Introduction
Hypertension is a common disease that may result in
pathological changes in important organs including the brain, heart
and the kidneys. Hypertension-associated brain damage can induce
cerebral dysfunction, which may manifest as cognitive decline
(1,2). Although it is generally believed that
hypertension-associated vascular events are the main causes of
dementia, many patients experience cognitive decline prior to a
stroke (3). In addition, there is
a delay between the onset of hypertension and cerebrovascular
disease. During this period, a series of pathological alterations
occur in the brain, including oxidative stress and cellular
apoptosis (4). Furthermore,
hypertension is known to enhance the levels of oxidative stress in
the brain and vascular tissues (4). Additionally, increased oxidative
stress can lower the level of neuronal activity and more
importantly, result in the massive entrance of Ca2+ into
neurons, thus activating proapoptotic mechanisms and leading to
neuronal loss (5). However, few
studies have assessed the age-related changes of hypertensive brain
damage in spontaneously hypertensive rats (SHR). Studies
investigating the pathophysiological mechanisms involved in
hypertension-associated cellular apoptosis and oxidative stress
injury may provide new drug targets to prevent the progression of
the pathological changes observed in hypertension.
SHRs are the model most extensively used for the
evaluation of hypertensive brain damage and the potential
treatments. The time-dependent rise in arterial blood pressure and
the occurrence of brain atrophy, loss of nerve cells and glial
reaction share, to an extent, similarities with the process
occurring in human hypertensive brains (6). SHRs, therefore, represent a
reasonable model of hypertension-associated brain damage. The
hippocampus is a brain region involved in learning and memory
(7), and is particularly sensitive
to ischemia and represents a predictive site for assessing brain
damage (8). However, the majority
of investigations on the sensitivity of the hippocampus to
hypertension and/or ischemia have been performed in acute
conditions or following abrupt elevation of blood pressure
(9). Only few studies have
evaluated the influence of chronic hypertension or ischemia on the
structure of the hippocampus (6).
Furthermore, the SHR models used in previous studies were
~6–26-week-old, which is not representative of the advanced
hypertensive period (3,10,11).
Peroxisome proliferator-activated receptor (PPAR)-γ is a
ligand-activated transcription factor belonging to the nuclear
hormone receptor superfamily, and is involved in the oxidative
stress response (12). Oxidative
stress is able to attenuate PPAR-γ expression and activity in
vascular endothelial cells through the suppression of PPAR-γ
transcription (13). In addition,
the PPAR-γ agonist rosiglitazone has been shown to protect QZG
cells against oxidative stress injury through modulating the PPAR-γ
pathway (14). Thus, PPAR-γ may
participate in oxidative stress-related injury.
The present study assessed whether hypertension is
able to induce apoptosis and/or necrosis in the hippocampus of
SHRs, and the associated age-related alterations. In addition, the
oxidative stress pathway was investigated as to whether it was
involved in this process and its potential underlying
mechanisms.
Materials and methods
Animals
16, 32 and 64-week-old male normotensive
Wistar-Kyoto (WKY) rats and SHRs were purchased from Shanghai
Laboratory Animal Center (n=6 per age group; Shanghai,
China). They were housed under humidity (50–60%), temperature
(21–23°C) and light cycle (12 h light/dark) controlled conditions,
with free access to food and water. All procedures were conducted
according to the guidelines established by the Institutional Animal
Care and Use Committee and the National Institutes of Health
(Bethesda, MD, USA). The study was approved by the ethics committee
of The Second Affiliated Hospital or Xi'ab Jiaotong University
(Xi'an, China).
Blood pressure measurements
Systolic blood pressure (SBP) was measured by an
indirect tail-cuff method (BP-2000; Visitech Systems, Inc., Apex,
NC, USA). Briefly, unanesthetized rats were placed in a holding
device mounted on a thermostatically controlled warming plate to
make the pulsations of the tail artery detectable. Tail cuffs were
fixed on the animals, which were allowed to acclimate to the cuffs
for 10 min prior to each pressure recording session.
Brain tissue preparation
Rats were anesthetized with 10% chloral hydrate. For
western blot analysis, rats (n=3) were perfused
transcardially with 0.9% saline (pH 7.4), following which the
brains were rapidly removed and stored in sample protectors (Takara
Biotechnology, Inc., Dalian, China) until use. For
immunohistochemistry and the terminal deoxynucleotidyl
transferase-mediated dUTP end-labeling (TUNEL) assay, rats
(n=3) were perfused intraventricularly with 0.9% saline (pH
7.4) and 4% formaldehyde. The brains were subsequently removed and
postfixed in 4% formaldehyde overnight. Targeted brain pieces were
chosen, dehydrated, embedded in paraffin (Beyotime Institute of
Biotechnology, Haimen, China) and cut into 10-µm-thick
sections. A total of 3 sections containing both the cortex and
hippocampus were selected from each rat for immunohistochemical and
TUNEL analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the hippocampus with
the use of TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and the RNA samples were transcribed to
cDNA using a PrimeScript RT Master Mix kit (Takara Biotechnology,
Inc.) according to the manufacturer's instructions. RT-qPCR was
performed with SYBR ExScript RT-PCR Kit (Takara Biotechnology,
Inc.) on an iQ Multicolor Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The primers used were as
follows: PPAR-γ, forward 5′-GGAGCCTAAGTTTGAGTTTGCTGTG-3′ and
reverse 5′-TGCAGCAGGTTGTCTTGGATG-3′; caspase-3, forward
5′-GAGACAGACAGTGGAACTGACGATG-3′ and reverse
5′-GGCGCAAAGTGACTGGATGA-3′; Bax, forward 5′-GCGTCCACCAAGAAGCTGA-3′
and reverse 5′-ACCACCCTGGTCTTGGATCC-3′; Bcl-2, forward
5′-GACTGAGTACCTGAACCGGCATC-3′ and reverse
5′-CTGAGCAGCGTCTTCAGAGACA-3′; and β-actin, forward
5′-GGAGATTACTGCCCTGGCTCCTA-3′ and reverse
5′-GACTCATCGTACTCCTGCTTGCTG-3′. The primers were designed and
synthesized by Takara Biotechnology, Inc. Amplification was
performed at 95°C for 30 sec, followed by 40 cycles of 95°C for 3
sec and 60°C for 30 sec. Cycle threshold values were obtained from
the Bio-Rad iQ5 2.0 Standard Edition optical System software
(Bio-Rad Laboratories, Inc.). Relative quantification was performed
using the comparative method (2−ΔΔCq) (15) and data was presented as the mean ±
standard deviation of three separate experiments conducted in
triplicate.
Immunohistochemistry
In brief, sections were deparaffinized and
rehydrated according to standard protocols. Subsequently, sections
were treated with 3% H2O2 for 20 min,
followed by incubation with 0.3% Triton X-100 for 30 min and 10%
goat serum for 1 h at room temperature. The slides were then
incubated overnight at 4°C with rabbit polyclonal anti-glial
fabrillary acidic protein (GFAP; 1:2,000; ab16997; Abcam,
Cambridge, UK). Following washing in phosphate-buffered saline
(PBS), the sections were incubated with biotinylated goat
anti-rabbit IgG (1:100; A10547; Invitrogen; Thermo Fisher
Scientific, Inc.). The immuno-complexes were detected using
3,3′-diaminobenzidine. The series of sections obtained from the
different groups were processed in the same way to avoid
alterations in the intensity of the staining occurring as a result
of different incubation conditions. For the negative control, the
primary antibody was replaced with PBS. Activated astrocytes
express high levels of GFAP and have altered morphology (larger
cell bodies and thick branches). Using Image-Pro Plus version 6.0
(Media Cybernetics, Inc., Rockville, MD, USA) to count activated
astrocytes, a threshold was set up for positive cell area, and
within these area limits, each nucleated cell exhibiting GFAP
labeling was counted. Labeled astrocytes were investigated in the
right and left hippocampus in 5–6 sections per animal, using three
animals per group.
Western blot analysis
For western blot analysis, the brain tissues were
homogenized and the total proteins were extracted using
radioimmunoprecipitation assay lysis buffer. The protein
concentrations were determined using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology). A total of 20 µg of
each sample was separated on 12% sodium dodecyl sulfate
polyacrylamide gels, transferred to polyvinylidene fluoride
membranes (both from Beyotime Institute of Biotechnology), and
blocked in 10% non-fat milk at room temperature for 2 h. Membranes
were incubated overnight at 4°C with rabbit polyclonal antibodies
against PPAR-γ (1:400; ab66343; Abcam), rabbit polyclonal
antibodies against gp47phox (bs3261), Bax (bs2538),
Bcl-2 (bs1511), caspase-3 (bs7004; all 1:800; Bio-World, Dublin,
OH, USA) and a mouse monoclonal antibody against inducible nitric
oxide synthase (iNOS; 1:500; sc-7271; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The blots were then washed with
Tris-buffered saline-Tween-20 (0.1%) 3 times, and incubated with
secondary antibodies horseradish peroxidase (HRP)-conjugated goat
anti-rabbit immunoglobulin G (1:5,000; BS13278; Bio-World) and
HRP-conjugated goat anti-mouse immunoglobulin G (1:5,000; BS12478;
Bio-World) for 2 h at room temperature. Following washing, protein
bands were detected with chemiluminescent HRP substrate
(SuperSignal West Pico; Thermo Fisher Scientific, Inc.) for 5 min
at room temperature in the dark and exposed to X-ray film (Fujifilm
Corp, Tokyo, Japan). The band intensities were analyzed using
Quantity One software 4.6.2 (Bio-Rad Laboratories, Inc.) and
normalized to the β-actin loading control.
TUNEL assay
For the TUNEL assay, a cell death detection kit was
used (Promega Corp, Madison, WI, USA). Briefly, the sections were
deparaffinized with xylene and rehydrated according to standard
protocols, and were then incubated with proteinase-K for 20 min at
room temperature followed by 3 washes in PBS. Subsequently, the
sections were covered with equilibration buffer for 10 min at room
temperature, followed by incubation with the rTdT incubation buffer
(50 µl) in the dark for 1 h at 37°C. The slides were then
immersed in 2X SSC buffer for 15 min and washed with PBS. Sections
were counterstained with 4′,6′-diamino-2-phenylindole (DAPI;
1:1,000; Sigma-Aldrich, St. Louis, MO, USA), washed in PBS and
mounted. Fluorescence microscopy was conducted using an Olympus
BX51 microscope equipped with a mercury lamp. TUNEL-positive cells
were normalized to the DAPI stained cells. The immunoreactive cells
from 9 random fields (3 fields per sample, 3 samples per group)
were counted using a 20x objective by an observer blinded to the
treatment groups.
Statistical analysis
The data were presented as the mean ± standard
deviations and analyzed using SPSS software, version 16.0 (SPSS,
Inc., Chicago, IL, USA). A two-way analysis of variance was used to
determine the significant differences between strain and age
followed by a Tukey-Kramer post-hoc test to evaluate the
statistical significance between the hypertensive state and age
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Blood pressure
The SBP levels in the 16, 32 and 64-week-old SHR
groups averaged 127.1±6.9, 184.4±8.1 and 193.9±11.3 mmHg,
respectively. In aged-matched WKY rats, the SBP levels were
significantly lower (102.9±4.7, 103.5±3.6 and 110.0±9.8 mmHg,
respectively; P<0.01). A progressive augmentation of SBP was
also observed in the SHR group with increasing age. Compared with
the 16-week-old group, the 32 and 64-week-old groups showed
significantly upregulated SBP levels (P<0.01). However, there
was no significant difference between the two groups. Systolic
pressure values were similar in the WKY groups of different
ages.
Quantitative analysis of activated
astrocytes
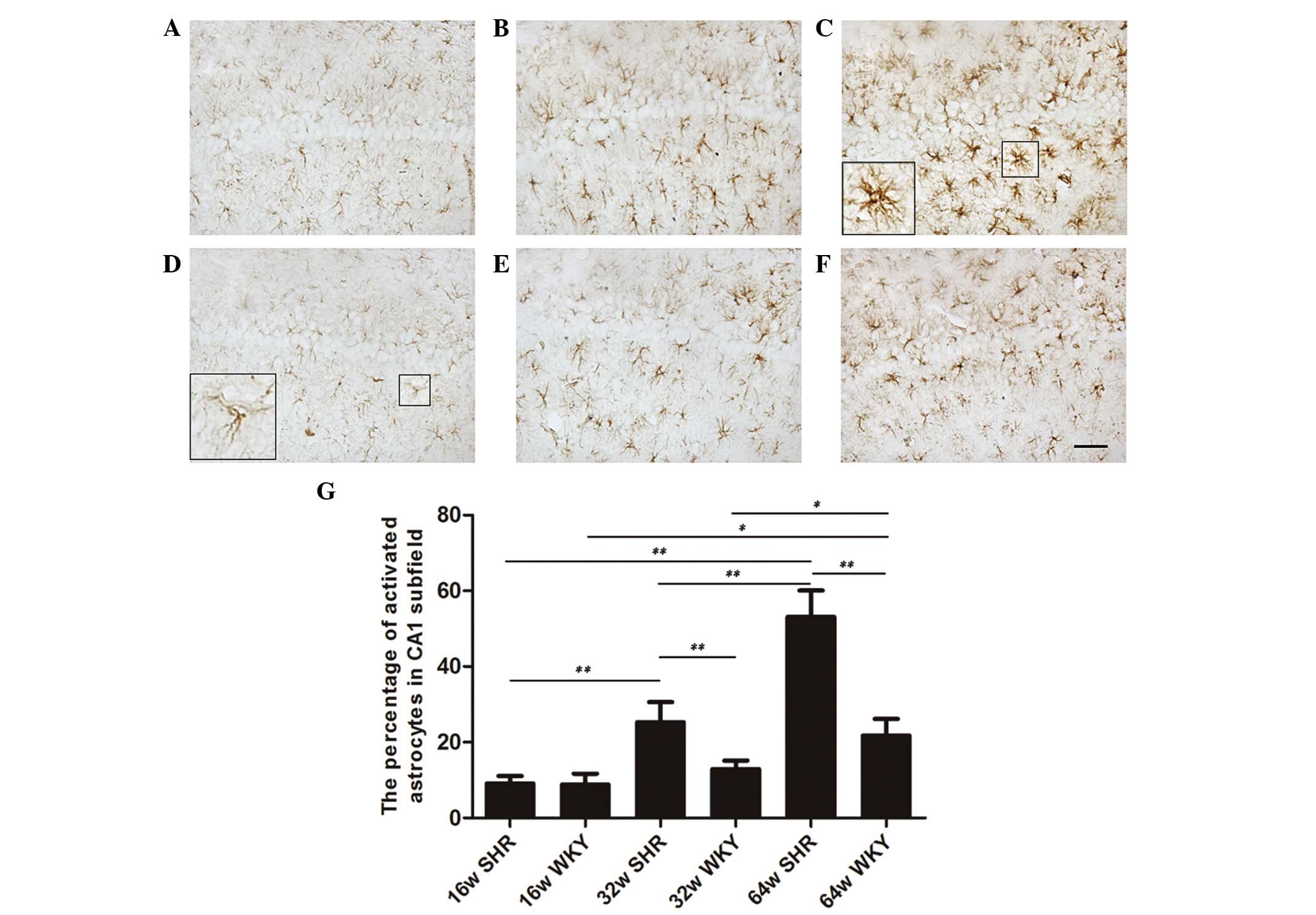
Activated astrocytes are markers of brain damage.
The sections processed for GFAP immunohistochemistry exhibited
thin, shallow, dark-brown astrocytes with slender branches in the
WKY group (Fig. 1D). The SHR group
exhibited increased occurrence of activated astrocytes, with large
cell bodies and thick branches (Fig.
1C). Although the number of astrocytes was similar in the SHR
and WKY groups of different ages, the ratio of activated astrocytes
was increased progressively with age in the CA1 subfield in the SHR
and WKY groups. The WKY group had 8.8, 12.9 and 21.8% activated
astrocytes in the 16, 32 and 64-week-old groups, respectively
(Fig. 1D–F). Compared with the
age-matched WKY group, the SHRs exhibited a significant increase in
the proportion of activated astrocytes (9.1, 25.3 and 53.1%,
respectively; Fig. 1A–C). Apart
from the 16-week-old group, the 32 and 64-week-old SHR groups were
significantly different compared with the age-matched WKY groups
(P<0.01). In addition, there were significant differences
between the three SHR groups (P<0.01). The parameters
investigated were similar in the 16 and 32-week-old WKY rats. The
64-week-old WKY group was significantly different compared with the
16 and 32-week-old WKY rats (P<0.05; Fig. 1G).
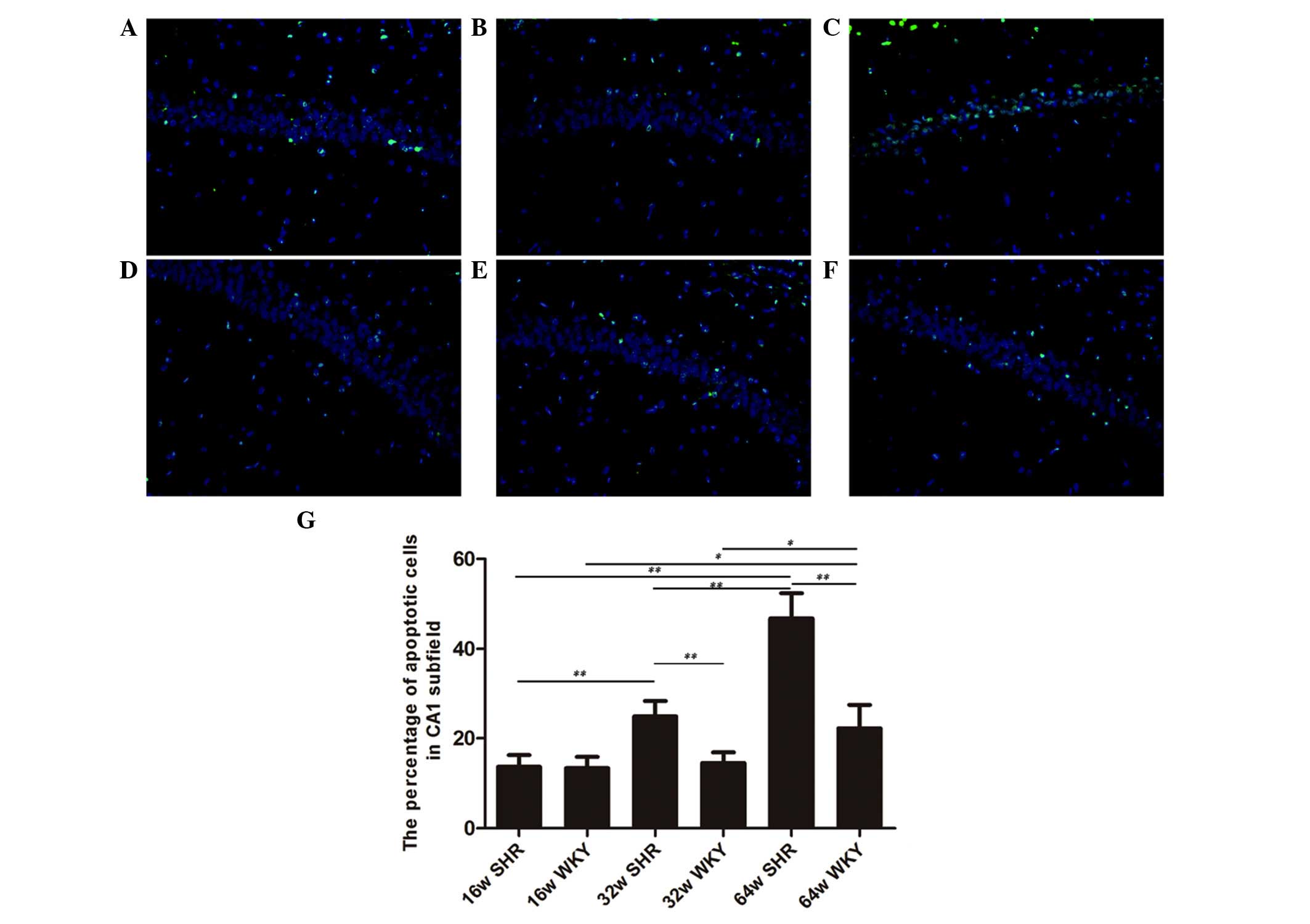
Quantitative analysis of TUNEL-positive
cells
Sections processed for TUNEL staining revealed a few
profiles of TUNEL-positive nuclei in the hippocampus of the 16 week
SHR and WKY rats (13.7 and 13.4%, respectively; Fig. 2A and D). No statistically
significant alteration was observed between the 16 week SHR and WKY
rats. The apoptotic ratio of cells was reduced in the SHR group in
comparison with the age-matched WKY rats. In the hippocampus of the
32-week-old SHRs, 24.9% of nuclei were TUNEL-positive (Fig. 2B). This number was reduced to 14.5%
in the 32-week-old WKY group (Fig.
2E). A total of 46.8% TUNEL-positive nuclei were observed in
the CA1 subfield of the 64-week-old SHRs (Fig. 2C), while the 64-week-old WKY group
exhibited 22.2% TUNEL-positive nuclei (Fig. 2F). Apart from the 16-week-old
group, the 32 and 64-week-old SHR groups were significantly
different compared with the age-matched WKY groups (P<0.01). In
addition, there were significant differences between the three SHR
groups (P<0.01; Fig. 2G).
RT-qPCR assay
The expression of PPAR-γ mRNA in the SHR group
reduced progressively with increasing age (P<0.01, compared
within SHR groups). Compared with the age-matched WKY group, the 32
and 64-week-old SHR groups demonstrated a significantly reduced
level of PPAR-γ mRNA expression (P<0.01). In addition, aging
resulted in an age-dependent reduction of PPAR-γ mRNA expression
levels in the WKY group. The 64-week-old WKY group were observed to
be significantly different compared with the 16-week-old WKY group
(P<0.05; Fig. 3A).
The expression levels of Bax and caspase-3 mRNA
increased progressively with increasing age (P<0.01, compared
within SHR groups). The 32 and 64-week-old SHR groups were
significantly different compared with the age-matched WKY groups
(P<0.01; Fig. 3B and C).
Compared with the 16-week-old group, the 64-week-old group
exhibited a significant difference in caspase-3 mRNA expression
levels (P<0.01; Fig. 3B). Aging
resulted in an age-dependent reduction of Bcl-2 mRNA expression
levels in the SHR group (P<0.01, 16 vs. 32 and 64 weeks).
Compared with the age-matched WKY groups, the Bcl-2 mRNA expression
levels were observed to be significantly different in the 32 and
64-week-old SHR groups (P<0.01; Fig. 3D).
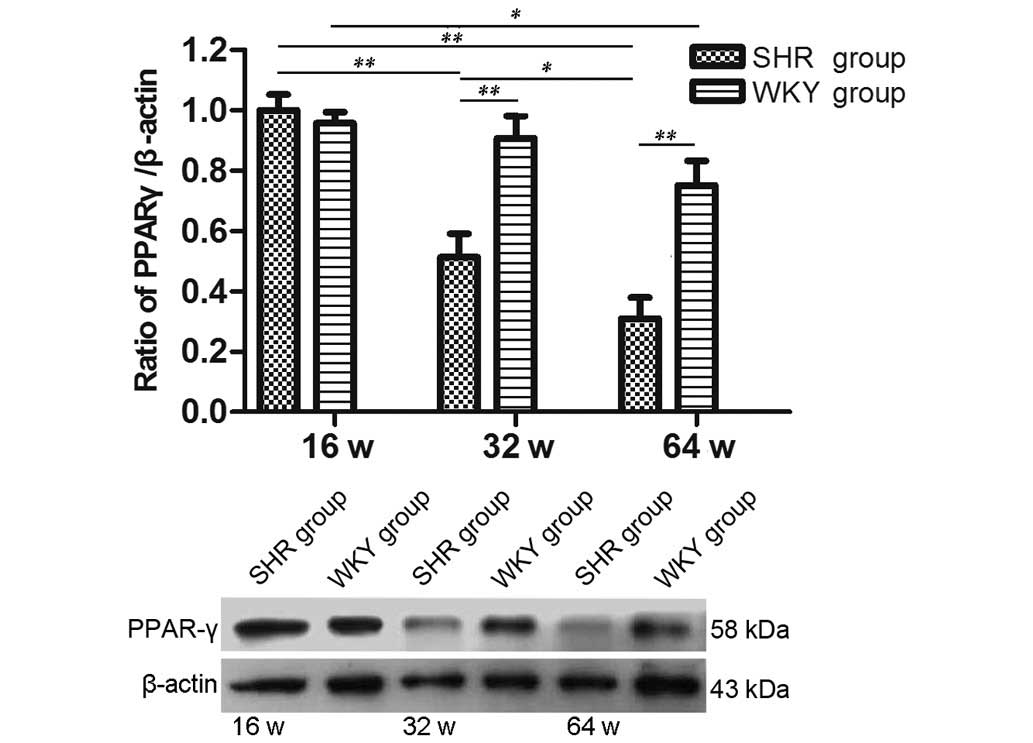
Western blot analysis
As shown in Fig. 4,
the protein expression levels of PPAR-γ were progressively reduced
with increasing age in the SHR groups (P<0.01, 16 vs. 32 week;
P<0.05, 32 vs. 64 week). Apart from the 16-week-old group, the
32 and 64-week-old SHR groups showed a significant difference
compared with the age-matched WKY groups (P<0.01). In addition,
aging resulted in an age-dependent reduction of PPAR-γ protein
expression levels in the WKY groups. However, only the 64-week-old
WKY group was significantly different compared with the 16-week-old
WKY group (P<0.05).
iNOS and gp47phox are markers used for
assessing oxidative stress in brain tissues. As shown in Fig. 5, aging resulted in a significant
upregulation of the protein expression levels of iNOS (P<0.01,
16 vs. 32 week; P<0.05, 32 vs. 64 week) and gp47phox
(P<0.01, compared within SHR groups) in the SHR groups. Compared
with the age-matched WKY group, the 32 and 64-week-old SHR groups
exhibited significant differences in iNOS (P<0.05 and P<0.01,
respectively) and gp47phox (P<0.01) expression
levels. In addition, the 64-week-old WKY group showed significantly
increased expression of gp47phox and iNOS protein
compared with the 16-week-old WKY group (P<0.05).
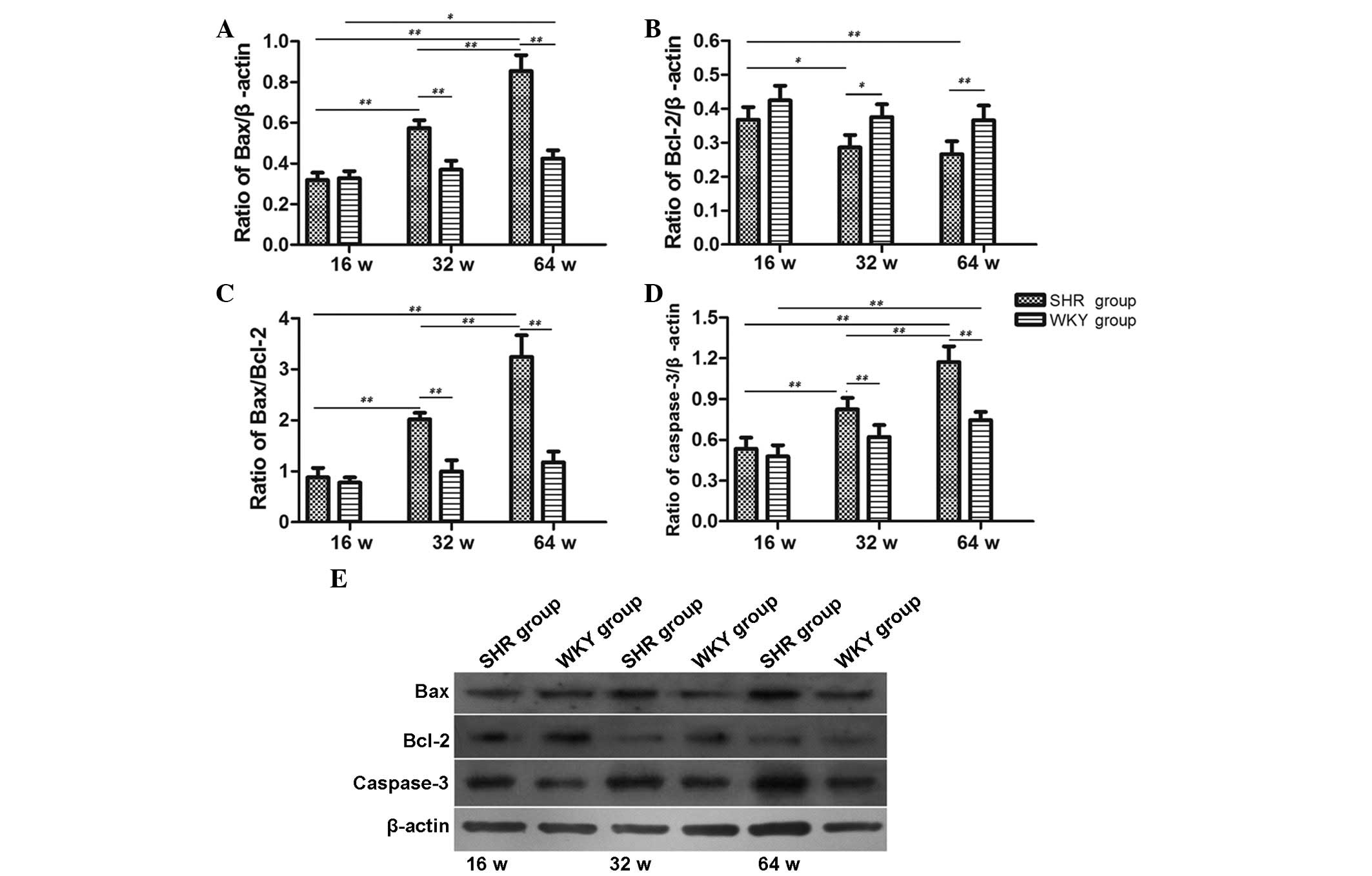
The expression of Bax, Bcl-2 and
caspase-3
As shown in Fig. 6,
the protein expression levels of Bax, caspase-3 and the ratio of
Bax/Bcl-2 were progressively increased with increasing age in SHR
group (P<0.01). Apart from the 16-week-old group, the levels in
the 32 and 64-week-old SHR groups were all significantly different
compared with the age-matched WKY groups (P<0.01). In addition,
the expression levels of Bax and caspase-3 protein in the
64-week-old WKY group were significantly increased compared with
the 16-week-old WKY group (P<0.05, P<0.01, respectively).
However, the expression of Bcl-2 protein was progressively reduced
with increasing age in the SHR groups (P<0.05, 16 vs. 32 week;
P<0.01, 16 vs. 64 week), but there was not a significant
difference between the 32 and 64-week-old SHR groups. Compared with
the age-matched WKY group, the 32 and 64-week-old SHR groups
exhibited significantly reduced Bcl-2 protein expression
(P<0.05, P<0.01, respectively).
Discussion
The main findings of the current study was that SHRs
exhibited an age-dependent increase in apoptotic cells in the
hippocampus and that this was accompanied by increased levels of
oxidative stress and reduced expression of PPAR-γ. In addition, the
degree of hypertensive brain damage, as indicated by activated
astrocytes, was increased with increasing age, and the abnormally
elevated Bax/Bcl-2 ratio may be involved in this process. To the
best of our knowledge, the majority of previous studies assessing
age-related oxidative stress levels and damage have been conducted
in young/adult SHR models, ranging from 6–26 weeks of age (3,10,11).
Few studies have examined the degree of age-related brain damage in
the hippocampi of 32 and 64-week-old SHRs. The current study
indicates that both aging and hypertension are able to exacerbate
brain damage in SHRs, which was shown by the increased proportion
of apoptotic cells and the expression of oxidative stress markers.
The reduced expression of PPAR-γ may contribute to the age-related
brain damage observed in SHRs.
SHRs represent a model of chronic hypertension,
sharing several similarities with human hypertension and exhibiting
behavioral and brain morphological alterations when compared with
age-matched normotensive animals (16). Previous studies have observed
significant reductions in the volume of grey matter, and a
reduction in the number of neurons in the CA1 subfield of
hippocampi in 6–7 month old SHRs, indicating that the volume
reduction in grey matter potentially reflects neuronal loss
occurring in certain hippocampal regions in SHRs (17,18).
These previous studies suggest that depressed neuronal activity in
SHRs occurs around 6 months of age (6,19).
In the present study, no significant difference was observed in the
ratio of TUNEL-positive cells between 16-week-old SHR and WKY
groups, while the 32 and 64-week-old SHR groups showed a
significant increase in the proportion of TUNEL-positive cells in
the CA1 subfield of the hippocampus. The number of neurons in a
given cerebral area is associated with the capability of the
nervous system to receive, analyze and store information (20). The observation of increased levels
of apoptotic cells in the hippocampus of SHR and aged WKY rats
suggests that brain function may undergo a progressive
impairment.
The specificity of the TUNEL technique for apoptosis
has been questioned, as it may detect both apoptotic and necrotic
nuclei (21). Although based on
the TUNEL data it is not possible to conclude whether the CA1
neurons were undergoing apoptosis and/or necrosis, the
demonstration of TUNEL-positive nuclei in the current study
suggests the occurrence of cell death in the hippocampi of SHRs
(22). This observation is
consistent with a previous report which showed that excessive
reactive oxygen species (ROS) induced by hypertension damaged
neuronal components and lead to neuronal loss via apoptosis or
necrosis (23). The demonstration
of GFAP-immunoreactive astrocytes in the present study strengthens
the suggestion of neuronal damage in the hippocampus of SHRs. GFAP
is a cell-specific marker which distinguishes astrocytes from other
glial cells. Increased expression of GFAP is considered to be an
indicator of brain injury (24).
The increased number of activated astrocytes in 32 and 64-week-old
SHRs potentially indicates the detrimental effects of hypertension
on the brain, and the attempt of astrocytes to protect the neuronal
microenvironment.
Oxidative stress is suggested to be an important
factor in aging and numerous age-related diseases (25), including essential hypertension
(10). This is based on the
presence of increased production of ROS, reduced NO synthesis and
the reduced bioavailability of antioxidants, which have been
verified in experimental and human hypertension (10,26).
To date, oxidative stress has been demonstrated in patients with
hypertension (27,28) and experimental hypertensive models
(29–32). As the most active organ with the
greatest oxygen consumption and richness of fatty acids, the brain
is more susceptible to oxidative stress injury than other organs,
thus suggesting that oxidative stress may serve an important role
in hypertension-associated brain damage (33). This is supported by evidence that
the activation of cerebral nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase preceded cerebral inflammation and
cellular apoptosis (34).
gp47phox is an important subunit of the NADPH oxidase
complex, and functions in the spatial organization of the various
subunits of the enzyme (11,35).
It has been observed that the enhancement of NADPH oxidase activity
in SHRs was associated with an increase in gp47phox
expression in vessels (11), which
indicates that gp47phox is an important marker for
assessing oxidative stress injury in SHRs. In addition, iNOS has
been considered to be a marker of oxidative stress due to its
ability to generate toxic levels of nitric oxide, which
subsequently lead to cellular apoptosis or necrosis in the brain
(36,37). In the current study, the increase
in the protein expression levels of gp47phox and iNOS
provides additional support for increased ROS production and
oxidative stress in the brains of SHRs and aged WKY rats.
PPAR-γ is a ligand-activated transcription factor
and serves beneficial roles in inhibiting inflammation and tissue
injury (38). Recently, PPAR-γ has
been suggested to be involved in the oxidative stress response.
Oxidative stress can attenuate PPAR-γ expression and activity
through the suppression of PPAR-γ transcription (13). A previous study reported that once
activated, the PPAR-γ agonist was able to protect QZG cells against
oxidative stress injury (14).
Therefore, PPAR-γ may participate in oxidative stress-related
injury and exert beneficial effects. To the best of our knowledge,
a systematic analysis of the expression profile of PPAR-γ protein
in SHRs of different ages has not been conducted. Diep et al
(39) reported that PPAR-γ
expression increases with age during the development of
hypertension. Wu et al (40) observed that the basal protein
expression levels of PPAR-γ in vascular tissues did not differ
between the prehypertensive stage (5 weeks) or the evolving
hypertensive state (13 weeks) of SHRs compared with age-matched WKY
rats, while the protein levels of PPAR-γ were lower in 21-week-old
SHRs compared with age-matched WKY rats (40). In the present study, it was
observed that PPAR-γ expression was reduced with increasing age in
the hippocampi of SHRs. Whilst this conflicts with the previous
reports that PPAR-γ expression is elevated in the brains of SHRs
(41), the difference may due to
the increased age of the SHRs used in the present study. The
current study demonstrated that SHRs exhibit an age-dependent
reduction in PPAR-γ expression in the hippocampus, which may be
involved in the hypertension-associated oxidative stress
injury.
Bax is a member of the Bcl-2 family, and promotes
apoptosis, while Bcl-2 blocks cell death (42). In a previous study, 32-week-old
SHRs exhibited increased mRNA expression of Bax and reduced mRNA
expression of Bcl-2 (43). The
Bax/Bcl-2 ratio is a widely used parameter to determine cell
susceptibility to apoptosis, and in the present study, an
age-dependent increase in the Bax/Bcl-2 ratio was observed in the
32 and 64-week-old SHRs. The increase in oxidative stress markers
and apoptosis regulatory proteins observed in the hippocampi of
SHRs may be, at least in part, responsible for the increased
proportion of apoptotic cells in these rats. However, the present
study also observed that the 64-week-old WKY rats exhibited
significantly increased active astrocytes and TUNEL-positive cells
compared with 16 and 32-week-old WKY rats, which was accompanied by
increased levels of oxidative stress markers and proapoptotic
proteins. We hypothesized that the results reported in the present
study may arise for two reasons: i) aging is an important factor in
inducing cellular apoptosis; and ii) normotensive WKY rats may
develop obesity with as they age. Common mechanisms leading to
oxidative stress may underlie hypertension and obesity (44).
In conclusion, the present study demonstrated that
SHRs and aged WKY rats exhibited increased apoptotic cells in the
hippocampus, with this accompanied by increased levels of oxidative
stress, suggesting the possible role of oxidative stress in
hypertension-associated brain damage. Aging resulted in an
age-dependent increase in oxidative stress and apoptotic cells in
the hippocampi of SHRs. Reduced mRNA and protein expression of
PPAR-γ the hippocampus may be involved in hypertension-associated
oxidative stress injury. Further studies should be undertaken to
further dissect the mechanisms and signaling pathways involved.
Acknowledgments
The authors would like to thank Professor Yi Zhu for
his excellent technical assistance. The current study was supported
by the National Natural Science Foundation of China (grant no.
81070219).
References
|
1
|
Blackwood WS, Maudgal DP, Pickard RG,
Lawrence D and Northfield TC: Cimetidine in duodenal ulcer.
Controlled trial Lancet. 2:174–176. 1976.
|
|
2
|
Kilander L, Nyman H, Boberg M, Hansson L
and Lithell H: Hypertension is related to cognitive impairment: A
20-year follow-up of 999 men. Hypertension. 31:780–786. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sabbatini M, Tomassoni D and Amenta F:
Hypertensive brain damage: Comparative evaluation of protective
effect of treatment with dihydropyridine derivatives in
spontaneously hypertensive rats. Mech Ageing Dev. 122:2085–2105.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harrison DG and Gongora MC: Oxidative
stress and hypertension. Med Clin North Am. 93:621–635. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown RC and Davis TP: Calcium modulation
of adherens and tight junction function: A potential mechanism for
blood-brain barrier disruption after stroke. Stroke. 33:1706–1711.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabbatini M, Strocchi P, Vitaioli L and
Amenta F: The hippocampus in spontaneously hypertensive rats: A
quantitative microanatomical study. Neuroscience. 100:251–258.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geinisman Y, Detoledo-Morrell L, Morrell F
and Heller RE: Hippocampal markers of age-related memory
dysfunction: Behavioral, electrophysiological and morphological
perspectives. Prog Neurobiol. 45:223–252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt-Kastner R, Ophoff BG and Hossmann
KA: Pattern of neuronal vulnerability in the cat hippocampus after
one hour of global cerebral ischemia. Acta Neuropathol. 79:444–455.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barone FC, Price WJ, White RF, Willette RN
and Feuerstein GZ: Genetic hypertension and increased
susceptibility to cerebral ischemia. Neurosci Biobehav Rev.
16:219–233. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Touyz RM: Reactive oxygen species,
vascular oxidative stress and redox signaling in hypertension: What
is the clinical significance? Hypertension. 44:248–252. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amoureux S, Lorgis L, Sicard P, Girard C,
Rochette L and Vergerly C: Vascular BDNF expression and oxidative
stress during aging and the development of chronic hypertension.
Fundam Clin Pharmacol. 26:227–234. 2012. View Article : Google Scholar
|
|
12
|
Fuenzalida K, Quintanilla R, Ramos P,
Piderit D, Fuentealba RA, Martinez G, Inestrosa NC and Bronfman M:
Peroxisome proliferator-activated receptor gamma up-regulates the
Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial
stabilization and protection against oxidative stress and
apoptosis. J Biol Chem. 282:37006–37015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blanquicett C, Kang BY, Ritzenthaler JD,
Jones DP and Hart CM: Oxidative stress modulates PPAR gamma in
vascular endothelial cells. Free Radic Biol Med. 48:1618–1625.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li WL, Liang X, Wang X, Zhang XD, Liu R,
Zhang W, Chen HL, Qin XJ, Bai H and Hai CX: Protective effect of
the peroxisome proliferator-activated receptor (PPAR)-γ, ligand
rosiglitazone on tert-butyl hydroperoxide-induced QZG cell injury.
Exp Toxicol Pathol. 63:527–533. 2011. View Article : Google Scholar
|
|
15
|
Fleige S, Walf V, Huch S, Prgomet C, Sehm
J and Pfaffl MW: Comparison of relative mRNA quantification models
and the impact of RNA integrity in quantitative real-time RT-PCR.
Biotechnol Lett. 28:1601–1613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lehr RP Jr, Browning RA and Myers JH:
Gross morphological brain differences between Wistar-Kyoto and
spontaneously hypertensive rats. Clin Exp Hypertens. 2:123–127.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tajima A, Hans FJ, Livingstone D, Wei L,
Finnegan W, Demaro J and Fenstermacher J: Smaller local brain
volumes and cerebral atrophy in spontaneously hypertensive rats.
Hypertension. 21:105–111. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sabbatini M, Catalani Å, Consoli C,
Marletta N, Tomassoni D and Avola R: The hippocampus in
spontaneously hypertensive rats: An animal model of vascular
dementia? Mech Ageing Dev. 123:547–559. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomassoni D, Avola R, Mignini F, Parnetti
L and Amenta F: Effect of treatment with choline alphoscerate on
hippocampus microanatomy and glial reaction in spontaneously
hypertensive rats. Brain Res. 1120:183–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Napoleone P, Ferrante F, Ghirardi O,
Ramacci MT and Amenta F: Age-dependent nerve cell loss in the brain
of Sprague-Dawley rats: Effect of long term acetyl-L-carnitine
treatment. Arch Gerontol Geriatr. 10:173–185. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Charriaut-Marlangue C and Ben-Ari Y: A
cautionary note on the use of the TUNEL stain to determine
apoptosis. Neuroreport. 7:61–64. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamet P, Richard L, Dam TV, Teiger E,
Orlov SN, Gaboury L, Gossard F and Tremblay J: Apoptosis in target
organs of hypertension. Hypertension. 26:642–648. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lo EH, Dalkara T and Moskowitz MA:
Mechanisms, challenges and opportunities in stroke. Nat Rev
Neurosci. 4:399–415. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takamiya Y, Kohsaka S, Toya S, Otani M and
Tsukada Y: Immunohistochemical studies on the proliferation of
reactive astrocytes and the expression of cytoskeletal proteins
following brain injury in rats. Brain Res. 466:201–210. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kregel KC and Zhang HJ: An integrated view
of oxidative stress in aging: Basic mechanisms, functional effects
and pathological considerations. Am J Physiol Regul Integr Comp
Physiol. 292:R18–R36. 2007. View Article : Google Scholar
|
|
26
|
Tanito M, Nakamura H, Kwon YW, Teratani A,
Masutani H, Shioji K, Kishimoto C, Ohira A, Horie R and Yodoi J:
Enhanced oxidative stress and impaired thioredoxin expression in
spontaneously hypertensive rats. Antioxid Redox Signal. 6:89–97.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higashi Y, Sasaki S, Nakagawa K, Matsuura
H, Oshima T and Chayama K: Endothelial function and oxidative
stress in renovascular hypertension. N Engl J Med. 346:1954–1962.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou L, Xiang W, Potts J, Floyd M, Sharan
C, Yang H, Ross J, Nyanda AM and Guo Z: Reduction in extracellular
superoxide dismutase activity in African-American patients with
hypertension. Free Radic Biol Med. 41:1384–1391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dobrian AD, Schriver SD, Khraibi AA and
Prewitt RL: Pioglitazone prevents hypertension and reduces
oxidative stress in diet-induced obesity. Hypertension. 43:48–56.
2004. View Article : Google Scholar
|
|
30
|
Nishiyama A, Yao L, Nagai Y, Miyata K,
Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, et
al: Possible contributions of reactive oxygen species and
mitogen-activated protein kinase to renal injury in
aldosterone/salt-induced hypertensive rats. Hypertension.
43:841–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pechanova O, Zicha J, Kojsova S, Dobesová
Z, Jendeková L and Kunes J: Effect of chronic N-acetylcysteine
treatment on the development of spontaneous hypertension. Clin Sci
(Lond). 110:235–242. 2006. View Article : Google Scholar
|
|
32
|
Kushiro T, Fujita H, Hisaki R, Asai T,
Ichiyama I, Kitahara Y, Koike M, Sugiura H, Saito F, Otsuka Y and
Kanmatsuse K: Oxidative stress in the Dahl salt-sensitive
hypertensive rat. Clin Exp Hypertens. 27:9–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Poulet R, Gentile MT, Vecchione C, Distaso
M, Aretini A, Fratta L, Russo G, Echart C, Maffei A, De Simoni MG
and Lembo G: Acute hypertension induces oxidative stress in brain
tissues. J Cereb Blood Flow Metab. 26:253–262. 2006. View Article : Google Scholar
|
|
34
|
Yamamoto E, Tamamaki N, Nakamura T,
Kataoka K, Tokutomi Y, Dong YF, Fukuda M, Matsuba S, Ogawa H and
Kim-Mitsuyama S: Excess salt causes cerebral cellular apoptosis and
inflammation in stroke-prone hypertensive rats through angiotensin
II-induced NADPH oxidase activation. Stroke. 39:3049–3056. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Infanger DW, Sharma RV and Davisson RL:
NADPH oxidases of the brain: Distribution, regulation and function.
Antioxid Redox Signal. 8:1583–1596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang T, Gao L, Shi J, Lu J, Wang Y and
Zhang Y: Angiotensin-(1-7) modulates renin-angiotensin system
associated with reducing oxidative stress and attenuating cellular
apoptosis in the brain of hypertensive rats. Pharmacol Res.
67:84–93. 2013. View Article : Google Scholar
|
|
37
|
Yamagata K, Tagami M and Yamori Y:
Neuronal vulnerability of stroke-prone spontaneously hypertensive
rats to ischemia and its prevention with antioxidants such as
vitamin E. Neuroscience. 170:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Narala VR, Subramani PA, Narasimha VR,
Shaik FB and Panati K: The role of nitrated fatty acids and
peroxisome proliferator-activated receptor gamma in modulating
inflammation. Int Immunopharmacol. 23:283–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Diep QN and Schiffrin EL: Increased
expression of peroxisome proliferator-activated receptor-alpha and
-gamma in blood vessels of spontaneously hypertensive rats.
Hypertension. 38:249–254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu L, Wang R, De Champlain J and Wilson
TW: Beneficial and deleterious effects of rosiglitazone on
hypertension development in spontaneously hypertensive rats. Am J
Hypertens. 17:749–756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun L, Gao YH, Tian DK, Zheng JP, Zhu CY,
Ke Y and Bian K: Inflammation of different tissues in spontaneously
hypertensive rats. Sheng Li Xue Bao. 58:318–323. 2006.PubMed/NCBI
|
|
42
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
43
|
Li Y, Duan Z, Gao D, Huang S, Yuan H and
Niu X: The new role of LOX-1 in hypertension induced cellular
apoptosis. Biochem Biophys Res Commun. 425:735–740. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simao S, Gomes P, Pinto V, Silva E, Amaral
JS, Igreja B, Afonso J, Serrão MP, Pinho MJ and Soares-da-Silva P:
Age-related changes in renal expression of oxidant and antioxidant
enzymes and oxidative stress markers in male SHR and WKY rats. Exp
Gerontol. 46:468–474. 2011. View Article : Google Scholar : PubMed/NCBI
|