Introduction
Neuroblastoma, which are derived from the
sympathetic nervous system, represent the forth most common type of
extracranial malignant solid tumor in children (1). A variety of treatment approaches,
including surgery, immunotherapy, apoptosis-inducing therapy,
myeloablative chemotherapy and radionuclide therapy, are used in
the clinic for inhibiting the rapid growth of neuroblastoma
(2,3). In the spite of the development of
numerous anti-cancer drugs and therapies, the five-year survival
rate remains <75% owing to the high proliferation and migratory
ability of neuroblastoma (4,5).
Innovative therapeutic approaches using migratory inhibitors are
expected to improve patient survival due to enhanced efficacy as
well as reduced drug-associated toxicity.
1H-indole-2,3-dione (isatin) is a promising
heterocyclic drug with numerous beneficial biological activities,
including anti-bacterial, anti-fungal and anti-tumor properties
(6). Derivatives of isatin have
been demonstrated to exert inhibitory effects on tyrosine kinases
and cyclin-dependent kinases (CDKs) as well as anti-angiogenic
effects in tumor cells (7–11). Previous studies by our group
suggested that isatin has marked pro-apoptotic effects on the
SH-SY5Y neuroblastoma cell line in vitro and in vivo
(12,13). The present study investigated the
anti-proliferative and anti-invasive effects of isatin on SH-SY5Y
cells as well as the underlying molecular mechanisms.
Materials and methods
Cells and cell culture
The SH-SY5Y human neuroblastoma cell line was
purchased from Peking Union Medical College (Beijing, China). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin (both
Solarbio Science & Technology Co., Ltd., Beijing, China). The
cells were cultured at 37°C in an humidified atmosphere of 95% air
and 5% CO2. Upon 70% confluency, isatin [in a 5 mM stock
solution in 0.1% dimethly sulfoxide (DMSO); Sigma-Aldrich, St.
Louis, MO, USA] was added with final concentrations of 100, 200 or
400 µM. Following incubation for 48 h, the cells were
harvested and subjected to analysis.
Flow-cytometric analysis
The treated cells were harvested by centrifugation
and washed three times with phosphate-buffered saline. The cells
were fixed with ice-cold 75% ethanol for 18 h, stained with
propidium iodide (Sigma-Aldrich) and then analyzed by flow
cytometry (FACSCanto; BD Biosciences, Franklin Lakes, NJ, USA) to
detect the cell cycle. A minimum of 10,000 events were analyzed in
each experiment, and the results were analyzed using ModFit LT
software, version 3.2 (Verity Software House, Inc., Topsham, ME,
USA).
Invasion assay
The invasive potential of SH-SY5Y cells was examined
using Transwell inserts (Corning Inc., Corning, NY, USA). The
membranes were coated with Matrigel (BD Biosciences) for 30 min.
SH-SY5Y cells were trypsinized (Thermo Fisher Scientific, Inc.),
re-suspended in serum-free medium and counted following serum
starvation for 12 h. The bottom wells of the Transwell inserts were
filled with DMEM containing 10% FBS. Cells (2×105 in 200
µl serum-free medium) were added to the upper compartment of
each Transwell insert and incubated for 24 h in the absence or
presence of isatin (100 or 200 µM). Cells that failed to
migrate through the filter following incubation were removed using
a sterile cotton swab, while invaded cells on the lower side of the
filter were fixed with methanol (Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China) and stained with 0.1% crystal violet
(Solarbio Science & Technology Co., Ltd.). The number of
invaded cells in five random fields of the Transwell membrane was
counted under a microscope (CKX41; Olympus Corporation, Tokyo,
Japan).
Cell survival assay
An MTT assay was conducted in order to assess the
survival of SH-SY5Y cells. Cells (103 cells/well) were
seeded into 96-well plates and isatin was added to a final
concentration of 100 µM-400 µM 24 h later. Following
incubation for 48 h, the cells were incubated with MTT (1 mg/ml;
Sigma-Aldrich) for 3 h at 37°C, after which formazan crystals were
dissolved in 100 µl DMSO. The absorbance was measured at 490
nm using a microplate reader (Synergy H1; BioTek Instruments, Inc.,
Winooski, VT, USA). The suppression rate was calculated using the
following formula: Suppression rate = (1−A/C) × 100%, where A and C
represent the number of cells treated with or without isatin,
respectively. The MTT assay was performed six times.
Monolayer wound healing assay
Cells were seeded into individual wells of a
six-well culture plate and grown to confluence. In order to
suppress the contribution of cell proliferation, cells were grown
in serum-free medium for 12 h; furthermore, cells were treated with
mitomycin (10 µg/ml; Bio Basic Canada, Inc., Markham, ON,
Canada) for 3 h prior to wounding. A sterile 10-µl pipette
tip was then used to perform a longitudinal scratch in the
confluent monolayer. The cell debris and medium were removed by
aspiration and substituted with 2 ml fresh serum-free medium.
Images were captured at 0, 12, 24, 36 and 48 h after wounding
(corresponding to 12, 24, 36, 48 and 60 h post-treatment) using an
inverted microscope (CKX41; Olympus Corporation). Ten randomly
selected points along each wound, which were used to mark the
horizontal distance between the initial wound and the migrated
cells, was measured. All images were processed using Image-Pro Plus
6.0 software (Media Cybernetics, Rockville, MD, USA).
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SH-SY5Y cells cultured
in the presence or absence of isatin using RNAiso Plus (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocol. Total RNA was converted into cDNA using
the Transcriptor First Strand cDNA Synthesis kit (Roche
Diagnostics, Basel, Switzerland), under the following conditions:
50°C for 1 h followed by 85°C for 5 min. cDNA was subjected to qPCR
amplification in triplicate experiments using SYBR Green qPCR
Master Mix (Takara Biotechnology Co., Ltd.) in a Real-Time PCR
System (LightCycler® 96; Roche Diagnostics). The qPCR
amplification conditions were as follows: 95°C for 30 sec, followed
by 45 cycles of 95°C for 5 sec, 60°C for 20 sec and 72°C for 30
sec. Quantification relative to GAPDH was performed using the
2−ΔΔCq method (14).
Real-time PCR was performed using the following primers (Shanghai
Sunny Biotech Co., Ltd., Shanghai, China): GAPDH forward,
5′-AACAGCCTCAAGATCATCAGCAA-3′ and reverse,
5′-GACTGTGGTCATGAGTCCTTCCA-3′; MMP2 forward,
5′-TTCCCTCGCAAGCCCAAGTG-3′ and reverse, 5′-CTCCCAGCGGCCAAAGTTGA-3′;
MMP9 forward, 5′-GCTGACTCGACGGTGATGGG-3′ and reverse,
5′-GCCCCACTTCTTGTCGCTGT-3′.
Protein extraction and western blot
analysis
SH-SY5Y cells were harvested and treated with isatin
for 48 h. The cells were lysed in radioimmunoprecipitation assay
buffer (Solarbio Science & Technology Co., Ltd.) for 20 min on
ice. The homogenate was centrifuged for 5 min at 13,200 × g and the
protein concentration in the supernatant was quantified using the
bicinchoninic acid protein assay (Beyotime Institute of
Biotechnology, Inc., Shanghai, China). Following storage at −80°C,
30 µg protein from each sample was separated by 0.1% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (Sigma-Aldrich)
and electrotransferred onto polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). The membranes were individually
blocked with 5% bovine serum albumin (Amresco, LLC, Solon, OH, USA)
and then incubated with mouse anti-β-actin monoclonal antibody
(1:1,000; TA-09; Zhongshan Golden Bridge Biotechnology, Beijing,
China), rabbit anti-cyclin D1 monoclonal antibody (1:1,000; 2978;
Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
anti-matrix metalloproteinase (MMP)2 polyclonal antibody (1:1,000;
4022; Cell Signaling Technology), rabbit anti-MMP9 monoclonal
antibody (1:3,000; ab76003; Abcam, Cambridge, UK) and rabbit
anti-phosphorylated signal transducer and activator of
transcription 3 (pSTAT3) monoclonal antibody (1:2,000; Tyr705; Cell
Signaling Technology) for 2 h at room temperature, followed by
further incubation at 4°C overnight. The blots were washed three
times for 10 min each in Tris-buffered saline (Sangon Biotech Co.,
Ltd., Shanghai, China) containing 0.1% Tween 20 (TBST; Bio Basic
Canada, Inc.) and then incubated with secondary horseradish
peroxidase-conjugated goat anti-rabbit (1:2,000; BA1054; Boster
Biological Technology, Ltd., Wuhan, China) and goat anti-mouse
(1:5,000; ZB-2305; Zhongshan Golden Bridge Biotechnology)
monoclonal antibodies for 1 h at room temperature. Following three
washes in TBST for 10 min each, proteins were detected using an
Enhanced Chemiluminescence Plus kit (Cyanagen, Bologna, Italy) by a
chemiluminescence system (Fusion FX7; Vilber Lourmat, Collégien,
France). Densitometric analysis was performed with Quantity One
software, version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Each experiment was performed at least three times.
Values are expressed as the mean ± standard deviation. Statistical
analysis included one-way analysis of variance, which was performed
using SPSS software, version 20.0 (IBM SPSS, Armonk, NY, USA). When
the differences between average levels among several groups were
statistically significant, the Bonferroni multiple-comparisons test
was performed. P<0.05 was considered to indicate as
statistically significant difference.
Results
Isatin enhances the G1-phase
population of SH-SY5Y cells
Following administration of isatin for 48 h, cell
cycle analysis was performed by flow cytometry. The results
revealed that isatin significantly increased the proportion of
cells in G1 phase (P<0.01) and significantly
decreased the proportion of cells in S phase (P<0.01), while the
proportion of cells in the G2/M phase was not altered
(Table I). Compared with the 100
and 200 µM groups, treatment with 400 µM isatin
resulted in a significantly higher increase in G1-phase
and decrease in S-phase populations (P<0.01). These results
suggested that isatin significantly caused G1-phase
arrest in SH-SY5Y cells.
 | Table ICell cycle distribution of SH-SY5Y
cells treated with isatin for 48 h as determined by flow
cytometry. |
Table I
Cell cycle distribution of SH-SY5Y
cells treated with isatin for 48 h as determined by flow
cytometry.
| Group | Cell population (%)
|
|---|
| G1
phase | S phase | G2/M
phase |
|---|
| Control | 54.18±0.47 | 36.16±0.64 | 9.66±1.12 |
| 100 µM
isatin | 66.23±0.51a | 28.76±0.91a | 5.01±1.04 |
| 200 µM
isatin | 67.40±0.17a | 28.98±0.68a | 3.62±0.51 |
| 400 µM
isatin | 73.39±2.12a,b,c | 17.68±0.78a,b,c | 8.92±1.71 |
Isatin impedes the expression of cyclin
D1
As cyclin D1 activation regulates the transcription
of genes associated with cell proliferation (15), the present study investigated the
impact of isatin on the expression of cyclin D1. As shown in
Fig. 1, isatin significantly
reduced the protein expression of cyclin D1 compared with that in
the control group (P<0.01). Furthermore, cyclin D1 expresion in
the 400 µM isatin group was significantly decreased compared
with that in the 100 and 200 µM groups (P<0.01).
Isatin inhibits the invasive and
migratory capacity of SH-SY5Y cells
The impact of isatin on the invasion and migration
of neuroblastoma cells was assessed using in vitro Transwell
and wound-healing assays. As illustrated in Fig. 2A and B, 200 µM isatin
significantly restrained the invasiveness of SH-SY5Y cells
(P<0.01). Furthermore 200 µM isatin reduced the migratory
ability of these cells after 36 h (P=0.046) and 48 h of incubation
(P=0.035) (Fig. 2C and D). These
results suggested that isatin reduces the invasion and migration of
the SH-SY5Y human neuroblastoma cell line.
Isatin inhibits the proliferation of
SH-SY5Y cells
The effect of isatin on the proliferation of SH-SY5Y
cells was investigated using an MTT assay. As is shown in Table II, isatin significantly inhibited
the proliferation of SH-SY5Y cells (P<0.01), as compared with
the control. In addition, the OD490 and suppression rate
of SH-SY5Y cells were significantly decreased (P<0.05). Notably,
the 400 µM isatin group resulted in a more significant
decrease, as compared with the 100 µM group (P<0.05).
 | Table IIInhibitory effect of isatin on human
SHSY-5Y cells, as measured by an MTT assay. |
Table II
Inhibitory effect of isatin on human
SHSY-5Y cells, as measured by an MTT assay.
| Group |
OD490 | Suppression rate
(%) |
|---|
| Control | 0.2401±0.0280 | – |
| Isatin | | |
| 100 µM |
0.1976±0.0218a | 14.8±1.7a |
| 200 µM |
0.1832±0.0301a | 18.5±3.1a |
| 400 µM |
0.1796±0.0201a,b | 22.1±2.4a,b |
Isatin reduces the expression of MMP2 and
MMP9
As it is known that MMP-2 and MMP-9 expression is
relevant to metastasis and progression of neuroblastoma (16), the present study assessed the
impact of isatin on MMP2 and MMP9 mRNA and protein expression in
SH-SY5Y cells. As shown in Fig. 3A and
B, 100 µM isatin significantly reduced the mRNA
expression of MMP2 (P=0.010) and MMP9 (P=0.040); however, at this
concentration, isatin did not significantly affect the protein
expression of these MMPs (P=0.520 and P=0.661) (Fig. 3C–E). Of note, at 200 and 400
µM, isatin significantly inhibited the mRNA and protein
expression of MMP2 and MMP9 compared with that in the control
(P<0.01).
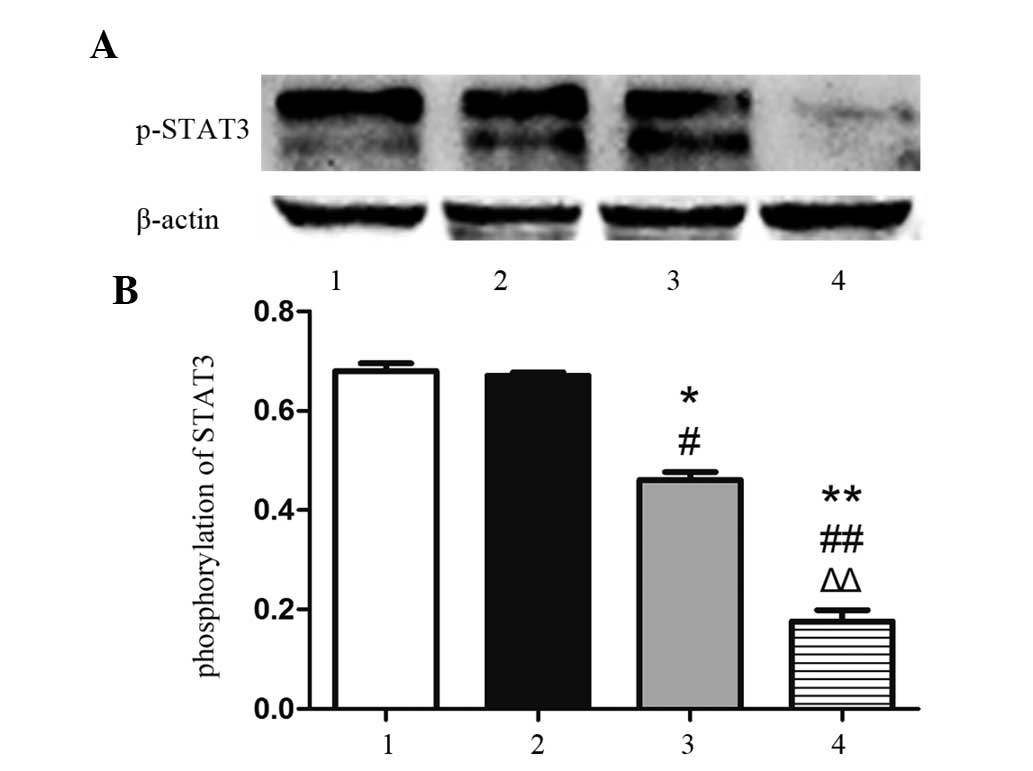
Isatin restrains the phosphorylation of
STAT3
As pSTAT3 has been suggested to be the active form
of STAT3 (17), the present study
determined the protein levels of pSTAT3 (Tyr705) by western blot
analysis. As shown in Fig. 4, the
phosphorylation of STAT3 in the 100-µM group was not
markedly affected, while 200 µM isatin significantly
inhibited the phosphorylation of STAT3 (P<0.05), and 400
µM isatin further diminished the activation of STAT3
(P<0.01).
Discussion
The results of the present study showed that isatin
caused cell-cycle arrest of SH-SY5Y cells in
G0/G1 phase. It is well established that
cyclins regulate various phases of the cell cycle (18,19).
Cyclin D1 is one of the key regulatory proteins for the
G1-S transition of the cell cycle. Overexpression of
cyclin D1 has been found in numerous types of solid tumor,
including neuroblastoma, bladder cancer, prostate cancer and breast
cancer (20–23). The results of the present study
suggested that isatin causes G1-phase arrest and
inhibits the proliferation of SH-SY5Y cells by downregulating
cyclin D1 expression.
The majority of neuroblastoma-associated mortalities
occur due to metastasis to lymph nodes and bones (24,25).
Therefore, inhibiting cancer-cell migration and invasion is crucial
in limiting metastasis (26).
Neuroblastoma-cell invasiveness and metastasis are dependent on the
ability of tumor cells to degrade the extracellular matrix (ECM) to
detach from the primary tumor and enter the bloodstream or
lymphatic system, followed by re-attachment at distant sites
(27). MMPs are an important class
of ECM-degrading enzymes, with gelatinases MMP2 and MMP9 known to
be correlated with metastatic, aggressive or invasive tumor
phenotypes (28–30). Therefore, the inhibition of MMP2
and MMP9 may be a useful strategy to inhibit metastasis formation
and cancer progression in early tumor stages. The results of the
present study showed that isatin inhibited the mRNA and protein
expression of MMP2 and MMP9, which implied that isatin distinctly
impedes the migration and invasion of neuroblastoma cells by
decreasing MMP2 and MMP9.
STAT3 has a major role in tumor formation, as it is
the point of convergence of multiple signaling pathways triggered
by growth factors, cytokines and oncogenes. Considerable evidence
has implicated STAT3 in the regulation of cellular apoptosis, tumor
proliferation, invasion/metastasis and angiogenesis (31–33).
Target genes of STAT3 include several members of the MMP family,
D-type cyclins, vascular endothelial growth factor (VEGF) and
B-cell lymphoma 2 (Bcl-2)/Bcl-2-associated X protein (Bax)
(34–37). The results of the present study
showed that isatin inhibited the expression of MMP family and
D-type cyclins. Previous studies by our group indicated that isatin
regulates Bcl-2/Bax and VEGF expression (12,13).
Hence, isatin may downregulate these genes by restraining the
phosphorylation of STAT3.
The present study confirmed that isatin is an
effective inhibitor of neuroblastoma-cell proliferation and
metastasis. It inhibits the proliferation of SH-SY5Y cells by
reducing the expression of cyclin D1 and impedes cell migration and
invasion by decreasing MMP2 and MMP9. The observed effects of
isatin on tumor-cell migration and proliferation are likely to be
associated with pSTAT3. Isatin is a promising candidate for the
clinical treatment of human neuroblastoma, which should be
evaluated in in vivo studies.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472542).
References
|
1
|
Beierle EA, Ma X, Stewart J, Nyberg C,
Trujillo A, Cance WG and Golubovskaya VM: Inhibition of focal
adhesion kinase decreases tumor growth in human neuroblastoma. Cell
Cycle. 9:1005–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rössler J, Monnet Y, Farace F, Opolon P,
Daudigeos-Dubus E, Bourredjem A, Vassal G and Geoerger B: The
selective VEGFR1-3 inhibitor axitinib (AG-013736) shows antitumor
activity in human neuroblastoma xenografts. Int J Cancer.
128:2748–2758. 2011. View Article : Google Scholar
|
|
3
|
Lee S, Qiao J, Paul P and Chung DH:
Integrin β1 is critical for gastrin-releasing peptide
receptor-mediated neuroblastoma cell migration and invasion.
Surgery. 154:369–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayashi M, Okabe K, Kato K, Okumura M,
Fukui R, Fukushima N and Tsujiuchi T: Differential function of
lysophosphatidic acid receptors in cell proliferation and migration
of neuroblastoma cells. Cancer Lett. 316:91–96. 2012. View Article : Google Scholar
|
|
6
|
Gencer N, Sonmez F, Demir D, Arslan O and
Kucukislamoglu M: Synthesis, structure-activity relationships and
biological activity of new isatin derivatives as tyrosinase
inhibitors. Curr Top Med Chem. 14:1450–1462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bürger S, Yafai Y, Bigl M, Wiedemann P and
Schliebs R: Effect of VEGF and its receptor antagonist SU-5416, an
inhibitor of angiogenesis, on processing of the β-amyloid precursor
protein in primary neuronal cells derived from brain tissue of
Tg2576 mice. Int J Dev Neurosci. 28:597–604. 2010. View Article : Google Scholar
|
|
8
|
Lane ME, Yu B, Rice A, Lipson KE, Liang C,
Sun L, Tang C, McMahon G, Pestell RG and Wadler S: A novel
cdk2-selective inhibitor, SU9516, induces apoptosis in colon
carcinoma cells. Cancer Res. 61:6170–6177. 2001.PubMed/NCBI
|
|
9
|
Uchiyama H, Sowa Y, Wakada M, Yogosawa M,
Nakanishi R, Horinaka M, Shimazaki C, Taniwaki M and Sakai T:
Cyclin-dependent kinase inhibitor SU9516 enhances sensitivity to
methotrexate in human T-cell leukemia Jurkat cells. Cancer Sci.
101:728–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moshinsky DJ, Bellamacina CR, Boisvert DC,
Huang P, Hui T, Jancarik J, Kim SH and Rice AG: SU9516: Biochemical
analysis of cdk inhibition and crystal structure in complex with
cdk2. Biochem Biophys Res Commun. 310:1026–1031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohammadi M, McMahon G, Sun L, Tang C,
Hirth P, Yeh BK, Hubbard SR and Schlessinger J: Structures of the
tyrosine kinase domain of fibroblast growth factor receptor in
complex with inhibitors. Science. 276:955–960. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou L, Ju C, Zhang J, Song J, Ge Y and Yue
W: Antitumor effects of Isatin on human neuroblastoma cell line
(SH-SY5Y) and the related mechanism. Eur J Pharmacol. 589:27–31.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song J, Hou L, Ju C, Zhang J, Ge Y and Yue
W: Isatin inhibits proliferation and induces apoptosis of SH-SY5Y
neuroblastoma cells in vitro and in vivo. Eur J Pharmacol.
702:235–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
Relative Gene Expression Data Using Real-Time Quantitative PCR and
the 2− ΔΔCT Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
15
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu HF, Lai KC, Hsu SC, Lin HJ, Kuo CL,
Liao CL, Yang JS and Chung JG: Involvement of matrix
metalloproteinases on the inhibition of cells invasion and
migration by emodin in human neuroblastoma SH-SY5Y cells. Neurochem
Res. 34:1575–1583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Molinari M: Cell cycle checkpoints and
their inactivation in human cancer. Cell Prolif. 33:261–274. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sankpal UT, Abdelrahim M, Connelly SF, Lee
CM, Madero-Visbal R, Colon J, Smith J, Safe S, Maliakal P and Basha
R: Small molecule tolfenamic acid inhibits PC-3 cell proliferation
and invasion in vitro and tumor growth in orthotopic mouse model
for prostate cancer. Prostate. 72:1648–1658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Comstock CE, Revelo MP, Buncher CR and
Knudsen KE: Impact of differential cyclin D1 expression and
localisation in prostate cancer. Br J Cancer. 96:970–979. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu S, Mott RT, Fry EA, Taneja P, Kulik G,
Sui G and Inoue K: Cooperation between Dmp1 loss and cyclin D1
overexpression in breast cancer. Am J Pathol. 183:1339–1350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaravinos A, Lambrou GI, Volanis D,
Delakas D and Spandidos DA: Spotlight on differentially expressed
genes in urinary bladder cancer. PLoS One. 6:e182552011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsioras K, Papastefanaki F, Politis PK,
Matsas R and Gaitanou M: Functional Interactions between
BM88/Cend1, Ran-binding protein M and Dyrk1B kinase affect cyclin
D1 levels and cell cycle progression/exit in mouse neuroblastoma
cells. PLoS One. 8:e821722013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matthay KK, Villablanca JG, Seeger RC,
Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT,
Brodeur GM, et al: Treatment of high-risk neuroblastoma with
intensive chemotherapy, radiotherapy, autologous bone marrow
transplantation and 13-cis-retinoic acid. Children's cancer group.
N Engl J Med. 341:1165–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Monclair T, Brodeur GM, Ambros PF, Brisse
HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK,
Nuchtern JG, et al: The International neuroblastoma risk group
(INRG) staging system: An INRG task force report. J Clin Oncol.
27:298–303. 2009. View Article : Google Scholar :
|
|
26
|
Steeg PS and Theodorescu D: Metastasis: A
therapeutic target for cancer. Nat Clin Pract Oncol. 5:206–219.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paz H, Pathak N and Yang J: Invading one
step at a time: The role of invadopodia in tumor metastasis.
Oncogene. 33:4193–4202. 2014. View Article : Google Scholar :
|
|
28
|
Hua H, Li M, Luo T, Yin Y and Jiang Y:
Matrix metalloproteinases in tumorigenesis: An evolving paradigm.
Cell Mol Life Sci. 68:3853–3868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaudhary AK, Singh M, Bharti AC, Asotra
K, Sundaram S and Mehrotra R: Genetic polymorphisms of matrix
metalloproteinases and their inhibitors in potentially malignant
and malignant lesions of the head and neck. J Biomed Sci.
17:102010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DeClerck YA, Perez N, Shimada H, Boone TC,
Langley KE and Taylor SM: Inhibition of invasion and metastasis in
cells transfected with an inhibitor of metalloproteinases. Cancer
Res. 52:701–708. 1992.PubMed/NCBI
|
|
31
|
Liu H, Jiang C, Xiong C and Ruan J: DEDC,
a new flavonoid induces apoptosis via a ROS-dependent mechanism in
human neuroblastoma SH-SY5Y cells. Toxicol In Vitro. 26:16–23.
2012. View Article : Google Scholar
|
|
32
|
Kamran MZ, Patil P and Gude RP: Role of
STAT3 in cancer metastasis and translational advances. Biomed Res
Int. 2013:4218212013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Walker SR, Xiang M and Frank DA: Distinct
roles of STAT3 and STAT5 in the pathogenesis and targeted therapy
of breast cancer. Mol Cell Endocrinol. 382:616–621. 2014.
View Article : Google Scholar
|
|
34
|
Overall CM and López-Otín C: Strategies
for MMP inhibition in cancer: Innovations for the post-trial era.
Nat Rev Cancer. 2:657–672. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhuang L, Lee CS, Scolyer RA, McCarthy SW,
Zhang XD, Thompson JF and Hersey P: Mcl-1, Bcl-XL and Stat3
expression are associated with progression of melanoma whereas
Bcl-2, AP-2 and MITF levels decrease during progression of
melanoma. Mod Pathol. 20:416–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang T, Niu G, Kortylewski M, Burdelya L,
Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola
D, et al: Regulation of the innate and adaptive immune responses by
Stat-3 signaling in tumor cells. Nat Med. 10:48–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bollrath J, Phesse TJ, von Burstin VA,
Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T,
Canli O, Schwitalla S, et al: gp130-mediated Stat3 activation in
enterocytes regulates cell survival and cell-cycle progression
during colitis-associated tumorigenesis. Cancer Cell. 15:91–102.
2009. View Article : Google Scholar : PubMed/NCBI
|