Introduction
Lung carcinoma is one of the most common malignant
tumors worldwide and the incidence nearly doubled in Asia over the
past ten years (1). The most
common type of lung carcinoma is non-small cell lung cancer
(NSCLC), which accounts for 80–85% of cases (2). In the past, due to the lack of
effective treatment or early diagnosis, patient postoperative
survival was poor, and tumor metastasis and recurrence determined
the survival condition of patients (3).
MicroRNAs (miRNAs) are small non-coding RNA
molecules, that are ~21–25 nucleotides in length (4). They can be assembled into a
ribonucleoprotein complex and are activated to target specific
mRNA, by binding with the complementary sequence of the targeted
gene. This results in the degradation or translational inhibition
of the targeted mRNA molecule, to reduce the expression of target
genes involved in cell proliferation, differentiation, apoptosis
and death processes (5). Previous
studies have observed that miRNAs are associated with the
occurrence, development and prognosis of cancer, and can be used as
molecular markers and guides in clinical diagnosis and treatment.
miR-21 is one of the most important miR molecules out of >700
types of miRs that have been identified (6). Furthermore, previous studies have
identified increased miR-21 expression levels in various types of
cancer including liver (7), breast
(8) and lung (9) cancer.
Phosphatase and tensin homolog (PTEN) is a newly
identified tumor-suppressor gene. It exists in a variety of healthy
tissues, however it can also be expressed in the tissues of
malignant tumors (10). At
present, it is clear that the association of multiple signaling
pathway factors and PTEN serves an important role in cell growth,
apoptosis and signal transduction, and participates in the
occurrence and development of tumor malignancies (11).
Triptolide is isolated from the Tripterygium
wilfordii plant. Components of Tripterygium wilfordii
include highly activated diterpene lactone epoxide compounds, such
as triptolide (12). It has
numerous pharmacological purposes, including anti-inflammatory,
immunosuppressive and anticancer activities (13). It is clinically used in the
treatment of arthritis and autoimmune disorders, various types of
cancer, organ transplant, kidney disease and asthma (14). In the present study, the antitumor
activity of triptolide and the possible effects on PTEN and miR-21
expression were investigated, as its effect in human NSCLC cells
remains unclear.
Materials and methods
Chemicals
RPMI-1640 medium and fetal bovine serum (FBS) was
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sigma-Aldrich (St. Louis, MO, USA). An annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
assay kit was obtained from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China).
Cell lines and cell growth
The human lung cancer cell line PC-9 was provided by
the Experimental Center of Hebei University (Baoding, China) and
maintained in RPMI-1640 medium with 10% FBS, 100 U/ml penicillin
and 100 mg/ml streptomycin (both from Wuhan Procell Lite Science
& Technology Co., Ltd., Wuhan, China) in 37°C incubator with 5%
carbon dioxide.
Cell viability
The in vitro effects of triptolide on cell
viability of PC-9 cells was determined by the MTT assay. PC-9 cells
were seeded at a density of 1×104 cells/well and plated
onto a 96-well plate. Cells were exposed to different
concentrations of triptolide (0, 10, 25 or 50 nM) for 3 days.
Subsequently, 20 µl 0.5% MTT solution with
phosphate-buffered saline (Wuhan Procell Lite Science &
Technology Co., Ltd.) was added to each well and incubated for 4 h
at 37°C with 5% carbon dioxide. Following the incubation period,
the culture medium was replenished with fresh medium and 200
µl dimethyl sulfoxide (Sigma-Aldrich) were added to each
well and shaken for 20 min at room temperature (PZ300; Wuhan LEHD
Ruihua Instruments Equipment Co., Ltd, Wuhan, China). The optical
density of each well was measured at 492 nm using a Multiskan MS
Plate Reader (LabSystems, Inc.; Thermo Fisher Scientific, Inc.
USA).
Annexin V-FITC/PI apoptosis assay
The in vitro effect of triptolide (T3652;
Sigma-Aldrich) on the cell viability of PC-9 cells was determined
by the annexin V-FITC/PI assay. PC-9 cells were seeded at the
density of 1×106 cells/well and plated onto a 6-well
plate. Cells were exposed to different concentrations of triptolide
(0, 10, 25 or 50 nM) for 2 days. Annexin V-FITC and PI were added
to the cells and were incubated for 10 min at room temperature in
the dark according to the manufacturer's instructions. Cell
apoptosis was examined by flow cytometry (LSRII; BD Biosciences,
San Jose, CA, USA).
Analysis of caspase-3 and 9
activation
PC-9 cells were seeded at a density of
1×106 cells/well and plated onto a 6-well plate. Cells
were exposed to different concentrations of triptolide (0, 10, 25
or 50 nM) for 2 days. PC-9 cells were prepared in cell lysis buffer
(1067–100; Amyjet Scientific Inc., Wuhan, China) for 30 min on ice
and then centrifuged at 12,000 × g for 10 min at 4°C. The
concentrations of the proteins of interest were determined using
the Pierce BCA Protein Assay kit (BD Biosciences). Equal quantities
of protein (30 µg) were mixed with the caspase-9 substrate
Ac-LEHD-pNA (E607115, Sangon Biotech Co., Ltd., Shanghai, China)
and the caspase-3 substrate Ac-DEVD-pNA (E607103-0200, Sangon
Biotech Co., Ltd.)), and incubated at 37°C for 2 h in the dark. The
optical density of each well was measured at 405 nm with a
spectrophotometer.
Reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)
Total RNA in PC-9 cells was extracted with TRIzol
reagent (Thermo Fisher Scientific, Inc.) and miRNAs were
specifically amplified with TaqMan microRNA assays (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Quantification of the miRNAs was performed using SYBR-Green qPCR.
The primers were used as follows: miR-21, F
5′-GGGGGTACCCTTCAGGAAGCTGGTTTC-3′ and R
5′-GGGGATATCTACATGTGAGGCAGGTTCTCAC-3′; U6, F
5′-CGCTTCGGCACATATACTA-3′ and R 5′-CGCTTCACGAATTTGCGTGTCA-3′.
Western blotting
PC-9 cells, treated with 25 nM triptolide or miR-21
plasmids, were prepared in cell lysis buffer for 30 min on ice and
then centrifuged at 12,000 × g for 10 min at 4°C. The protein
concentrations of the samples were determined by the Pierce BCA
protein assay kit according to the manufacturer's instructions.
Equal quantities of protein (30 µg) were loaded and
fractionated by 12% SDS-PAGE gel (Wuhan Procell Lite Science &
Technology Co., Ltd.) at 80 V for 15 min, and transferred to
nitrocellulose membranes (Santa Cruz Biotechnology Inc., Dallas,
TX, USA). Membranes were incubated with PTEN Antibody (F-1) (mouse
monoclonal immunoglobulin G1; cat no., sc-393186;
dilution, 1:1,500; Santa Cruz Biotechnology) and rabbit polyclonal
β-actin (cat no., D110007; dilution, 1:500; Sangon Biotech Co.,
Ltd.) primary antibodies overnight at 4°C. They were then incubated
with the appropriate conjugated to a bovine anti-mouse
immunoglobulin G-horseradish peroxidase-conjugated secondary
antibodies (cat no., sc-2371; dilution, 1:2,000; Santa Cruz
Biotechnology). Membranes were detected using
electrochemiluminescence detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and observed using a luminescent image
analyzer (Image Quant LAS4000 mini, GE Healthcare, Sweden).
Transfection
miR-21 (5′-AACAUCAGUCUGAUAAGCUAUU-3′) and negative
control plasmids (5′-CAGUACUUUUGUGUAGUACAA-3′) were designed and
purchased from Sangon Biotech Co., Ltd.. PC-9 cells were seeded at
a density of 1×106 cells/well and plated onto a 6-well
plate for 24 h. miR-21 (100 ng/ml) and negative control plasmids
(100 ng/ml) were transiently transfected into PC-9 cells using
Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. After 24 h of
transfection, PC-9 cells were used to perform this experiment.
Statistical analysis
Data are presented as the mean ± standard deviation
and analyzed using SPSS software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA). The statistical significance of results were
determined using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of triptolide on cell viability of
PC-9 cells
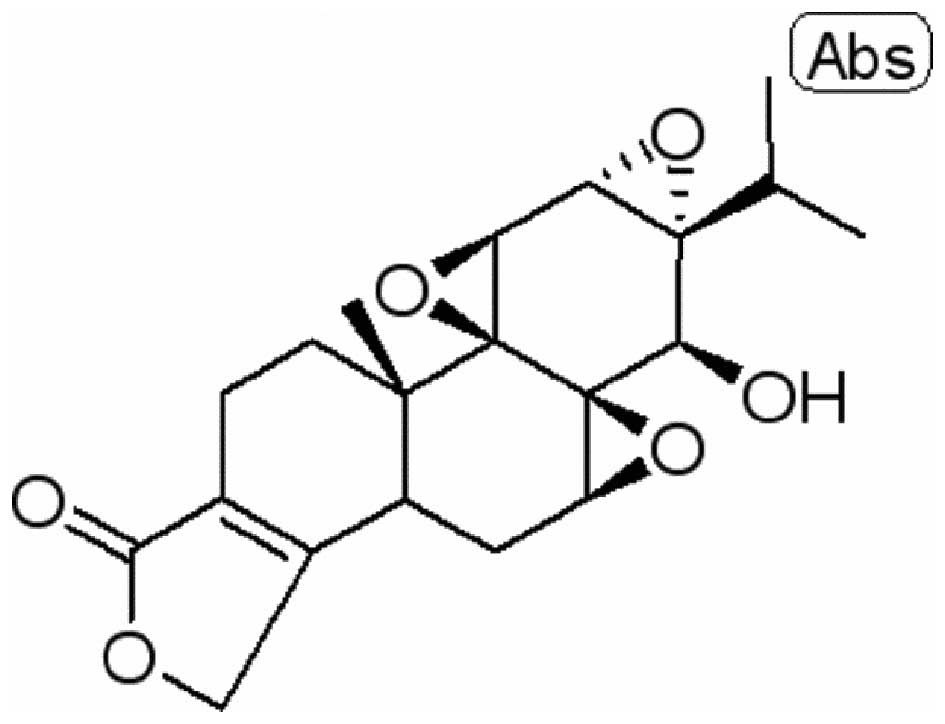
The chemical structure of triptolide (98% purity) is
presented in Fig. 1. For the
experimental purposes of the current study, triptolide was
dissolved in physiological saline. The study investigated the
predicted antitumor effect of triptolide on cell viability using
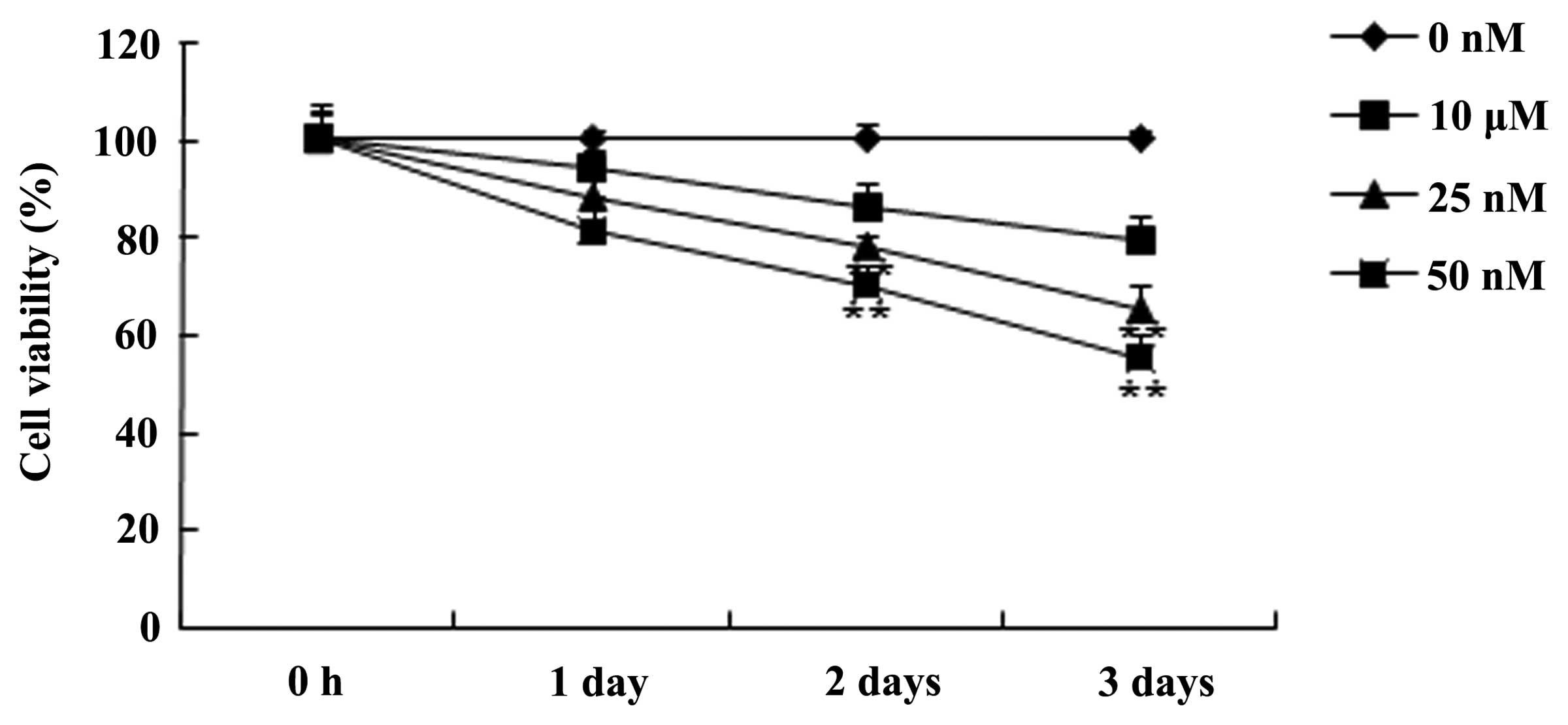
the MTT assay. Fig. 2 demonstrates
that the treatment with triptolide inhibited the cell viability of
PC-9 cells in a time- and dose-dependent manner. The data
additionally suggested that the cell viability of PC-9 cells was
effectively inhibited by 25 or 50 nM of triptolide after 2 or 3
days compared with the 0 nM control group (P<0.01). These data
suggested that triptolide may inhibit human NSCLC cell
viability.
Effect of triptolide on cell apoptosis of
PC-9 cells
To investigate the predicted antitumor effect of
triptolide on cell apoptosis in human NSCLC, an annexin V-FITC/PI
apoptosis assay was conducted. As demonstrated in Fig. 3, following a treatment period of 2
days, triptolide (25 or 50 nM) increased cell apoptosis compared
with the control group (P<0.01). These data suggested that
triptolide may promote human NSCLC cell apoptosis.
Effect of triptolide on caspase-3 and 9
activity in PC-9 cells
To further investigate the predicted antitumor
effect of triptolide on cell apoptosis in human NSCLC, caspase-3
and 9 were measured in PC-9 cells. As demonstrated in Fig. 4, caspase-3 and 9 were effectually
activated by triptolide treatment (25 or 50 nM) compared with the
control group (P<0.01). These data suggested that triptolide may
promote human NSCLC cell apoptosis by activation of the caspase-3
and 9 pathway.
Effect of triptolide on miR-21 expression
in PC-9 cells
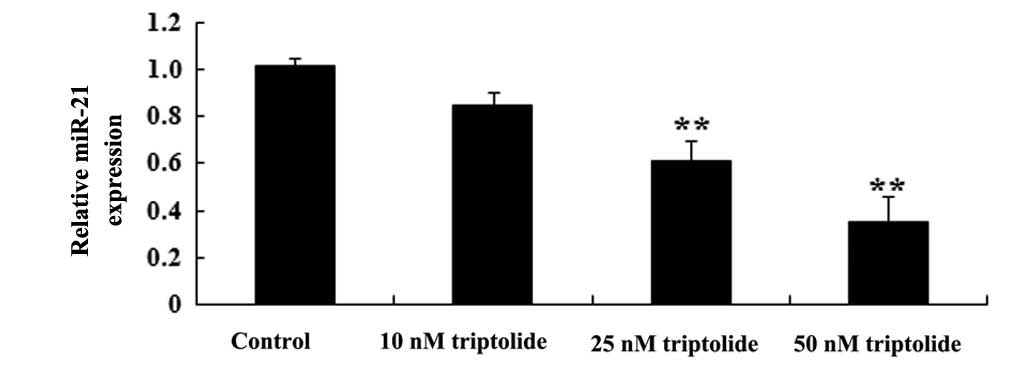
RT-qPCR was utilized to assess the predicted
antitumor effect of triptolide on miR-21 expression in PC-9 cells.
As demonstrated in Fig. 5, the
administration of triptolide (25 or 50 nM) suppressed miR-21
expression in PC-9 cells compared with the control group
(P<0.01). These data suggested triptolide may weaken miR-21
expression in human NSCLC cells.
Effect of triptolide on PTEN protein
expression in PC-9 cells
To identify the predicted antitumor effect of
triptolide on PTEN protein expression in human NSCLC, PTEN protein
expression levels were measured in PC-9 cells using western
blotting. As demonstrated in Fig.
6, pre-treatment with triptolide (25 or 50 nM) enhanced PTEN
protein expression levels in PC-9 cell compared with the control
group (P<0.01). These data suggested that triptolide may
increase PTEN protein expression levels in human NSCLC cells.
Upregulation of miR-21 influences the
effect of triptolide on cell viability of PC-9 cells
In order to further study the effects of triptolide
on cell viability, miR-21 and negative control plasmids were
transfected into PC-9 cells with Lipofectamine 2000 reagent. As
demonstrated in Fig. 7A, RT-qPCR
analysis indicated that miR-21 plasmids promoted miR-21 expression
in the PC-9 cells, following 2-day triptolide (25 nM) treatment,
compared with the triptolide-treated group (P<0.01). The
reduction of cell viability following triptolide treatment
(P<0.01 vs. untreated control) was partially reversed by miR-21
plasmid transfection. The miR-21 plasmid-transfected group
exhibited significantly increased cell viability compared with the
triptolide-treated group (P<0.01; Fig. 7B). These data suggested that
triptolide reduces proliferation in human NSCLC cells via
downregulation of miR-21.
Upregulation of miR-21 influences the
effect of triptolide on PTEN protein expression in PC-9 cells
In order to decipher the effect of triptolide on the
cell viability of PC-9 cells, miR-21 and negative control plasmids
were transfected into PC-9 cells. As demonstrated in Fig. 8, miR-21 plasmid transfection
suppressed PTEN protein expression levels in PC-9 cells, compared
with the 25 nM triptolide-treated group (P<0.01). These data
that suggested triptolide reduces proliferation in human NSCLC
cells via the upregulation of PTEN expression levels.
Discussion
Lung carcinoma is the type of malignant tumor with
the highest rate of mortality worldwide with 1.2 million diagnoses
per year of various types of which 80% are NSCLC (15). At the point of diagnosis, 65–70% of
cases of NSCLC are in the late phase of the disease. Due to this,
surgery is no longer a viable option as treatment, and chemotherapy
becomes the key treatment strategy (16). At present, the most effective drug
for NSCLC is platinum and the three generations of novel
platinum-based drugs introduced from 1990 onwards (17). The chemotherapy efficiency of the
late phase of NSCLC is 20–40%, with the median survival period
being <10 months (18). The
results of the present study indicated that the administration of
triptolide effectively attenuated cell viability, induced cell
apoptosis and activated caspase-3 and 9 in PC-9 cells. Previous
studies have demonstrated that triptolide induced cell apoptosis in
gastric (19), breast (20) and cholangiocarcinoma (21) cancer. The data from those studies
and the present study, suggest a potential antitumor effect of
triptolide on human NSCLC cells. However, this requires further
investigation.
miRs are a newly identified type of non-coding small
RNA, which exist in eukaryotes (22). miR molecules regulate targeted gene
expression through specific-protein mRNA degradation or inhibition
of translation, and are involved in cell growth, differentiation,
apoptosis, and other important cell processes (22,23).
miR-21 is one of the earliest discovered miRNA molecules, and its
gene is located in the 3′UTR of the vacuole membrane protein 1 gene
at chromosome 17q23.1 (24). This
region is usually amplified in neuroblastomas and colon, breast and
lung cancer (24). This is
consistent with miR-21 overexpression in various types of cancer
(25). Yang et al (26) indicated that the downregulation of
miR-21 expression suppresses NSCLC cell proliferation through the
upregulation of programmed cell death. Data from the current study
also suggested that triptolide reduced miR-21 expression in PC-9
cells. Li et al (27)
indicated that triptolide inhibits miR-21 expression levels,
enhances PTEN expression levels and modulates the sensitivity of
K562/A02 cells.
In the field of cancer research, researchers have
identified various PTEN gene mutations and deletions, that lead to
the formation of a defective protein, and abnormal protein
expression (28). Studying the
role of PTEN in tumor malignancies may contribute to an
understanding of cellular signal transduction mechanisms, and open
up a novel direction for genetic therapies (29). Studies have observed that PTEN is
abnormally expressed in various types of cancer (30). Research on PTEN and NSCLC
association have been reported (31) and in the present study, data
demonstrated that triptolide enhanced PTEN protein expression
levels in PC-9 cells compared with the control group. However,
upregulation of miR-21 expression levels suppressed the effect of
triptolide on cell viability and PTEN protein expression levels in
PC-9 cells.
In conclusion, the results of the present study
demonstrated that triptolide reduces the proliferation and enhances
the apoptosis of human NSCLC cells by targeting miR-21 via PTEN.
The current study enhances the understanding of triptolide
treatment and the regulatory mechanisms of miR-21 in cancer
progression.
Acknowledgments
This study was supported by the Hebei University
Special Funds for Medical Science Construction project (grant no.
2015B1001).
References
|
1
|
Pu Z, Yuan X, Zhang X, Chen Q and Xie H:
Meta-analysis on the association between CYP2D6*10 gene
polymorphism and disease free survival of breast cancer patients
receiving tamoxifen treatment in Asia. Bangladesh J Pharmacol.
9:652–662. 2014. View Article : Google Scholar
|
|
2
|
Couto P, Miranda D, Vieira R, Vilhena A,
De Marco L and Bastos-Rodrigues L: Association between CLOCK, PER3
and CCRN4L with non-small cell lung cancer in Brazilian patients.
Mol Med Rep. 10:435–440. 2014.PubMed/NCBI
|
|
3
|
Zhang HM, Yang FQ, Yan Y, Che JP and Zheng
JH: High expression of long non-coding RNA SPRY4-IT1 predicts poor
prognosis of clear cell renal cell carcinoma. Int J Clin Exp
Pathol. 7:5801–5809. 2014.PubMed/NCBI
|
|
4
|
Faltejskova P, Besse A, Sevcikova S,
Kubiczkova L, Svoboda M, Smarda J, Kiss I, Vyzula R and Slaby O:
Clinical correlations of miR-21 expression in colorectal cancer
patients and effects of its inhibition on DLD1 colon cancer cells.
Int J Colorectal Dis. 27:1401–1408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng J, Xue H, Wang T, Jiang Y, Liu B, Li
J, Liu Y, Wang W, Zhang B and Sun M: miR-21 downregulates the tumor
suppressor P12 CDK2AP1 and stimulates cell proliferation and
invasion. J Cell Biochem. 112:872–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu Y, Wang C, Li Y, Zhao J, Chen C, Zhou
Y, Tao Y, Guo M, Qin N, Ren T, et al: MiR-21 controls in situ
expansion of CCR6+ regulatory T cells through PTEN/AKT
pathway in breast cancer. Immunol Cell Biol. 93:753–764. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Yu X, Fu H, Wang H, Wang P, Zheng X
and Wang Y: MicroRNA-21 is involved in ionizing radiation-promoted
liver carcinogenesis. Int J Clin Exp Med. 3:211–222.
2010.PubMed/NCBI
|
|
8
|
Nassar FJ, El Sabban M, Zgheib NK, Tfayli
A, Boulos F, Jabbour M, El Saghir NS, Talhouk R, Bazarbachi A,
Calin GA, et al: miRNA as potential biomarkers of breast cancer in
the Lebanese population and in young women: A pilot study. PLoS
One. 9:e1075662014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Y, Chen X, Tian W, Yin X, Wang J and
Yang H: The role of TGF-β1-miR-21-ROS pathway in bystander
responses induced by irradiated non-small-cell lung cancer cells.
Br J Cancer. 111:772–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi H, Ohno S, Sasaki Y and Matsuura
M: Hereditary breast and ovarian cancer susceptibility genes
(review). Oncol Rep. 30:1019–1029. 2013.PubMed/NCBI
|
|
11
|
Nishimura R, Arima N, Toyoshima S, Ohi Y,
Anan K, Sagara Y, Mitsuyama S and Tamura K: Evaluation of PTEN loss
and PIK3CA mutations and their correlation with efficacy of
trastuzumab treatment in HER2-positive metastatic breast cancer: A
retrospective study (KBC-SG 1001). Mol Clin Oncol. 1:47–52.
2013.PubMed/NCBI
|
|
12
|
Jiang N, Dong XP, Zhang SL, You QY, Jiang
XT and Zhao XG: Triptolide reverses the Taxol resistance of lung
adenocarcinoma by inhibiting the NF-kappaB signaling pathway and
the expression of NF-kappaB-regulated drug-resistant genes. Mol Med
Rep. Nov 2–2015.Epub ahead of print.
|
|
13
|
Zhou H, Guo W, Long C, Wang H, Wang J and
Sun X: Triptolide inhibits proliferation of Epstein-Barr
virus-positive B lymphocytes by down-regulating expression of a
viral protein LMP1. Biochem Biophys Res Commun. 456:815–820. 2015.
View Article : Google Scholar
|
|
14
|
Ho JN, Byun SS, Lee S, Oh JJ, Hong SK, Lee
SE and Yeon JS: Synergistic antitumor effect of triptolide and
cisplatin in cisplatin resistant human bladder cancer cells. J
Urol. 193:1016–1022. 2015. View Article : Google Scholar
|
|
15
|
Kriegshäuser G, Fabjani G, Ziegler B,
Zöchbauer-Müller S, End A and Zeillinger R: Biochip-based detection
of KRAS mutation in non-small cell lung cancer. Int J Mol Sci.
12:8530–8538. 2011. View Article : Google Scholar
|
|
16
|
Shames DS, Girard L, Gao B, Sato M, Lewis
CM, Shivapurkar N, Jiang A, Perou CM, Kim YH, Pollack JR, et al: A
genome-wide screen for promoter methylation in lung cancer
identifies novel methylation markers for multiple malignancies.
PLoS Med. 3:e4862006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bognar CL, Simon SD, Gansl RC, Abramoff R,
Aisen M, Lopes Junior Gde L, Smaletz O, Peres SV and Tabacof J: The
impact of erlotinib use in non-small-cell lung cancer patients
treated in a private reference general hospital and in a private
cancer clinic from 2005 to 2011. Einstein (Sao Paulo). 13:215–220.
2015.In English, Portuguese. View Article : Google Scholar
|
|
18
|
Moravcikova E, Krepela E, Prochazka J,
Benkova K and Pauk N: Differential sensitivity to apoptosome
apparatus activation in non-small cell lung carcinoma and the lung.
Int J Oncol. 44:1443–1454. 2014.PubMed/NCBI
|
|
19
|
Wang BY, Cao J, Chen JW and Liu QY:
Triptolide induces apoptosis of gastric cancer cells via inhibiting
the overexpression of MDM2. Med Oncol. 31:2702014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao H, Ma J, Guo T and Hu R: Triptolide
induces apoptosis of breast cancer cells via a mechanism associated
with the Wnt/β-catenin signaling pathway. Exp Ther Med. 8:505–508.
2014.PubMed/NCBI
|
|
21
|
Ding X, Zhang B, Pei Q, Pan J, Huang S,
Yang Y, Zhu Z, Lv Y and Zou X: Triptolide induces apoptotic cell
death of human cholangiocarcinoma cells through inhibition of
myeloid cell leukemia-1. BMC Cancer. 14:2712014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rothschild SI: microRNA therapies in
cancer. Mol Cell Ther. 2:72014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li JS and Yao ZX: MicroRNAs: novel
regulators of oligodendrocyte differentiation and potential
therapeutic targets in demyelination-related diseases. Mol
Neurobiol. 45:200–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan X, Wang ZX and Wang R: MicroRNA-21: A
novel therapeutic target in human cancer. Cancer Biol Ther.
10:1224–1232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong B, Liu WW, Nie WJ, Li DF, Xie ZJ, Liu
C, Liu YH, Mei P and Li ZJ: MiR-21/RASA1 axis affects malignancy of
colon cancer cells via RAS pathways. World J Gastroenterol.
21:1488–1497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Meng H, Peng Q, Yang X, Gan R,
Zhao L, Chen Z, Lu J and Meng QH: Downregulation of microRNA-21
expression restrains non-small cell lung cancer cell proliferation
and migration through upregulation of programmed cell death 4.
Cancer Gene Ther. 22:23–29. 2015. View Article : Google Scholar
|
|
27
|
Li H, Hui L, Xu W, Shen H, Chen Q, Long L
and Zhu X: Triptolide modulates the sensitivity of K562/A02 cells
to adriamycin by regulating miR-21 expression. Pharm Biol.
50:1233–1240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bunney TD and Katan M: Phosphoinositide
signalling in cancer: Beyond PI3K and PTEN. Nat Rev Cancer.
10:342–352. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian T, Nan KJ, Guo H, Wang WJ, Ruan ZP,
Wang SH, Liang X and Lu CX: PTEN inhibits the migration and
invasion of HepG2 cells by coordinately decreasing MMP expression
via the PI3K/Akt pathway. Oncol Rep. 23:1593–1600. 2010.PubMed/NCBI
|
|
31
|
Poliseno L and Pandolfi PP: PTEN ceRNA
networks in human cancer. Methods. 77–78:41–50. 2015. View Article : Google Scholar
|