
32
|
Zhang K, Lu Y, Yang P, Li C, Sun H, Tao D,
Liu Y, Zhang S and Ma Y: HILI inhibits TGF-β signaling by
interacting with Hsp90 and promoting TβR degradation. PLoS One.
7:e419732012. View Article : Google Scholar
|
|
33
|
Shah MA and Schwartz GK: Cyclin-dependent
kinases as targets for cancer therapy. Update Cancer Ther.
1:311–332. 2006. View Article : Google Scholar
|
|
34
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: Role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CDKIs). Oncogene. 11:211–219. 1995.
|