Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of malignant tumor worldwide, and the third most common
cause of cancer-associated mortality, with surgical resection
remaining the most effective therapy (1). However, <15% of patients benefit
from this treatment due to the presence of multiple tumor nodules
(2). Therefore, it is imperative
to identify novel therapeutic strategies, including suicide gene
therapy, in which nucleic acids encoding specific therapeutic genes
are used as antitumor agents. Cancer gene therapy offers potential
for decreasing tumor-associated mortality rates. However, it has
been clinically limited by non-targeted and insufficient gene
transfer (3). Ultrasound-targeted
microbubble destruction-targeted gene delivery to the tumor tissue,
and the targeted co-delivery of genes synergistically improves
antitumor effects (3).
The present study evaluated the use of gene therapy
to target HCC, using the herpes simplex virus thymidine
kinase/ganciclovir (HSV-TK/GCV) suicide gene system via its
'bystander effect'. It is not a requirement that all tumor cells
are directly targeted, and the occurrence of the bystander effect
in HSV-TK/GCV therapy may represent an important therapeutic
opportunity (4). There is
compelling evidence demonstrating that gap junctional intercellular
communication (GJIC) is directly involved (5,6). Gap
junctions are formed by connexins, a family of homologous proteins,
which directly link the cytoplasms of adjacent cells to allow the
passage of ions (4). Connexins can
also act as tumor suppressor genes (7). GJIC is involved in tissue
homeostasis, whereas the expression of connexin32 (Cx32) remains
expressed and is critical for intercellular communication (8). Alternatively, a number of classes of
chemicals, including gemcitabine (9) and cAMP (10), have been reported to increase Cx26
and Cx43 and, subsequently, GJIC. An ideal wide-spectrum chemical
inducer of GJIC is trans-retinoic acid (ATRA), which results in
upregulated expression levels of Cx43 and GJIC (11,12).
Therefore, the present study hypothesized that
treatment of tumor cells with ATRA augments the bystander effect of
the HSV-TK/GCV system and results in improved tumor cell
death-inducing effects by enhancing GJIC. In the present study, the
effect of ATRA on the bystander-mediated cell death of HepG2 cells
were examined in vitro and in vivo, and whether
facilitating gap junction communication through Cx32 overexpression
can increase the therapeutic efficacy of suicide gene therapy.
Materials and methods
Chemicals and reagents
Rabbit anti human Cx32 antibody was purchased from
Bioworld Technology, Inc. (St. Louis Park, MN, USA). ATRA and (GCV)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). The total
expression plasmid vector of the enhanced green fluorescent protein
and HSV-TK I type (pIRES2-EGFP-HSV-TK) was constructed previously
at the Institute of Ultrasound Imaging of Chongqing Medical
University (Chongqing, China) (13). UTG 1025, a self-made ultrasonic
gene transfection instrument and lipid microbubbles were provided
by the Institute of Ultrasound Imaging of Chongqing Medical
University (Chongqing, China).
Cell line and experimental animals
The HepG2 human hepatoma cell line was obtained from
the Cell Resource Center, Chinese Academy of Medical Sciences,
Peking Union Medical College (Beijing, China), and cultured in high
glucose Dulbecco's modified Eagle's medium containing 10% fetal
bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA), at
37°C in 5% CO2. The viability of the HepG2 cells,
determined by trypan blue (0.4%; Sigma-Aldrich) exclusion, was
>95%.
A total of 32 male athymic BALB/c nu/nu mice (4–6
weeks old, weighing 20±2 g) were purchased from the Laboratory
Animal Center of Chongqing Medical University. They were housed at
a temperature of 25°C, under a 12-h light/dark cycle, in specific
pathogen-free conditions and received food and water ad
libitum. Each mice was inoculated subcutaneously with 0.2 ml
HepG2 cells (5×106 cells per mouse) in suspension in the
right flank. The experimental animals, with tumor diameters
measuring 0.5–1.0 cm, were randomly divided into the following four
groups (n=8 per group): (A) phosphate-buffered saline (PBS), (B)
HSV-TK, (C) ATRA and (D) HSV-TK+ATRA. Each group contained eight
mice. All protocols were approved by the Animal Experimentation
Ethics Committee of Chongqing Medical University, in compliance
with the recommended National Institutes of Health guidelines for
the care and use of animals for scientific purposes (14).
Plasmid preparation and the combining of
microbubbles with the plasmid
The pIRES2-EGFP-HSVTK gene plasmid for transfection
was extracted and purified using a Tiangen kit (cat. no. RM204–01;
Tiangen Biotech Co., Ltd., Beijing, China); the concentration of
the isolated plasmid DNA was determined at an absorbance of
260/280=1.9 by ultraviolet spectrophotometry (U-0080D; Hitachi
High-Technologies Corp., Tokyo, Japan), and resuspended to a final
concentration of 1 µg/µl in ddH2O
(Beyotime Institute of Biotechnology, Shanghai, China). The
recombinant plasmid was evaluated using biomaging systems
(GelGDoc2000; Bio-Rad Laboratories GmbH, München, Germany). The
method used for the preparation of the gene-loaded lipid
microbubbles was performed, according to the criteria described by
Wang et al (15). The
prepared blank lipid microbubbles and plasmid were then mixed (1
mg/ml) and incubated at 37°C for 30 min, and the gene-loaded lipid
microbubbles were produced.
Ultrasound microbubble-mediated
transfection with the pIRES2-EGFP-HSVTK gene
The plasmid concentration was adjusted to 0.2
µg/ml, mixed with the lipid microbubbles and incubated at
37°C for 30 min, as described above. The microbubbles containing
the pIRES2-EGFP-HSVTK plasmid were added to each well and exposed
to ultrasonic (UTG 1025, Institute of Ultrasound Imaging of
Chongqing Medical University) radiation (1 MHz; 0.5
W/cm2; 30 sec). In order to obtain the positive clone
(TK+) HepG2 cells (density, 80–90%), the
HSV-TK-transfected cells were selected using G418 culture media
(800 mg/ml; HyClone Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses of the transfection
and expression of the pIRES2-EGFP-HSV-TK gene
At 2 weeks following the observation of HepG2 cells
stably expressing HSV-TK, total RNA was extracted from the HepG2
cells and quantified using RNA Isolation Solvent (Omega Bio-tek,
Inc., Doraville, GA, USA) according to the manufacturer's
instructions and reverse transcribed, using an RT-PCR kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. The RNA (1 µg) was used to synthesize cDNA (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The relative
transcription of HSV-TK was determined by performing
semi-quantitative PCR analyses of HSV-TK, TK+ cells
(positive control) and β-actin (internal control) by qPCR
amplification using a Rotor-Gene 6000 PCR machine (Corbett Life
Science, Mortlake, Australia). The forward primer was 5′-CAG CAA
GAA GCC ACG GAAGT-3′ and the reverse primer was 5′-AGC ACC CGC CAG
TAA GTCAT-3′ (Sangon Biotech Co., Ltd., Shanghai, China). For qPCR
amplification, a 20 µl reaction volume, containing 10
µl 2X Taq PCR Master mix (Thermo Fisher Scientific, Inc.), 1
µl 1X each primer, 2 µl 2-fold diluted cDNA and 6
µl RNase-free water (Thermo Fisher Scientific, Inc.) was
used. The amplification conditions were as follows: 94°C for 2 min,
followed by 28 cycles of 94°C for 15 min, 58°C for 10 min and 72°C
for 30 min, and a final step at 72°C for 10 min. The expected
lengths of the HSV-TK was 1,327 bp. A total of 10 µl of each
PCR product was loaded onto a 1.5% agarose gel (0.5 µg/ml
ethidium bromide; Sigma-Aldrich) and separated by electrophoresis.
The expression of HSV-TK was quantified using Image-Pro Plus 5.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Assessment of the effects of ATRA and GCV
on the growth of mixed cells
Prior to proceeding with comparisons of the
HSV-TK/GCV-mediated bystander effect, it was important to ensure
that ATRA itself had no effect on in vitro growth rates or
cell death in the cell lines used in the present study. The HepG2
cells were plated at a density of 5×103 cells/well. The
cell culture media, containing varying concentrations of ATRA (0,
10−4. 10−5, 10−6, 10−7
and 10−8 mol/l) were replaced every day. Following 3
days incubation at 37°C, 20 µl MTT assay mix was added to
each well. The plates were incubated for 4 h at 37°C, and the
absorbance was measured at 570 nm (U-0080D), following which the
optical density (OD570) was calculated. The same method to used to
assess the cytotoxicity of GCV at varying concentrations (0, 20,
40, 60, 80 and 100 µg/ml). The inhibition ratio was
calculated as follows: Inhibition ratio = (1 -
ODexperiment/ODcontrol) × 100%. Each assay was repeated three
times.
ATRA treatment to assess the bystander
effect of HSV-TK in vitro
The HSV-TK-transfected (TK+) cells were
mixed with untransfected HSV-TK (TK) cells at concentrations
between 0 and 100%. These mixtures were plated in 96-well culture
plates at a density of 4,000 cells/well in 100 µl media with
(10−6 mol/l). or without ATRA. There were four duplicate
wells plated for each mixture of cells. When the cells were ~20–30%
confluent, with the majority of cells showing visible contact with
adjacent cells, the medium was removed and replaced with complete
medium containing GCV (40 µg/ml). The cells were incubated
for 3 days at 37°C with or without ATRA (10−6 mol/l).
The assessment of cell number was determined using an MTT (20
µl, 37°C, 0.5 mg/ml) colorimetric cell proliferation assay,
which measures viable cell dehydrogenase activity. The experiment
was repeated three times.
In vivo experiments
ATRA administration in vivo
The mice were subcutaneously injected with HepG2
cells, as described above, and when the tumor diameter reached
0.5–1.0 cm, the microbubbles containing the HSV-TK plasmid (200
µl; 0.1 µg/µl) were injected into the tumor
foci of groups B and D (once every 3 days, five times).
Subsequently, the ultrasonic gene trans-fection instrument was used
to irradiate the tumor (1 MHz; 2 W/cm2; 5 min), and GCV
(100 mg/kg·day) was administered into the peritoneal cavity
following irradiation for 14 consecutive days. In groups C and D,
ATRA (1 mg/kg·day; no antitumor effect) was administered into the
peritoneal cavity consecutively for 14 days. PBS (200 µl)
was injected into the tumor foci in group A.
Tumor sizes were measured every 3 days, and the
volumes were calculated using the following equation: [(longest
diameter) × (shortest diameter)2 / 2]. The tumor
inhibition rate was calculated as follows: Inhibition rate = (PBS
group - mean tumor volume in treatment group) / mean tumor volume
in control group × 100%.
Immunohistochemical analysis
The mice were anesthetized with pentobarbital (0.03
mg/100 g; Sigma-Aldrich) prior to sacrifice following treatment in
each group. Tumor tissues were fixed in 10% zinc-buffered formalin
(Sigma-Aldrich), embedded in paraffin (Sigma-Aldrich), sectioned (4
µm), and stained with hematoxylin and eosin (Beyotime
Institute of Biotechnology). To avoid nonspecific staining, avidin
and biotin in the tissues were blocked using a blocking kit.
Following the blocking reaction, the slides were incubated with
biotin-conjugated anti-Cx32 (1:100) at 4°C overnight. Goat
anti-rabbit immunoglobulin G (IgG; cat. no. BS13271; Bioworld
Technology, Inc.) was used as the negative control, at 37°C for 20
min. The reaction was visualized using a SABC Standard kit (cat.
no. SA1022; Wuhan Boster Biological Technology, Ltd., Wuhan,
China), followed by counterstaining with hematoxylin. Serial
sections were fixed in 10% zinc-buffered formalin and stained with
hematoxylin and eosin. The sections were then incubated in PBS
containing diaminoben-zidine (Beyotime Institute of Biotechnology)
for 5 min, and examined under a microscope (CKX 41SF; Olympus,
Tokyo, Japan).
Protein extraction and western blot
analysis
The proteins were extracted using protein extraction
reagent (Beyotime Institute of Biotechnology) 48 h following
transfection, and stored at −20°C, as described previously
(16). The protein extracts were
obtained using a Membrane and Cytosol Protein Extraction kit
(P0033; Beyotime Institute of Biotechnology), and protein
concentration was determined using a Bradford assay kit (cat. no.
P0006; Beyotime Institute of Biotechnology). Proteins were resolved
by electrophoresis on 8% SDS-PAGE gels (Beyotime Institute of
Biotechnology) and transferred onto polyvinylidene difluoride
membranes (Thermo Fisher Scientific, Inc.). The membranes were
immunoblotted with polyclonal rabbit anti-human anti-Cx32 (cat. no.
BS3527; Bioworld Technology, Inc.; 1:500–1:1,000 dilution)
overnight at 4°C. The secondary antibody comprised horseradish
peroxidase-conjugated monoclonal goat anti-rabbit (cat. no.
BS13271; 1:10,000; Bioworld Technology, Inc.). The bands were
analyzed using a GelGDoc2000 imaging system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and the protein levels were quantified by
their relative OD.
Statistical analysis
The SPSS 17.0 statistical software package (SPSS,
Inc., Chicago, IL, USA) was used to perform statistical analysis.
The data are expressed as the mean ± standard deviation. Analysis
of variance was used to assess the inhibition rate. A least
significant difference t-test was used for pairwise
comparisons. Kaplan-Meier's method was applied for survival
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of TK-specific mRNA and
cytotoxicity of ATRA and GCV
The mRNA expression of TK was observed in the HepG2
cells transfected using ultrasound microbubble-mediated with
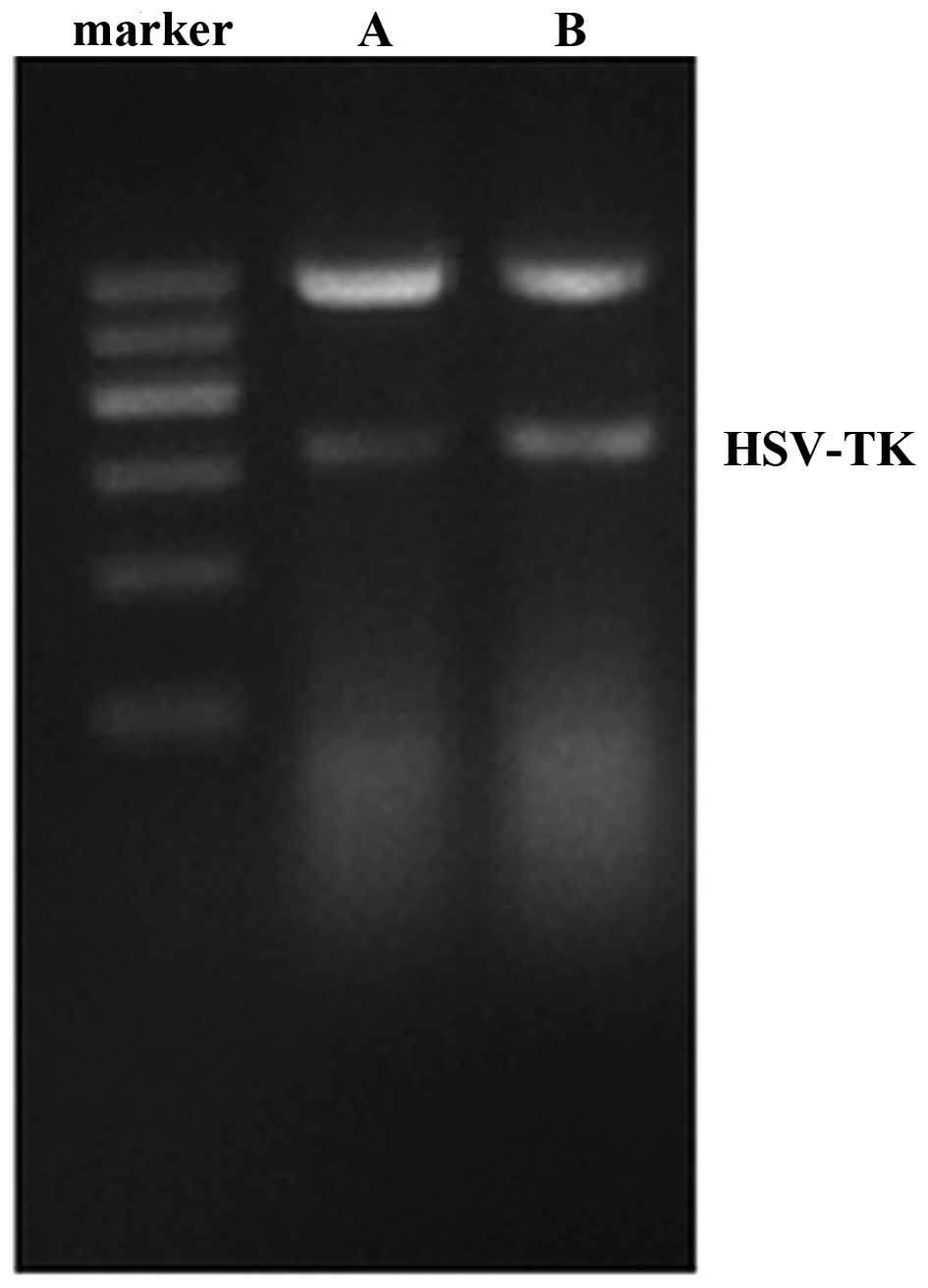
pIRES2-EGFP-HSVTK, compared with the positive control (Fig. 1). The rates of growth inhibition of
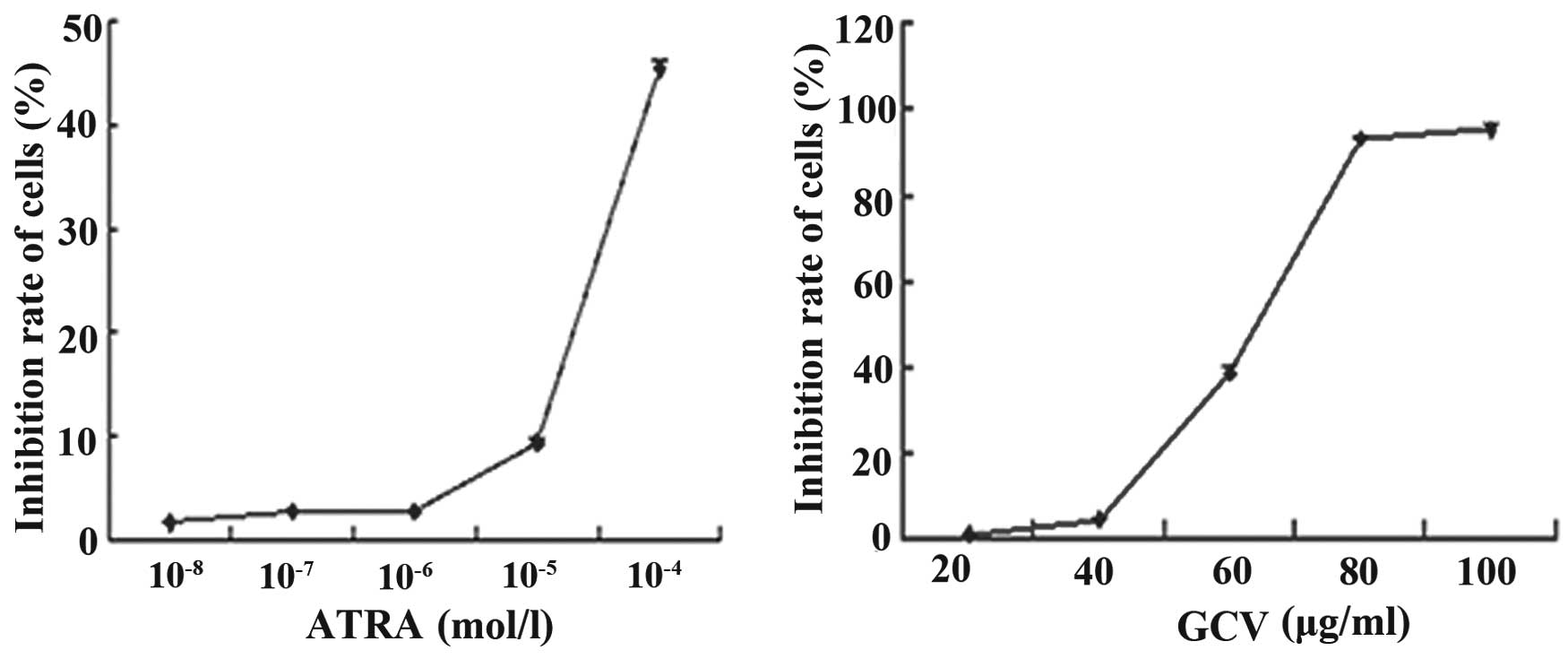
the cells were 1.74±0.04, 2.75±0.76, 2.80±0.09, 9.37±0.32 and
45.38±0.72% at ATRA concentrations of 10−8,
10−7, 10−6, 10−5 and
10−4 mol/l, respectively (Fig. 2A). No apoptosis was observed in the
cells exposed to ATRA alone, at concentrations as high as
10−6 mol/l, compared with the cells growing in the
absence of ATRA (P>0.05), which showed no growth inhibitory
effects. Toxic effects were observed when the concentration of ATRA
was >10−6 mol/l (P<0.05).
The rates of growth inhibition of the cells were
1.57±0.05, 4.95±0.10, 39.12±1.50, 94.30±0.93 and 95.38±1.12% at GCV
concentrations of 20, 40, 60, 80 and 100 µg/ml, respectively
(Fig. 2B). No significant
difference in growth was observed in the cells exposed to GCV at
concentrations up to 40 µg/ml, compared with the cells
growing in the absence of GCV (P>0.05).
In vitro analysis of the bystander
effect
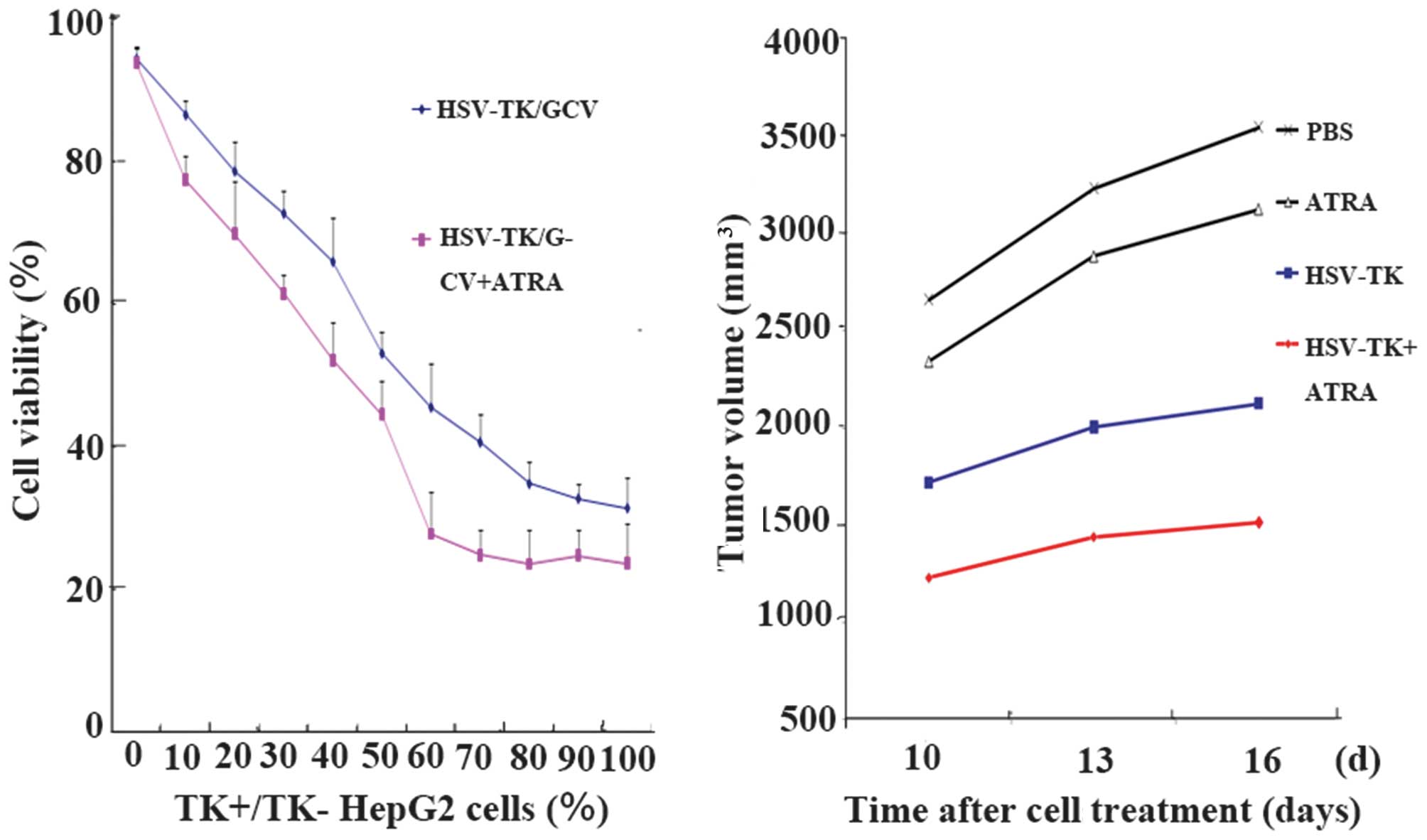
To compare the potency of the bystander effect with
or without ATRA, experiments were performed, in which
HSV-TK+ and HSV-TK cells were mixed at varying ratios,
followed by exposure to 40 µg/ml GCV, with or without
10−6 mol/l ATRA. ATRA alone had no effect on the number
of cells and this concentration of GCV had no effect on the growth
of the cells. In the same proportion of HSV-TK+ cells, a
significant decrease in cell viability was observed in the
ATRA-treated cells, compared with cells with ATRA treatment
(P<0.05; Fig. 3).
In vivo experiments
Treatment effect
As tumor size increased, the mice exhibited an
emaciated body, appetite loss, dull furs, activity reduction and
body weight loss. However, the growth of the mice in the treatment
group was significantly improved, compared with that in the control
group. Analysis of the tumor inhibition rates revealed that the
tumor sizes in group D (HSV-TK+ATRA) were significantly lower
(P<0.05), compared with those in the other groups. Compared with
the HSV-TK group, the tumor sizes in the HSV-TK+ATRA group were
smaller at all time points (P<0.05). The tumor inhibition rates
of the PBS, HSV-TK, ATRA and HSV-TK+ATRA groups were 0, 37.97±4.35,
6.92±7.41 and 59.40±6.17%, respectively (Fig. 3).
Histopathological changes and
expression levels of Cx32 in tumor tissues
Compared with the other groups (Fig. 4A–C), higher levels of tumor cell
necrosis were observed in the HSV-TK+ATRA group (Fig. 4D; P<0.05). Lower levels of cell
necrosis and karyopyknosis were observed in the HSV-TK group,
although these levels were higher, compared with the levels of cell
necrosis in the PBS and ATRA groups. In analyzing the
immunohistochemical staining, the brown-yellow or dark brown
staining of the cytoplasm was considered positive (predominantly
localized to the membrane of cells). Compared with the untreated
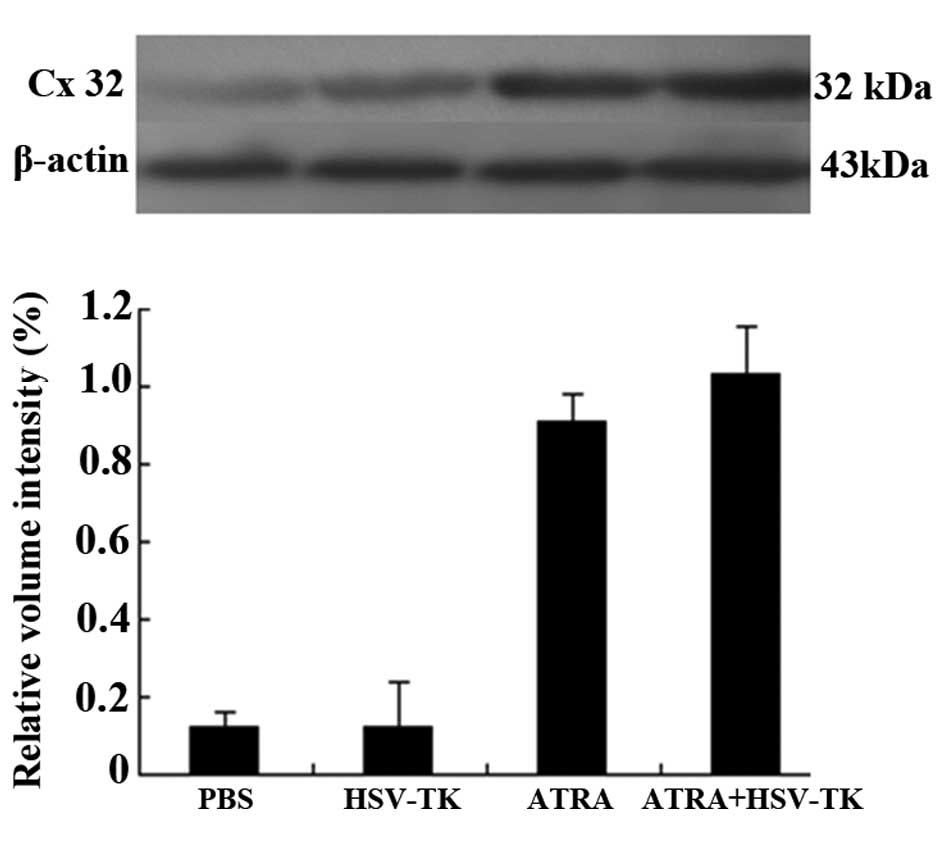
group, the protein expression of Cx32 was significantly increased
in the ATRA-treated tumor groups (P<0.01; Fig. 4E and F). Similar results were
obtained in the analysis of protein expression of Cx32 using
Western blot analysis (P<0.05), in which the protein levels of
Cx32 were significantly increased in the ATRA and HSV-TK+ATRA
groups, compared with the other groups (P<0.01; Fig. 5).
 | Figure 4Hepatic tumor tissues stained with
hematoxylin and eosin following treatment. Compared with the (A)
PBS, (B) HSV-TK and (C) ATRA groups, higher levels of tumor cell
necrosis were found in the (D) HSV-TK+ATRA group (P<0.05).
Levels of cell necrosis and karyopyknosis were lower in the HSV-TK
group, which showed higher levels of necrosis than the PBS and ATRA
groups (P<0.05). Original magnification, x200; scale bar=50
µm. Compared with the (E) untreated group, protein levels of
Cx32 were higher in the (F) ATRA-treated group (P<0.01). (G)
Quantification of results. Compared with the other groups, there
were fewer hepatic tumor cells in the HSV-TK+ATRA group
(P<0.05). (H) Cx32 protein level was increased in the
ATRA-treated tumor group (P<0.01), compared with the control
group (untreated group). Original magnification, x400; scale bar=25
µm. Cx32, connexin32BS, phosphate-buffered saline; ATRA,
trans-retinoic acid; HSV-TK, herpes simplex virus-thymidine
kinase. |
Discussion
HCC is one of the most common types of malignancy,
which has a poor prognosis worldwide and frequently recurs
following surgical or nonsurgical treatments, including
transarterial chemoembolization, radiofrequency ablation,
percutaneous ethanol injection therapy and chemotherapy (17). Newly established treatment methods,
including gene therapy, can be combined with traditional treatments
to destroy the tumor tissues. Prodrug/suicide gene therapy, which
delivers a 'suicide' gene to target cells and renders them
sensitive to a specific prodrug, is a promising strategy for the
treatment of malignant tumors (18). The HSV-TK/GCV system is one of the
most well-characterized systems and has been successfully used
in vitro and in vivo for the treatment of
malignancies (19–21). Numerous studies have shown that the
treatment effect of the HSV-TK/GCV suicide gene system is closely
associated with the transfection efficiency of the TK gene and the
bystander effect (22,23).
The bystander effect of this system is explained by
gap junctions, which transfer the GCV-triphosphate and other toxic
metabolites among neighboring cells (24). GJIC has long been considered to be
important in maintaining the bystander effect, wherein significant
tumor regression can be achieved via bystander effects mediated by
GJICs (25) composed of Cx
protein.
GJIC is involved in the bystander effect of the
HSV-TK/GCV system, possibly by allowing the passage of
phosphorylated GCV metabolites between HSVTK + and HSVT cells. Gap
junctions are formed and maintained by Cx. The normal expression
and correct localization of Cx in the cell membrane between
neighboring cells is necessary to functionally channel GJIC.
Therefore, GJIC is the primary mechanism for the bystander effect
of the suicide gene. However, in the liver, Cx32 is the major and
specific Cx, and is often aberrantly located or reduced in tumor
states, which leads to loss of GJIC function (26). GJIC has long been considered to be
important in maintaining homeostasis and the control of cell growth
(27). GJIC predominantly involves
three connexins, Cx26, Cx32 and Cx43, depending on the cell type or
cell position in the lobule (8).
The expression of Cx32 is relatively specific in liver cells and is
also one of the major gap junction proteins in hepatoma (28). In the present study, the expression
of Cx32 was markedly increased in groups C and D, and the apoptotic
effect of the microbubble-mediated pIRES2-EGFP-HSV-TK suicide gene
transfection via ultrasonic radiation was enhanced in vitro
and in vivo, compared with groups A and B.
ARTA is important in a broad spectrum of biological
processes, including inhibition of proliferation, regulation of
apoptosis, induction of differentiation and control of development
(29). ATRA is an ideal chemical
inducer of GJIC and has a wide range of biological actions. Through
binding to its receptors and a post-translational mechanism of
action, ATRA antagonizes the effects of two serine/threonine
protein kinase families, protein kinase C and mitogen-activated
protein kinase. This results in the phosphorylation of Cx43 and/or
other Cx proteins, including Cx26 (30). In addition, it has been shown that
ATRA enhances the tumoricidal effect of HSVTK/GCV suicide gene
therapy against Daoy MB cells, by strengthening the bystander
effect in vitro and in vivo (31). Similarly, the results of the
present study revealed that ATRA significantly increased the
expression of Cx32 in the HepG2 cells (Fig. 4F), and significantly enhanced the
bystander effect of the pIRES2-EGFP-HSV-TK/GCV system in the HepG2
cells (Figs. 2 and 3).
The results of the present study suggested that the
HSV-TK/GCV suicide gene therapy system, mediated by Cx32 and
combined with ATRA as an adjuvant, may have important implications
for HCC treatment. The results of the in vitro experiments
demonstrated a markedly enhanced apoptotic effect in the
ATRA-treated cells, compared with ATRA-untreated cells, at the same
proportion of HSVTK+ cells. In vivo, the tumor
inhibition rate was 37.97% when treated with the HSV-TK suicide
gene only, whereas the tumor inhibition rate was 59.4% in the
HSV-TK+ATRA group. In addition the expression of Cx32 in tumor
tissues or cells treated with ATRA was increased, with localization
in a normal position. However, despite the experiments performed in
the present study, a number of questions remain, including how ATRA
increased the expression of Cx, and whether other Cx proteins are
involved in the antitumor effect. However, the potential side
effects of ATRA may be reduced through use of the HSV-TK/GCV
suicide gene system.
In the present study, an ultrasound mircobubble was
used as the gene vector. The ultrasound microbubble-mediated
delivery system has been used as a novel and effective gene
delivery method (32–34). The ultrasound microbubbles-mediated
HSV-TK suicide gene system not only improves gene targeting, but
also increases the gene transfection efficiency due to the features
of ultrasound and microbubbles. Ultrasound-targeted microbubble
destruction technology is expected to become a novel gene delivery
technique and may provide a novel strategy for targeted cancer
therapy.
In conclusion, the results of the present study
supported the suggested that the bystander effect ATRA, combined
with delivery of the pIRES2-EGFP-HSV-TK/GCV system, can be an
effective treatment for HCC. The mechanism appears to involve the
induction of death of the proliferative HepG2 cells by enhancing
the function of GJIC. The bystander effect may provide additional
beneficial effects to that of promoter selectivity by eliminating
neighboring, but uninfected, target cells (35). The clinical use of this type of
gene therapeutic regimen require further investigation, and the
formulation of the correct combination of therapeutic regimens and
prediction of curative effects are also essential.
Acknowledgments
The present study was supported by the National
Natural Scientific Foundation of China (grant no. 81272570), the
National Natural Science Fund for Young Scholars (grant no.
81301975) and the Natural Science Foundation of Hubei Province of
China (grant nos. 2014CFB310 and 2015CFB615).
References
|
1
|
Liao YJ, Fang CC, Yen CH, Hsu SM, Wang CK,
Huang SF, Liang YC, Lin YY, Chu YT and Arthur Chen YM: Niemann-Pick
type C2 protein regulates liver cancer progression via modulating
ERK1/2 pathway: Clinicopathological correlations and therapeutical
implications. Int J Cancer. 137:1341–1351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qu L, Wang Y, Gong L, Zhu J, Gong R and Si
J: Suicide gene therapy for hepatocellular carcinoma cells by
survivin promoter-driven expression of the herpes simplex virus
thymidine kinase gene. Oncol Rep. 29:1435–1440. 2013.PubMed/NCBI
|
|
3
|
Yu BF, Wu J, Zhang Y, Sung HW, Xie J and
Li RK: Ultrasound targeted HSVTK and Timp3 gene delivery for
synergistically enhanced antitumor effects in hepatoma. Cancer Gene
Ther. 20:290–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao J, Zhang G, Qiu P, Liu X, Wu Y, Du B,
Li J, Zhou J, Li J and Tan Y: Tanshinone IIA increases the
bystander effect of herpes simplex virus thymidine
kinase/ganciclovir gene therapy via enhanced gap junctional
intercellular communication. PLoS One. 8:e676622013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wygoda MR, Wilson MR, Davis MA, Trosko JE,
Rehemtulla A and Lawrence TS: Protection of herpes simplex virus
thymidine kinase-transduced cells from ganciclovir-ediated
cytotoxicity by bystander cells: The Good Samaritan effect. Cancer
Res. 57:1699–1703. 1997.PubMed/NCBI
|
|
6
|
Lawrence TS, Rehemtulla A, Ng EY, Wilson
M, Trosko JE and Stetson PL: Preferential cytotoxicity of cells
transduced with cytosine deaminase compared to bystander cells
after treatment with 5-flucytosine. Cancer Res. 58:2588–2593.
1998.PubMed/NCBI
|
|
7
|
McLachlan E, Shao Q, Wang HL, Langlois S
and Laird DW: Connexins act as tumor suppressors in
three-dimensional mammary cell organoids by regulating
differentiation and angiogenesis. Cancer Res. 66:9886–9894. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maes M, Crespo Yanguas S, Willebrords J
and Vinken M: Models and methods for in vitro testing of hepatic
gap junctional communication. Toxicol In Vitro. 30:569–577. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia-Rodríguez L, Pérez-Torras S, Carrió
M, Cascante A, García-Ribas I, Mazo A and Fillat C: Connexin-26 is
a key factor mediating gemcitabine bystander effect. Mol Cancer
Ther. 10:505–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banoub RW, Fernstrom M, Malkinson AM and
Ruch RJ: Enhancement of gap junctional intercellular communication
by dibutyryl cyclic AMP in lung epithelial cells. Anticancer Res.
16:3715–3719. 1996.PubMed/NCBI
|
|
11
|
Trottier C, Colombo M, Mann KK, Miller WH
Jr and Ward BJ: Retinoids inhibit measles virus through a type I
IFN-dependent bystander effect. FASEB J. 23:3203–3212. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolf G: Tissue-specific increases in
endogenous all-trans retinoic acid: Possible contributing factor in
ethanol toxicity. Nutr Rev. 68:689–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou S, Li S, Liu Z, Tang Y, Wang Z, Gong
J and Liu C: Ultrasound-targeted microbubble destruction mediated
herpes simplex virus-thymidine kinase gene treats hepatoma in mice.
JExp Clin Cancer Res. 29:1702010. View Article : Google Scholar
|
|
14
|
Wu L, Fu Z, Zhou S, Gong J, Liu CA, Qiao Z
and Li S: HIF-1α and HIF-2α: Siblings in promoting angiogenesis of
residual hepatocellular carcinoma after high-intensity focused
ultrasound ablation. PLoS One. 9:e889132014. View Article : Google Scholar
|
|
15
|
Wang ZX, Wang ZG, Ran HT, Ren JL, Zhang Y,
Li Q, Zhu YF and Ao M: The treatment of liver fibrosis induced by
hepatocyte growth factor-directed, ultrasound-targeted microbubble
destruction in rats. Clin Imaging. 33:454–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aoi A, Watanabe Y, Mori S, Takahashi M,
Vassaux G and Kodama T: Herpes simplex virus thymidine
kinase-mediated suicide gene therapy using nano/microbubbles and
ultrasound. Ultrasound Med Biol. 34:425–434. 2008. View Article : Google Scholar
|
|
17
|
Wang P, Sheng L, Wang G, Wang H, Huang X,
Yan X, Yang X and Pei R: Association of transarterial
chemoembolization with survival in patients with unresectable
hepatocellular carcinoma. Mol Clin Oncol. 2:203–206.
2014.PubMed/NCBI
|
|
18
|
Vachani A, Moon E, Wakeam E and Albelda
SM: Gene therapy for mesothelioma and lung cancer. Am J Respir Cell
Mol Biol. 42:385–393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Määttä AM, Samaranayake H, Pikkarainen J,
Wirth T and Ylä-Herttuala S: Adenovirus mediated herpes simplex
virus-thymidine kinase/ganciclovir gene therapy for resectable
malignant glioma. Curr Gene Ther. 9:356–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakinoki K, Nakamoto Y, Kagaya T,
Tsuchiyama T, Sakai Y, Nakahama T, Mukaida N and Kaneko S:
Prevention of intra-hepatic metastasis of liver cancer by suicide
gene therapy and chemokine ligand 2/monocyte chemoattractant
protein-1 delivery in mice. J Gene Med. 12:1002–1013. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu DS, Zhao W, Huang HZ, Hu XW, Liu XQ and
Tang HK: Synthetic radiation-inducible promoters mediated
HSV-TK/GCV gene therapy in the treatment of oral squamous cell
carcinoma. Oral Dis. 16:445–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Tan Q, Ding Z and Liu D: Mechanism
of DADS in the bystander effect of HSV-TK/GCV suicide gene therapy
system in lens epithelial cells. Zhong Nan Da Xue Xue Bao Yi Xue
Ban. 36:329–334. 2011.In Chinese. PubMed/NCBI
|
|
23
|
Yang J, Liu TJ, Jiang YX and Lu Y: ATRA
enhances the bystander effect of suicide gene therapy driven by the
specific promoter LEP 503 in human lens epithelial cells. Mol Vis.
18:2053–2066. 2012.PubMed/NCBI
|
|
24
|
Yang L, Chiang Y, Lenz HJ, Danenberg KD,
Spears CP, Gordon EM, Anderson WF and Parekh D: Intercellular
communication mediates the bystander effect during herpes simplex
thymidine kinase/ganciclovir-based gene therapy of human
gastrointestinal tumor cells. Hum Gene Ther. 9:719–728. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sato T, Neschadim A, Lavie A, Yanagisawa T
and Medin JA: The engineered thymidylate kinase (TMPK)/AZT
enzymeprodrug axis offers efficient bystander cell killing for
suicide gene therapy of cancer. PLoS One. 8:e787112013. View Article : Google Scholar
|
|
26
|
Tang N, Wang Q, Wu D, Zhang S, Zhang Y and
Tao L: Differential effects of paclitaxel and docetaxel on gap
junctions affects their cytotoxicities in transfected HeLa cells.
Mol Med Rep. 8:638–644. 2013.PubMed/NCBI
|
|
27
|
Cronier L, Crespin S, Strale PO, Defamie N
and Mesnil M: Gap junctions and cancer: New function for an old
story. Antioxid Redox Signal. 11:323–338. 2009. View Article : Google Scholar
|
|
28
|
Thévenin AF, Kowal TJ, Fong JT, Kells RM,
Fisher CG and Falk MM: Proteins and mechanisms regulating
gap-junction assembly, internalization and degradation. Physiology
(Bethesda). 28:93–116. 2013. View Article : Google Scholar
|
|
29
|
Lin SC, Dollé P, Ryckebüsch L, Noseda M,
Zaffran S, Schneider MD and Niederreither K: Endogenous retinoic
acid regulates cardiac progenitor differentiation. Proc Natl Acad
Sci USA. 107:9234–9239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Liu TJ, Jiang YX and Lu Y: ATRA
enhances the bystander effect of suicide gene therapy driven by the
specific promoter LEP 503 in human lens epithelial cells. Mol Vis.
18:2053–2066. 2012.PubMed/NCBI
|
|
31
|
Li S, Gao Y, Pu K, Ma L, Song X and Liu Y:
All-trans retinoic acid enhances bystander effect of suicide-gene
therapy against medulloblastomas. Neurosci Lett. 503:115–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Panje CM, Wang DS and Willmann JK:
Ultrasound and microbubble-mediated gene delivery in cancer:
Progress and perspectives. Invest Radiol. 48:755–769. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sorace AG, Saini R, Rosenthal E, Warram
JM, Zinn KR and Hoyt K: Optical fluorescent imaging to monitor
temporal effects of microbubble-mediated ultrasound therapy. IEEE
Trans Ultrason Ferroelectr Freq Control. 60:281–289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sorace AG, Warram JM, Umphrey H and Hoyt
K: Microbubble-mediated ultrasonic techniques for improved
chemotherapeutic delivery in cancer. J Drug Target. 20:43–54. 2012.
View Article : Google Scholar :
|
|
35
|
Chen Y, Wang G, Kong D, Zhang Z, Yang K,
Liu R, Zhao W and Xu Y: Double-targeted and double-enhanced suicide
gene therapy mediated by generation 5 polyamidoamine dendrimers for
prostate cancer. Mol Carcinog. 52:237–246. 2013. View Article : Google Scholar
|