Introduction
Inflammatory bowel disease (IBD) is an umbrella term
for a range of diseases, of which ulcerative colitis (UC) and
Crohn's disease (CD) are the two prevailing entities, and is
characterized by chronic inflammation of the colon (1). Patients with IBD have intermittent
disease flare-ups interspersed with periods of remission (2). The highest reported prevalence values
for IBD are in Europe (UC, 505/100,000 persons; CD, 322/100,000
persons) and North America (UC, 249/100,000 persons; CD,
319/100,000 persons) (3). The
life-time risk of colorectal cancer (CRC) in a patient with IBD is
2–4 times greater than the risk of the control population, which is
~5% (4). IBD compromises the
quality of life of patients (5)
and significantly increases the risk of developing CRC compared
with the general population (6).
The current treatment for IBD remains unsatisfactory and utilizes
drugs, such as mesalamine, glucocorticoids, azathioprine and
anti-tumor necrosis factor (TNF-α) agents (including infliximab and
adalimumab). These agents are not universally effective, and their
use is further restricted by the occurrence of side effects,
including bone marrow suppression, pancreatitis, opportunistic
infections and malignancies (7,8).
Therefore, there is a requirement for the identification of novel
immunomodulators for remission induction or maintenance in patients
with IBD.
5-Fluorouracil (5-FU) is a widely used anticancer
agent, whose anti-tumor mechanism remains unclear; however it
appears to interfere with the DNA synthesis and mRNA translation
(9). In addition, 5-FU has been
reported to inhibit protein synthesis and secretion (10). Thus, investigations regarding 5-FU
primarily focus on the potential treatment of various types of
cancer (11). Furthermore, the
role of 5-FU in intestinal physiology, and response to injury and
inflammation in vivo remain poorly defined. Notably, a
previous study suggested that 5-FU may decrease abnormal immune
cytokine responses, and thus relieve pathophysiological disorders,
through its anti-metabolic and immunosuppressive effect in a model
of acute pancreatitis (12). In
the present study, we hypothesize that 5-FU exhibits an
anti-inflammatory effect in colitis.
In order to investigate the possible
anti-inflammatory effect of 5-FU treatment and the mechanisms
underlying it in colitis, a dextran sodium sulfate (DSS)-induced
mouse model of colitis was utilized for experimental purposes.
Materials and methods
Induction of colitis and treatment
Female BALB/c mice, aged 6–8 weeks and weighing 20
g, were purchased from Shanghai SLAC Laboratory Animal Co., Ltd.,
(Shanghai, China) and maintained at 22°C with a 12-h light/dark
cycle and ad libitum access to water and a standard rodent
diet. Mice were randomly divided into four groups: Normal control,
DSS-treated, 5-FU-treated and DSS + 5-FU-treated groups (n=20 per
group). Colitis was induced in the mice with 4% DSS (molecular
weight, 36–50 kDa; MP Biomedicals, LLC, Santa Ana, CA, USA)
dissolved in tap water administered ad libitum (days 1–7).
Control mice were administered tap water ad libitum (days
1–7). 5-FU (Sigma-Aldrich, St. Louis, MO, USA) was administered
intraperitoneally at a dosage of 15 mg/kg body weight once daily
for the first 3 days of DSS treatment. Control mice received
phosphate-buffered saline (PBS; Sangon Biotech Co., Ltd., Shanghai,
China) via intra-peritoneal injection once daily for the first
three days, at the same volume of 5-FU. Half the mice from each
group were sacrificed via intraperitoneal injection of 4%
pentobarbital on day 3 and the other half on day 7. Colon tissue
was excised and fixed in 4% paraformaldehyde (Sangon Biotech Co.,
Ltd.), or frozen in liquid nitrogen and stored at −80°C. The Animal
Welfare Committee of Shanghai East Hospital approved all
experimental procedures.
Clinical assessment
Multiple clinical parameters were measured
throughout the present study, including the percentage of initial
weight, stool consistency and fecal bleeding. These were recorded
and graded for severity on a scale between 0 (less severe) and 3
(most severe), as previously described (13). In order to calculate the disease
activity score, each parameter was then rescored on a scale between
0 and 3. Final scoring was as follows: Percentage of initial weight
(0, >99%; 1, 92–99%; 2, 85–91%; and 3, <85%); stool
consistency (0, normal; 1, slightly soft; 2, loose; and 3, liquid);
bleeding on hemoccult test (0, negative; 1, faint blue; 2, blue; 3,
red) (Sangon Biotech Co., Ltd.). The mean of these parameters
represented the disease activity score.
Gross and histological analysis
Inflammation-induced reduction in colon length was
used as a marker for the severity of acute DSS-induced inflammation
of the colon (14). Half the mice
from each group were sacrificed on day 3 and the other half on day
7. For all mice, the entire colon was removed and measured
following sacrifice. The extracted colon tissue was spread on a
plastic sheet, fixed with 4% paraformaldehyde and embedded in a
paraffin block. Sections of paraffin-embedded tissue
(4-µm-thick) were subjected to hematoxylin and eosin
(H&E; Beyotime Institute of Biotechnology, Shanghai, China)
staining for the evaluation of colitis severity. Histological
sections were scored for severity based on the following parameters
(ranging between 0 and 3): Crypt damage; leucocyte infiltration;
and submucosal edema and haemorrhage. The score was further
multiplied by the extent of involvement (×1, <10%; ×2, 10–25%;
×3, >25%). A value of four was added to the histological score
if there was evidence of transmural involvement (15). The scoring was graded in a blinded
manner by two independent investigators.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the colon tissue of
mice with induced colitis using Tripure Isolation Reagent (Roche
Diagnostics, Basel, Switzerland) and RNA quality was assessed by
the A260/A280 ratio using a BioPhotometer (Eppendorf, Hamburg,
Germany). Total RNA (1 µg) was reverse transcribed using a
Prime Script RT Reagent kit with gDNA Eraser (Perfect Real Time;
Takara Bio, Inc., Otsu, Shiga, Japan). RT-qPCR was conducted using
the SYBR Green Master Mix kit (Takara Biotechnology Co., Ltd.,
Dalian, China), according to the manufacturer's protocol. The
relative quantity of target mRNA was determined using the
quantification cycle (Cq) method (16) by normalizing target mRNA Cq values
to those for β-actin. The primer sequences used were as follows:
TNF-α, F 5′-GTCGTAGCAAACCACCAAGTG-3′ and R
5′-CAGATTTGTGTTGGTCCTTC-3′; interleukin-1β (IL-1β), F
5′-AGGCTGCTCTGGGATTC-3′ and R 5′-GCCACAACAACTGACGC-3′; interferon γ
(IFN-γ), F 5′-TGTAGTGAGGAACAAGCCAGAG-3′ and R
5′-TACATTTGCCGAAGAGCC-3′; IL-10, F 5′-ATGCTGCCTGCTCTTACTGAC-3′ and
R 5′-CGGTTAGCAGTATGTTGTCCAG-3′; and β-actin, F
5′-GTCCACCTTCCAGCAGATGT-3′ and R 5′-AGGGAGACCAAAGCCTTCAT-3′.
RT-qPCR was performed on an Applied Biosystems Step One Real-Time
PCR System (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Myeloperoxidase (MPO) assay
Neutrophil infiltration in the colon was monitored
by measuring the MPO activity (17). Briefly, segments of colon were
homogenized in PBS at 50 mg/ml (50 mmol/l, pH 6.0) with 0.5%
hexadecyltrimethylammonium bromide (Sangon Biotech Co., Ltd.).
Samples were frozen and thawed three times, and then centrifuged at
30,000 × g for 20 min for 4°C. The supernatants were diluted 1:30
with assay buffer consisting of PBS, 0.167 mg/ml o-dianisidine
(Sigma-Aldrich) and 0.0005% H2O2 (Sangon
Biotech Co., Ltd.). The colorimetric reaction was measured using a
Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.).
MPO activity was calculated as follows: MPO activity = (A450 ×
13.5) / tissue weight (g), where A450 is the change in the
absorbance at a wavelength of 450 nm between 1 and 3 min after the
initiation of the reaction. The coefficient 13.5 was determined
empirically for 1 U MPO activity to represent the amount of enzyme
that will reduce 1 mol peroxide/min.
Immunohistochemistry
For immunohistochemical staining, slides were
deparaffinized in xylene (Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China), rehydrated and immersed in 1.5%
H2O2 in PBS for 30 min. Slides were then
incubated for 1 h with horseradish peroxidase-conjugated
avidin-biotin complex. Subsequently, slides were incubated
overnight at 4°C with rat anti-mouse CD4 (14-9766; eBioscience,
Inc., San Diego, CA, USA) or rabbit anti-mouse MPO (ab9535; Abcam,
Cambridge, UK) primary antibodies diluted 1:50 in PBS containing 5%
bovine serum albumin (BSA; Sangon Biotech Co., Ltd.) and 10% goat
serum (Thermo Fisher Scientific, Inc.). Following washing three
times with PBS for 5 min, biotinylated secondary goat anti-rabbit
(1:100; A0277; Beyotime Institute of Biotechnology) or rabbit
anti-rat (1:100; ab6733; Abcam) polyclonal antibodies were added to
the sections and incubated at room temperature for 1 h.
Streptavidin-horseradish peroxidase was then added and, following a
40-min incubation, the sections were stained with
3,3′-diaminobenzidine from a ChemMate and EnVision detection kit
(Gene Tech Biotechnology Co., Ltd., Shanghai, China) and
counterstained with hematoxylin. CD4+ T cells were
counted in a blinded manner in 10 intercrypt spaces per mouse using
an Olympus BX41-32P02-FLB3 microscope (Olympus Corporation, Tokyo,
Japan).
Western blot analysis
Colon tissue was collected, immediately placed in
liquid nitrogen and pulverized with a mortar. Tissue was
homogenized using lysis buffer (50 mM Tris-HCl, pH 7.6; 150 mM
NaCl; 1 mM EDTA; 1% (m/v) NP-40; 0.2 mM phenylmethylsulfonyl
fluoride; 0.1 mM NaF; and 1.0 mM dithiothreitol). Whole cell
extracts were prepared by lysing the cells in cold
radioimmunoprecipitation assay buffer containing a mixture of
proteasome inhibitors (Beyotime Institute of Biotechnology).
Lysates were then centrifuged at 15,100 × g for 20 min at 48°C, and
the supernatant was collected. The homogenate was centrifuged at
48°C for 15 min at 13,000 × g, and the supernatant was then
collected. The concentration of proteins was detected using a BCA
assay with a Varioskan Lux Multimode Microplate Reader (Thermo
Fisher Scientific, Inc.). Protein samples were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis at 80 V for 2 h
at room temperature and then transferred to nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA), which were blocked
with 1% BSA in PBS for 1 h at 37°C. The blots were incubated with
specific primary antibodies overnight at 48°C, followed by an IRDye
800-conjugated secondary anti-body for 1 h at 37°C. Protein
expression was detected using the Odyssey Infrared Imaging System
(LI-COR, Inc., Lincoln, NE, USA). All blots were stripped and
reprobed with polyclonal β-actin antibody to ascertain equal
loading of proteins. Densitometric analysis was performed using
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Briefly, the linear range was detected and the background was
subtracted prior to normalization to β-actin, which was considered
to be 1. Once normalized values were determined for each replicate,
the respective means, P-values and fold changes were
calculated.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical significance of differences between treatment
and control groups was determined by Student's t-test. Data were
analyzed with one-way analysis of variance, followed by Student's
t-test for experiments involving 2 groups or Dunnett's t-test for
experiments involving >2 groups. Statistical analyses were
performed using SPSS 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Administration of 5-FU attenuates the
severity of acute DSS-induced colitis
The effect of 5-FU on the severity of acute
DSS-induced colitis was assessed. Mice were treated with 5-FU or
PBS with the administration of 4% DSS in the drinking water. 5-FU +
DSS-treated mice experienced significantly less weight loss
(P<0.05; Fig. 1A), bleeding
(P<0.05; Fig. 1B) and loss of
stool consistency (P<0.05; Fig.
1C) compared with the DSS-treated mice. These clinical markers
suggest reduced DSS-induced inflammatory changes in the
5-FU-treated mice. Consequently, the disease activity score was
significantly lower in the 5-FU + DSS-treated mice compared with
the DSS group (P<0.05), particularly at day 7 (P<0.01;
Fig. 1D).
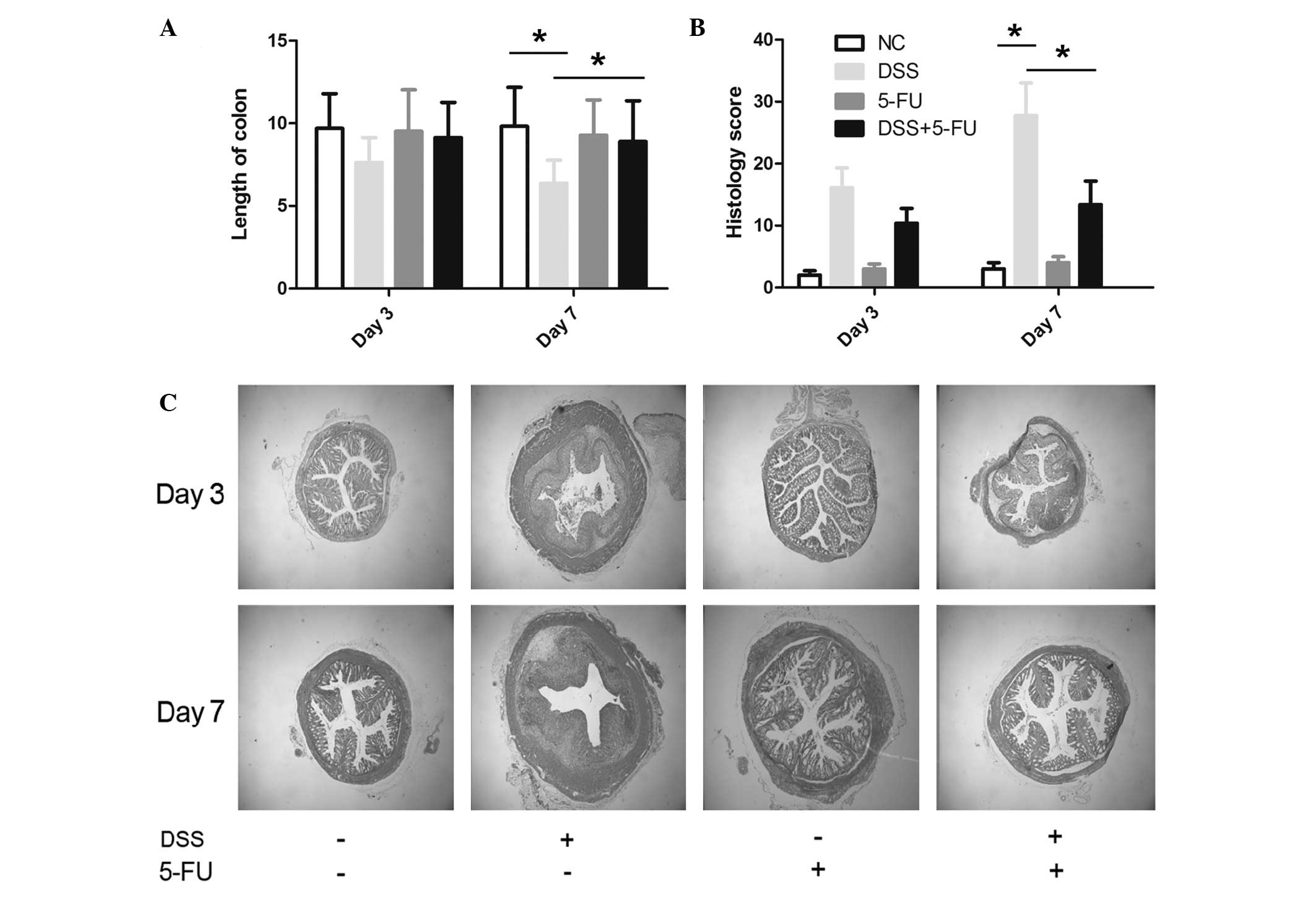
In accordance with the clinical data, gross and
histological parameters, including crypt damage, leucocyte
infiltration, colon length and histological score, favored the 5-FU
+ DSS-treated mice, suggesting less severe disease compared with
the DSS-treated mice. Accordingly, the mean length of colon in the
5-FU + DSS-treated mice (8.35±1.23 cm) was significantly longer
than in the control group (6.48±0.89 cm; P<0.05; Fig. 2A), suggesting decreased
inflammation. In addition, the mean histological score from colon
tissue in the 5-FU group was significantly lower than the DSS group
(P<0.05; Fig. 2B). Fig. 2C demonstrates that 5-FU treatment
prevented the abnormal architecture change in the colon that
occurred following DSS treatment. The 5-FU control group did not
indicate a distorted colon architecture (Fig. 2C). Furthermore, the dose of 15
mg/kg 5-FU resulted in no significant histological or biochemical
damage to the liver and kidney of the mice compared with the
DSS-treated group (Fig. 3).
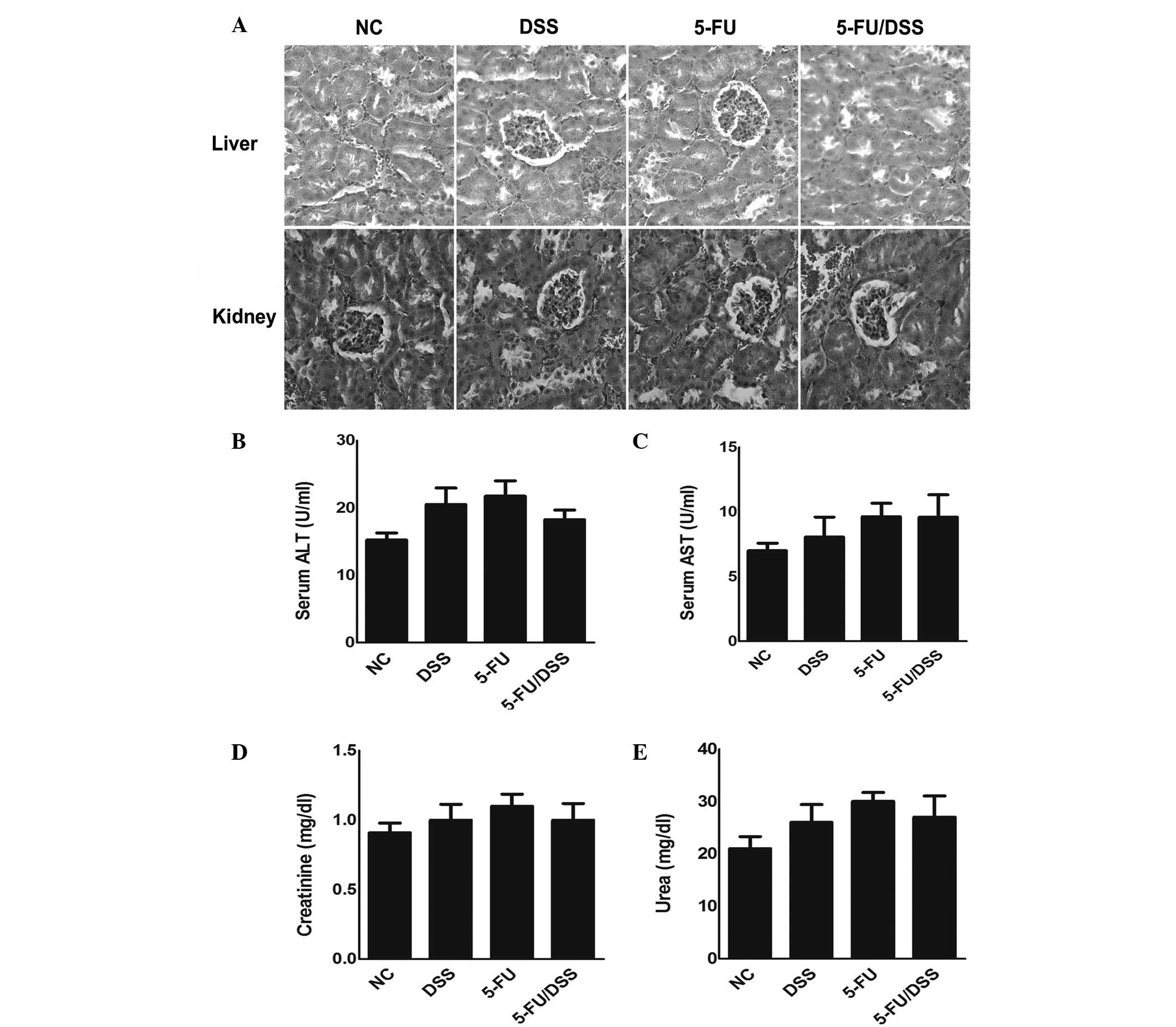 | Figure 3Effect of 5-FU (15 mg/kg) on
histological and biochemical changes in liver and kidney tissue of
mice. (A) Representative pictures of hematoxylin and eosin staining
(magnification, ×400). Change of (B) ALT, (C) AST, (D) creatinine
and (E) urea in control, DSS-treated, 5-FU-treated and DSS +
5-FU-treated mice. Data are expressed as the mean ± standard error
of the mean (n=10). NC, normal control; DSS, dextran sodium
sulfate; 5-FU, 5-fluorouracil; ALT, alanine transaminase; AST,
aspartate aminotransferase. |
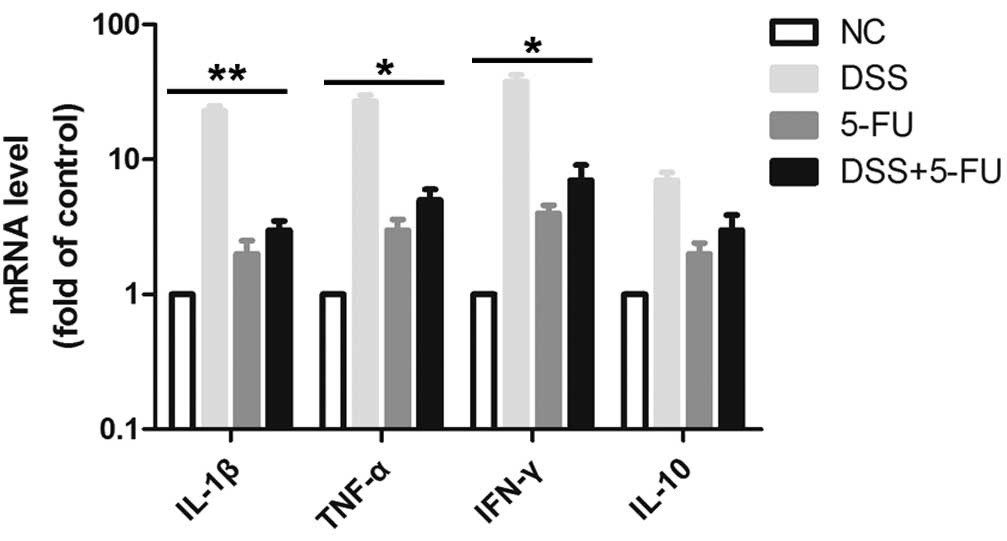
5-FU decreases the levels of
proinflammatory cytokines in the colon of DSS-induced mice at day
7
Oral administration of DSS is toxic to the colonic
epithelium and triggers inflammation by disrupting the
compartmentalization of commensal bacteria in the gut with high
levels of proinflammatory cytokines, such as TNF-α, IL-1β and IFN-γ
(18,19). The release of cytokines is
considered to be an indicator of the inflammatory response. Thus,
the mRNA expression levels of TNF-α, IL-1β and IFN-γ in the colon
tissue were detected using RT-qPCR. As demonstrated in Fig. 4, treatment with DSS increased the
release of TNF-α, IL-1β and IFN-γ in mice compared with the normal
control group. Notably, in mice treated with 5-FU, the DSS-induced
secretion of TNF-α (P<0.05), IL-1β (P<0.01) and IFN-γ
(P<0.05) were significantly reduced, indicating that 5-FU may
inhibit inflammation in intestinal epithelial cells. The expression
levels of the anti-inflammatory cytokine IL-10 did not
significantly change.
5-FU reduces the expression levels of MPO
and the number of CD4+ cells in the intestinal mucosal
of DSS-induced mice at day 7
Another trait of colitis is the invasion of immune
cells to the intestinal mucosal with increased expression levels of
MPO and CD4 (20). There was a
marked increase in lymphocytic infiltration and loss of glandular
architecture in the DSS-treated group compared with the 5-FU +
DSS-treated group (Fig. 5A).
Induction of colitis by DSS resulted in an increased MPO activity
in the colon tissue compared with the normal control group
(Fig. 5B). Additionally,
administration of 5-FU to DSS-treated mice significantly reduced
the MPO activity compared with the DSS-treated group (P<0.01;
Fig. 5C). Similarly, 5-FU +
DSS-treated mice demonstrated significantly decreased
CD4+ T cell infiltration compared with the DSS-treated
group (P<0.01; Fig. 5B).
5-FU inhibits the activation of NF-κB in
the intestinal mucosa of DSS mice model
NF-κB is a critical transcription factor in the
inflammatory response. It functions as a pro-inflammatory factor
and participates in the pathophysiology of intestinal inflammatory
diseases (21). Previous studies
detected activated NF-κB in colonic mucosal tissue sections of
patients with IBD compared with healthy subjects (22,23).
Furthermore, NF-κB was identified as one of the primary factors
governing the formation of the molecular network leading to various
cellular functions associated with IBD (24). For example, numerous
NF-κB-dependent pro-inflammatory mediators, such as IL-1β, TNF-α,
IL-12p40 and IL-23p19, are elevated in patients with IBD and, thus,
represent therapeutic targets (25). Additionally, previous studies
demonstrated that 5-FU inhibits the activation of NF-κB and its
subsequent nuclear translocation (26,27).
To investigate the mechanism of 5-FU's anti-inflammatory activity,
the effect of 5-FU on the activation of the NF-κB pathway in
intestinal epithelial cells was investigated. Changes in the
expression levels of phosphorylated (p)NF-κB-p65 in the intestinal
mucosa of DSS-treated mice were evaluated by western blotting. As
demonstrated in Fig. 6, pNF-κB-p65
was upregulated in DSS-treated mice compared with the normal
control group. 5-FU treatment significantly reduced this effect
compared with the DSS-treated group (P<0.05).
Discussion
The present study provides evidence that 5-FU
modulates the immune response in acute mouse colitis by inhibition
of cytokine generation, suppression of toxin-induced damage and
aggravation of inflammation via reduction of the NF-κB activation.
In the presence of 5-FU, DSS treatment induced less severe clinical
symptoms of colitis, a lesser extent of epithelial damage and
decreased inflammation compared with the DSS-treated mice. These
results indicate that 5-FU, as an important immunemodulatory agent,
participates in the epithelial response to injury and
inflammation.
5-FU is a derivant of pyrimidine and is classified
as an anti-metabolic agent. It interferes with the synthesis of DNA
and RNA in healthy and tumor cells, and it is widely used in the
treatment of CRC (9). Furthermore,
5-FU inhibits the synthesis of proteins, thus serving as a
proteinase inhibitor, and exerts its action throughout the whole
process of acute colitis (9). 5-FU
decreases the synthesis and secretion of cytokines, therefore, it
may alleviate the damage to mucosal tissues (12). Furthermore, Chen et al
(12) demonstrated that 5-FU
modulates the pro-inflammatory cytokine response in experimental
acute pancreatitis by minimizing the abnormal immune cytokines
production and relieving the pathophysiological disorders. In the
present study, 5-FU downregulated the serum levels of IL1β, IFN-γ
and TNF-α, and decreased the percentage of neutrophils in
pancreatitis. An alternative study revealed that 5-FU treatment
combined with octreotide inhibited the serum levels of TNF-α in a
mouse model of severe acute pancreatitis, which is a serious
systemic inflammatory disease with a high mortality rate (28).
In the present study, increased MPO activity and
CD4+ expression levels were observed in the intestinal
mucosa of DSS-treated mice. The decreased MPO activity that
resulted from 5-FU treatment may suppress the neutrophil influx
into the lamina propria and act to control disease severity through
reduced secretion of pro-inflammatory cytokines, including CXCL8
and IL-17, which have been widely implicated in pathological
intestinal inflammation (29).
Another effect of 5-FU was the reduction of
CD4+ T cell accumulation in the mucosa. This may cause
downregulation of pathological immune cell activation, including
restraining the ability of immune cells to present antigens during
colitis, a notable event previously reported during other
inflammatory diseases, such as rheumatoid arthritis (30).
The present study demonstrated that 5-FU reduced
pNF-κB-p65 protein expression levels in DSS-induced colitis, which
was in agreement with previous studies (25,26).
NF-κB is an important transcription factor in the pathophysiology
of several inflammatory diseases, owing to its ability to induce
the expression of numerous pro-inflammatory mediators, such as
cytokines, chemokines and adhesion molecules (31). A reduction in NF-κB activation
prevents the increasing production of pro-inflammatory
cytokines.
Previous studies have indicated that patients
administered with 5-FU may develop a certain degree of mucositis,
characterized by decreased villi length and crypt cell homeostasis
and accompanied with severe symptoms, including nausea and vomiting
(32,33). Furthermore, gastrointestinal
mucositis developed in mice that received 5-FU treatment (34). However, in the current study, no
intestinal mucositis was detected, possibly due to the low dose of
15 mg/kg used, which was in contrast to the dose of 400 mg/kg used
by Pritchard et al (35).
This dose resulted in histopathological changes to the gut,
quantified as loss of crypt and villus cellularity. A previous
study demonstrated that 5-FU may cause diarrhea accompanied by
changes in the expression of inflammatory cytokines, including
significantly increased TNF-α, IL-1β, IL-6, Il-17A and IL-22,
throughout the entire colon of mice (36). The difference in the regulation of
cytokine production may be a result of the mice species or the dose
of 5-FU used in the present study.
In conclusion, the results of the current study
demonstrated that low dose 5-FU (15 mg/kg) is capable of inhibiting
NF-κB activation and effectively reducing inflammation in a mouse
model of DSS-induced colitis without obvious side effects. 5-FU may
therefore be a candidate therapeutic agent for the treatment of
inflammatory bowel disease.
References
|
1
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ordás I, Eckmann L, Talamini M, Baumgart
DC and Sandborn WJ: Ulcerative colitis. Lancet. 380:1606–1619.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema
HW and Kaplan GG: Increasing incidence and prevalence of the
inflammatory bowel diseases with time, based on systematic review.
Gastroenterology. 142:46–54. 2012. View Article : Google Scholar
|
|
4
|
Grivennikov SI: Inflammation and
colorectal cancer: colitis-associated neoplasia. Semin
Immunopathol. 35:229–244. 2013. View Article : Google Scholar
|
|
5
|
Bernklev T, Jahnsen J, Lygren I, Henriksen
M, Vatn M and Moum B: Health-related quality of life in patients
with inflammatory bowel disease measured with the short form-36:
Psychometric assessments and a comparison with general population
norms. Inflamm Bowel Dis. 11:909–918. 2005. View Article : Google Scholar
|
|
6
|
Harpaz N and Talbot IC: Colorectal cancer
in idiopathic inflammatory bowel disease. Semin Diagn Pathol.
13:339–357. 1996.
|
|
7
|
Sandborn WJ: Azathioprine: State of the
art in inflammatory bowel disease. Scand J Gastroenterol Suppl.
225:92–99. 1998. View Article : Google Scholar
|
|
8
|
Targownik LE and Bernstein CN: Infectious
and malignant complications of TNF inhibitor therapy in IBD. Am J
Gastroenterol. 108:1835–1842; quiz 1843. 2013. View Article : Google Scholar
|
|
9
|
Álvarez P, Marchal JA, Boulaiz H, Carrillo
E, Vélez C, Rodríguez-Serrano F, Melguizo C, Prados J, Madeddu R
and Aranega A: 5-Fluorouracil derivatives: A patent review. Expert
Opin Ther Patents. 22:107–123. 2012. View Article : Google Scholar
|
|
10
|
Bielecki K, Wiedmann M, Meyer F, Kimura W
and Mössner J: Effect of 5-fluorouracil on secretion and synthesis
of pancreatic digestive enzymes: Studies in isolated pancreatic
acini and perfused pancreas derived from normal rats and from rats
with acute necrotizing pancreatitis. Pancreas. 9:518–525. 1994.
View Article : Google Scholar
|
|
11
|
Rich TA, Shepard RC and Mosley ST: Four
decades of continuing innovation with fluorouracil: Current and
future approaches to fluorouracil chemoradiation therapy. J Clin
Oncol. 22:2214–2232. 2004. View Article : Google Scholar
|
|
12
|
Chen X-L, Ciren S-Z, Zhang H, Duan LG and
Wesley AJ: Effect of 5-FU on modulation of disarrangement of
immune-associated cytokines in experimental acute pancreatitis.
World J Gastroenterol. 15:2032–2037. 2009. View Article : Google Scholar :
|
|
13
|
Luther J, Owyang SY, Takeuchi T, Cole TS,
Zhang M, Liu M, Erb-Downward J, Rubenstein JH, Chen CC, Pierzchala
AV, et al: Helicobacter pylori DNA decreases pro-inflammatory
cytokine production by dendritic cells and attenuates dextran
sodium sulphate-induced colitis. Gut. 60:1479–1486. 2011.
View Article : Google Scholar
|
|
14
|
Song JL, Qian Y, Li GJ and Zhao X:
Anti-inflammatory effects of kudingcha methanol extract (Ilex
kudingcha C.J. Tseng) in dextran sulfate sodium-induced ulcerative
colitis. Mol Med Rep. 8:1256–1262. 2013.PubMed/NCBI
|
|
15
|
Obermeier F, Dunger N, Strauch UG, Hofmann
C, Bleich A, Grunwald N, Hedrich HJ, Aschenbrenner E,
Schlegelberger B, Rogler G, et al: CpG motifs of bacterial DNA
essentially contribute to the perpetuation of chronic intestinal
inflammation. Gastroenterology. 129:913–27. 2005. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Bradley PP, Priebat DA, Christensen RD and
Rothstein G: Cellular and extracellular myeloperoxidase in pyogenic
inflammation. J Invest Dermatol. 78:206–209. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berndt BE, Zhang M, Chen GH, Huffnagle GB
and Kao JY: The role of dendritic cells in the development of acute
dextran sulfate sodium colitis. J Immunol. 179:6255–6262. 2007.
View Article : Google Scholar
|
|
19
|
Kitajima S, Takuma S and Morimoto M:
Changes in colonic mucosal permeability in mouse colitis induced
with dextran sulfate sodium. Exp Anim. 48:137–143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L, Ren F, Yun Z, An Y, Wang C and Yan
X: Determination of the effects of lactoferrin in a preclinical
mouse model of experimental colitis. Mol Med Rep. 8:1125–1129.
2013.
|
|
21
|
Karrasch T and Jobin C: NF-kappaB and the
intestine: Friend or foe? Inflamm Bowel Dis. 14:114–124. 2008.
View Article : Google Scholar
|
|
22
|
Schreiber S, Nikolaus S and Hampe J:
Activation of nuclear factor kappa B inflammatory bowel disease.
Gut. 42:477–484. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rogler G, Brand K, Vogl D, Page S,
Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Schölmerich J and
Gross V: Nuclear factor kappaB is activated in macrophages and
epithelial cells of inflamed intestinal mucosa. Gastroenterology.
115:357–369. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berndt U, Bartsch S, Philipsen L, Danese
S, Wiedenmann B, Dignass AU, Hämmerle M and Sturm A: Proteomic
analysis of the inflamed intestinal mucosa reveals distinctive
immune response profiles in Crohn's disease and ulcerative colitis.
J Immunol. 179:295–304. 2007. View Article : Google Scholar
|
|
25
|
Pizarro TT and Cominelli F: Cytokine
therapy for Crohn's disease: Advances in translational research.
Annu Rev Med. 58:433–444. 2007. View Article : Google Scholar
|
|
26
|
Islam S, Hassan F, Tumurkhuu G, Ito H,
Koide N, Mori I, Yoshida T and Yokochi T: 5-Fluorouracil prevents
lipopolysaccharide-induced nitric oxide production in RAW 264.7
macrophage cells by inhibiting Akt-dependent nuclear factor-kappaB
activation. Cancer Chemother Pharmacol. 59:227–233. 2007.
View Article : Google Scholar
|
|
27
|
Melen-Mucha G, Balcerczak E, Mucha S,
Panczyk M, Lipa S and Mirowski M: Expression of p65 gene in
experimental colon cancer under the influence of 5-fluorouracil
given alone and in combination with hormonal modulation. Neoplasma.
51:319–324. 2004.PubMed/NCBI
|
|
28
|
Zhou MT, Chen BC and Sun HW: Continuous
regional arterial infusion with fluorouracil and octreotide
attenuates severe acute pancreatitis in a canine model. PLoS One.
7:e373472012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fournier BM and Parkos CA: The role of
neutrophils during intestinal inflammation. Mucosal Immunol.
5:354–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aarvak T and Natvig JB: Cell-cell
interactions in synovitis: Antigen presenting cells and T cell
interaction in rheumatoid arthritis. Arthritis Res. 3:13–17. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayden MS, West AP and Ghosh S: NF-kappaB
and the immune response. Oncogene. 25:6758–6780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Keefe DM, Gibson RJ and Hauer-Jensen M:
Gastrointestinal mucositis. Semin Oncol Nurs. 20:38–47. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Azevedo OG, Oliveira RA, Oliveira BC,
Zaja-Milatovic S, Araújo CV, Wong DV, Costa TB, Lucena HB, Lima RC
Jr, Ribeiro RA, et al: Apolipoprotein E COG 133 mimetic peptide
improves 5-fluorouracil-induced intestinal mucositis. BMC
Gastroenterol. 12:352012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saegusa Y, Ichikawa T, Iwai T, Goso Y,
Okayasu I, Ikezawa T, Shikama N, Saigenji K and Ishihara K: Changes
in the mucus barrier of the rat during 5-fluorouracil-induced
gastrointestinal mucositis. Scand J Gastroenterol. 43:59–65. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pritchard DM, Potten CS and Hickman JA:
The relationships between p53-dependent apoptosis, inhibition of
proliferation, and 5-fluorouracil-induced histopathology in murine
intestinal epithelia. Cancer Res. 58:5453–5465. 1998.PubMed/NCBI
|
|
36
|
Sakai H, Sagara A, Matsumoto K, Hasegawa
S, Sato K, Nishizaki M, Shoji T, Horie S, Nakagawa T, Tokuyama S
and Narita M: 5-Fluorouracil induces diarrhea with changes in the
expression of inflammatory cytokines and aquaporins in mouse
intestines. PLoS One. 8:e547882013. View Article : Google Scholar : PubMed/NCBI
|