Introduction
Esophageal squamous cell carcinoma (ESCC), a highly
lethal malignancy, is the eighth most common cancer worldwide and
the sixth most common cause of cancer-associated mortality
(1). Furthermore, ESCC has become
one of the most common types of malignant tumor in China, Japan and
Southeast Africa (2,3). In China, ESCC is the predominant
subtype and contributes to ~90% of all esophageal cancers (ECs)
(4,5). Despite the use of multimodal
treatments, such as radical surgery, chemotherapy and radiotherapy,
the overall prognosis for ESCC remains poor, with 5-year survival
rates of 5–45% (6–8). Although previous studies have
demonstrated that alterations of numerous oncogenes and
tumor-suppressor genes are involved in ESCC, the underlying
molecular and genetic mechanism of esophageal carcinogenesis
remains largely unknown (9).
Long non-coding RNAs (lncRNAs), with transcripts
>200 nt in length, which were initially recognized to represent
random transcriptional noise, have been implicated in numerous
biological behaviors, such as epigenetic regulation, chromatin
modification, transcription and post-transcriptional processing
(10–12). Increasing evidence has revealed the
contribution of lncRNAs as proto-oncogenes, tumor suppressor genes
and drivers of metastatic transformation (13–15).
lncRNA-Low Expression in Tumor (lncRNA-LET), a
recently identified lncRNA located at chromosome 15q24.1, was
initially established to be downregulated in hepatocellular
carcinoma (16). Recently, it was
demonstrated to be vital in the development and progression of
gallbladder cancer (GBC) (17).
However, the prognostic role of lncRNA-LET in cancer remains
unknown and to date, to the best of our knowledge, no data were
available regarding the lncRNA-LET expression level and biological
role in human ESCC.
In the present study, the expression level of
lncRNA-LET was demonstrated to be significantly decreased in ESCC
tissues when compared with that of adjacent healthy tissues. Its
correlation with clinicopathological factors in ESCC patients was
also evaluated. Using ESCC cell lines, overexpression of lncRNA-LET
by lentivirus-mediated gene transfection was investigated and
observed to induce apoptosis, and inhibit invasion and
proliferation. In addition, the present study verified that
overexpression of lncRNA-LET induced the activation of p53. Thus,
the current study indicates that lncRNA-LET has a significant role
in ESCC development and may be considered as a potential prognostic
factor for the prediction of clinical outcomes in ESCC
patients.
Materials and methods
ESCC specimens
A total of 48 ESCC patients that underwent
esophagectomy at The First Affiliated Hospital of Nanjing Medical
University (Nanjing, China) between 2012 and 2013 were enrolled in
the present study. Tumor specimens and paired healthy esophageal
tissue specimens, obtained from a site distant to the cancerous
lesion, were immediately snap-frozen in liquid nitrogen and stored
at −80°C until total RNA was extracted. No radiotherapy or
chemotherapy was conducted in these patients prior to surgery. The
clinical data, including age, gender, pathological stage, grade,
tumor location and lymph node metastasis were acquired from the
medical records. Patients were classified according to criteria set
by the World Health Organization (18) and were staged according to the
tumor-lymph node-metastasis (TNM) classification system, in which T
refers to the size of the ESCC and whether it has invaded nearby
tissue, N refers to whether or not regional lymph nodes are
involved, and M refers to distant metastasis (19). The study was approved by the
Research Ethics Committee of Nanjing Medical University. Informed
consent was obtained from all of the patients.
Cell culture
Human ESCC cell lines, Eca109 and TE-1 were
purchased from the Institute of Biochemistry and Cell Biology of
the Chinese Academy of Sciences (Shanghai, China). Cells were
cultured in RPMI-1640 medium (both purchased from Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA), 100
U/ml penicillin and 100 mg/ml streptomycin (Gibco: Thermo Fisher
Scientific, Inc.), within a humidified atmosphere containing 5%
CO2 at 37°C. The duration of the culture was 7 days, and
the medium was changed every 2 days.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA from the tissues and cells was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Briefly, 1 ml Trizol was
used to lyse cells (5×106 cells/well), then 0.2 ml of
chloroform was added and the cells were incubated at room
temperature for 3 min. After centrifugation at 12,000 × g at 4°C
for 15 min, the RNA aqueous phase was transferred to a fresh tube.
Then, 0.5 ml isopropanol per 1 ml TRIzol was added, and cells were
incubated for 10 min at room temperature, followed by
centrifugation at 10,000 × g at 4 °C for 10 min. After washing the
RNA pellet with 75% ethanol, the RNA was dissolved in 0.03 ml
RNase-free water and incubated for 10 min at 55°C. RNA was reverse
transcribed into cDNA using the PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). The following thermal
cycling protocol was used for reverse transcription: 37°C for 15
min for 3 cycles, followed by 85°C for 5 sec. The cDNA template was
amplified by qPCR using the SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. Briefly, 5 µl 10-fold cDNA was mixed with 10
µl SYBR Premix Ex Taq and 4 nmol of primer in a volume of 20
µl. The following thermal cycle was followed: 30 sec at
95°C, followed by 40 cycles of 95°C for 5 sec, and 60°C for 35
sec.
The relative levels of LET were determined by
RT-qPCR using gene specific primers. Ornithine decarboxylase
antizyme (OAZ-1) served as an internal control, and the lncRNA-LET
values were normalized to OAZ-1. The RT-qPCR reactions and data
collection were performed on a StepOne Plus™ Real-Time PCR system
(Thermo Fisher Scientific, Inc.). The relative expression fold
change of mRNAs was calculated using the 2−ΔΔCq method
(20). The primer sequences
(Sangon Biotech Co., Ltd., Shanghai, China) were as follows:
Forward, 5′-CGA GGA CAG AGC CGC CTT-3′ and reverse,
5′-GACAAACCCAGGCGAGATGA-3′ for OAZ-1; forward,
5′-GTTGTTGTTGCATTGGGGT-3′ and reverse, 5′-AAGATGGAGAGTGGAGCCT-3′
for lncRNA-LET.
Plasmid and transfection
The sequence of LET was synthe-sized and subcloned
into PLL3.7-EF-1a-SV40pA (Genewiz, Inc., Suzhou, China), and the
expression level of LET was detected by RT-qPCR.
Cells were grown on six-well plates to 70%
confluence and then transfected with the PLL3.7-EF-1a-SV40pA using
10 mg/ml polybrene (HanBio, Shanghai, China) according to the
manufacturer's instructions. Cells were harvested after 48 h for
RT-qPCR and western blot analysis.
Cell migration and invasion assay
Cells (5×105 cells/well) were seeded in
six-well plates and cultured in RPMI-1640 medium. After 48 h, cell
layers were wounded using the tip of 200 µl pipette. After
washing cells 3 times with PBS, the serum-free RPIM-1640 medium was
added to the plates and incubated within a humidified atmosphere
containing 5% CO2 at 37°C for 48 h. Wound closure was
observed under a light microscope (DFC500; Leica, Wetzlar, Germany)
and measured using AxioVision version 4.7 software (Carl Zeiss
Meditec, Dublin, CA, USA).
For the invasion assays, transwell apparatus was
used with a polycarbonate membrane (pore size, 8 µm) Boyden
chamber insert (EMD Millipore, Billerica, MA, USA) to measure cell
motility. The transfected cells and wild-type cells were treated
with trypsin/EDTA solution (Sigma-Aldrich) and washed once with
serum-containing RPMI-1640 medium. A total of 1×105
cells in 0.2 ml serum-free RPMI-1640 medium were seeded on
transwell apparatus. Each insert was precoated with 45 µg
Matrigel (BD Biosciences; San Jose, CA, USA). Prior to examination,
the chambers were incubated for 24 h at 37°C in a 5% CO2
incubator, in culture medium with 10% FBS in the lower chambers.
The cells on the upper surface were scraped using cotton buds and
washed away with PBS, whereas the invaded cells on the lower
surface were fixed in 100% precooling methanol (Sigma-Aldrich) for
10 min, stained with 0.05% crystal violet (Sigma-Aldrich) for 30
min, then rinsed in phosphate-buffered saline (PBS; Thermo Fisher
Scientific, Inc.) and subjected to microscopic inspection (DFC500;
Leica). Finally, the values for invasion were obtained by counting
three fields per membrane. Experiments were independently repeated
in triplicate.
Cell proliferation assay
The 5-ethynyl-20-deoxyuridine (EdU) detection kit
(Guangzhou RiboBio Co., Ltd., Guangzhou, China) was used to
evaluate cell proliferation. According to the manufacturer's
instructions, cells were grown on six-well plates to 70%
confluence, treated with 50 µM EdU for 2 h at 37°C and fixed
with PBS containing 4% paraformaldehyde (Sigma-Aldrich) for 30 min
at room temperature. After cells were incubated with 2 mg/ml
glycine (Abcam, Cambridge, UK) for 5 min at room temperature, they
were treated with 0.5% Triton X-100 (Sigma-Aldrich) for 10 min and
stained with 1X Apollo reaction cocktail (Guangzhou RiboBio Co.,
Ltd.) for 30 min at room temperature. After one wash with 0.5%
Triton X-100 in PBS, 1X Hoechst 33342 (Thermo Fisher Scientific,
Inc.) was used to incubate cells at room temperature for 30 min.
Images were captured under a confocal laser scanning microscope
(LEXT OLS3100; Olympus America, Inc., Center Valley, PA, USA). The
assay was repeated in triplicate.
Flow cytometric analysis
The effect of LET treatment on cell apoptosis was
determined by flow cytometry. Cells transfected with
PLL3.7-EF-1a-SV40pA were plated in six-well plates for 48 h. The
cells harvested and fixed in 70% ice-cold ethanol for 24 h were
collected and analyzed for cell apoptosis using a flow cytometer
(FACSCalibur; BD Biosciences). In addition, the cells were
harvested and stained with Annexin V/propidium idodide (PI), using
the Annexin V-fluorescein isothiocyanate apoptosis detection kit
(KGI Biotechnology Co., Ltd., Nanjing, China).
Western blot analysis
Cells harvested from six-well culture plates were
lysed using mammalian protein extraction reagent RIPA (Beyotime
Institute of Biotechnology, Haimen, China) supplemented with
protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland)
and phenylmethylsulfonyl fluoride (Roche Diagnostics). The lysates
were then collected and subjected to ultrasonication (Q700
Sonicator; Misonix, Inc., Farmingdale, NY, USA) and centrifugation
at 14m000 × g for 20 min. The supernatants were collected, and
protein content was determined using a Bradford assay (Abcam).
Protein extractions (50 µg) were separated by 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (90 min at 100
V; Abcam), then transferred to Immobilon-P polyvinylidene fluoride
membranes (EMD Millipore) and incubated with specific antibodies. A
GAPDH antibody served as a control. An enhanced chemiluminescence
chromogenic substrate (SignalFire ECL Reagent; Cell Signaling
Technology, Inc., Danvers, MA, USA) was used to visualize the bands
with a chemiluminescent detection system (Pierce ECL Substrate
Western Substrate; Thermo Fisher Scientific, Inc.) and then exposed
in a Molecular Imager ChemiDoc XRS system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The intensity of the bands was quantified
using Image J software (version 1.42; National Institutes of
Health, Bethesda, MD, USA). The rabbit anti-human GAPDH monoclonal
antibody (1:5,000; cat. no. 14C10) and rabbit anti-human p53
polyclonal antibody (1:1,000; cat. no. 9282) were purchased from
Cell Signaling Technology, Inc.. The secondary goat anti-rabbit
horseradish peroxidase-conjugated polyclonal antibody (1:2,000;
cat. no. ab6721) was purchased from Abcam. All experiments were
performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
(IBM SPSS, Armonk, NY, USA). Statistical significance was evaluated
by Student's t-test or a χ2 test as appropriate.
Survival analysis was performed using the Kaplan-Meier method, and
the log-rank test was used to compare the differences between
patient groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
lncRNA-LET expression level is
downregulated in ESCC specimens, and is correlated with
pathological grade, tumor stage and lymph node metastasis
The expression levels of lncRNA-LET in 48 pairs of
human ESCC and adjacent non-tumor tissue samples were examined by
RT-qPCR and normalized to GAPDH. The lncRNA-LET expression level
was identified to be significantly downregulated in tumor tissue
samples when compared with non-tumor tissue samples. Furthermore,
correlation analysis of lncRNA-LET expression levels with
clinicopathological features of ESCC patients indicated that the
expression level of lncRNA-LET was significantly correlated with
the pathological grade (P=0.043), clinical stage (P=0.034) and
lymph node metastasis (P=0.024); while there was no significant
correlation between lncRNA-LET expression level and gender, age or
tumor location (Table I). These
results indicate that lncRNA-LET may be involved in the progression
and metastasis of ESCC.
 | Table IlncRNA-LET expression and
clinicopathological characteristics in esophageal squamous cell
carcinoma. |
Table I
lncRNA-LET expression and
clinicopathological characteristics in esophageal squamous cell
carcinoma.
| Characteristic | Cases | lncRNA-LET expression
| P-value |
|---|
| Low | High |
|---|
| Gender | | | | 0.883 |
| Male | 27 | 16 | 11 | |
| Female | 21 | 12 | 9 | |
| Age (years) | | | | 0.915 |
| <60 | 34 | 20 | 14 | |
| ≥60 | 14 | 8 | 6 | |
| Histological
grade | | | | 0.043 |
| Well
differentiateda | 15 | 5 | 10 | |
| Moderately
differentiatedb | 16 | 10 | 6 | |
| Poorly
differentiatedc | 17 | 13 | 4 | |
| T stage | | | | 0.034 |
| T1-2d | 18 | 7 | 11 | |
| T3-4e | 30 | 21 | 9 | |
| Lymph node
metastasis | | | | 0.024 |
| Negative | 22 | 9 | 13 | |
| Positive | 26 | 19 | 7 | |
| Tumor location | | | | 0.575 |
| Upper and middle
1/3 | 31 | 19 | 12 | |
| Lower 1/3 | 17 | 9 | 8 | |
lncRNA-LET inhibits ESCC cell migration
and invasion in vitro
To investigate the biological role of lncRNA-LET in
ESCC progression, Eca109 and TE-1 cells were transfected with
PLL3.7-EF-1a-SV40pA-LET. The transfection efficiency was validated
using RT-qPCR (Fig. 1). Using a
wound healing assay, the relative migrating distance of cells was
identified to be significantly reduced in wild-type ESCC cells as
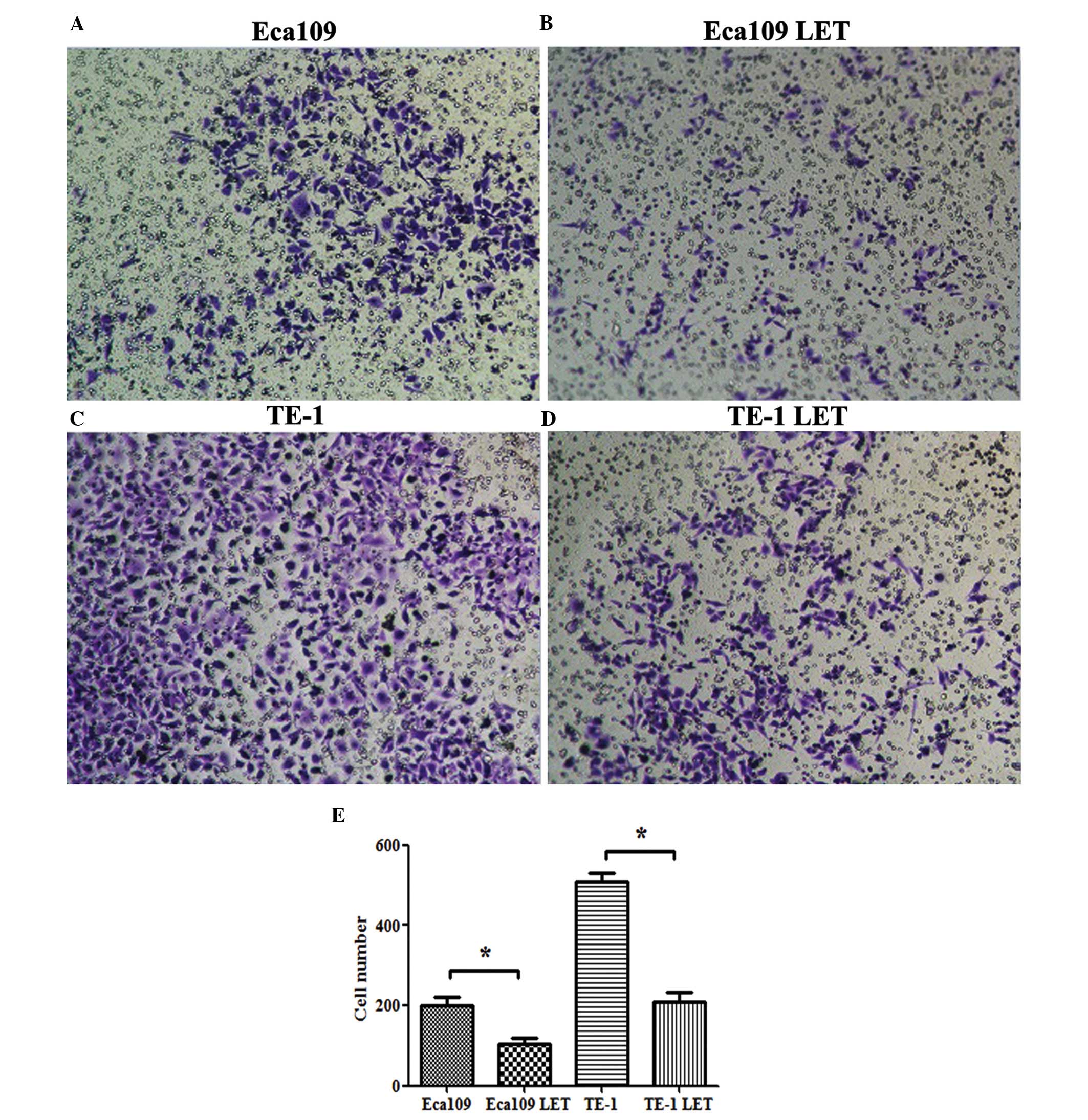
compared with the PLL3.7-EF-1a-SV40pA-LET-inf ected cells (Fig. 2). In addition, a Matrigel-coated
transwell assay demonstrated that the numbers of PLL3.7-EF-1a-SV4
0pA-LET-infected Eca109 (104 cells) and TE-1 (208 cells) cells that
invaded through the Matrigel were significantly less than those of
the wild-type Eca109 (200 cells) and TE-1 (509 cells) cells
(P<0.05; Fig. 3). These results
indicate that lncRNA-LET suppresses the invasive phenotype of ESCC
cells in vitro.
lncRNA-LET inhibits ESCC cell
proliferation in vitro
To determine whether LET overexpression affected
ESCC growth, the EdU assay was performed on ESCC cells infected
with PLL3.7-EF-1a-SV40pA-LET. The percentages of EdU-positive cells
(cells in the S phase of the cell cycle) in the
PLL3.7-EF-1a-SV40pA-LET-infected groups were not identified to be
significantly lower than those of the wild-type groups (P>0.05;
Fig. 4).
lncRNA-LET inhibits ESCC cell
proliferation via inducing apoptosis
To evaluate the effect of lncRNA-LET on tumor cell
apoptosis, Eca109 and TE-1 cells were employed as the model system.
It was hypothesized that lncRNA-LET overexpression induced cell
apoptosis in ESCC cells. The apoptotic rate of Eca109 and TE-1
cells transfected with PLL3.7-EF-1a-SV40pA-LET was identified to be
significantly increased when compared with those of the wild-type
ESCC cells (Fig. 5). This
indicates that upregulation of LET induces ESCC cell apoptosis
in vitro.
lncRNA-LET induces activation of the p53
protein
Recent studies have demonstrated that numerous
lncRNAs may participate in the regulation of cell growth by
modulating the p53 signaling pathway (21–24).
To further investigate whether, and the mechanism by which, LET
induces ESCC cell growth arrest and apoptosis, the protein level of
p53 was examined following transfection of PLL3.7-EF-1a-SV40pA-LET
in wild-type ESCC cells. The results of western blot analysis
indicated that the expression level of p53 was significantly
increased in ESCC cells transfected with PLL3.7-EF-1a-SV40pA-LET
when compared with that of wild-type ESCC cells (Fig. 6). These data indicate that LET
functions as a tumor suppressor gene by activating p53 in ESCC
cells.
Discussion
Recent studies have revealed that lncRNAs
participate in a multitude of biological processes, such as
chromatin modification, transcription and post-transcriptional
processing (10,12,25,26).
In addition, previous studies have demonstrated that dysregu-lation
of lncRNAs may also affect epigenetic information and provide a
cellular growth advantage, resulting in a wide range of diseases,
particularly in progressive and uncontrolled tumor growth (27,28).
HOX transcript antisense RNA, a well known lncRNA involved in tumor
pathogenesis, has been consistently upregulated and identified as a
strong prognosis marker of patient outcomes in various types of
human cancer (14,28-30).
H19, encoded by an imprinted gene, has been verified to be
upregulated in tumors and to possess oncogenic properties (31-33).
Maternally expressed gene 3 (MEG3) is located at chromosome 14q32,
and a loss of MEG3 expression has been observed in a number of
primary human tumors, including glioma, hepatocellular cancers,
non-small cell lung cancer, and gastric cancer (34-37).
Although evidence of the carcinogenicity of these lncRNAs is
strong, the molecular mechanism regarding tumor development and the
promotion of metastasis is not fully understood.
lncRNA-LET, as a novel lncRNA molecule, was
initially well known for its downregulation in primary
hepatocellular carcinoma, wherein lncRNA-LET suppresses cancer
invasiveness and metastasis (16).
Furthermore, downregulation of lncRNA was examined in squamous-cell
lung carcinoma and colon carcinoma tissues and compared with their
paired healthy primary tissues (16). In addition, there were findings
indicating that lncRNA-LET may represent a prognostic marker and
potential therapeutic target for GBC (17). Due to the observation that
lncRNA-LET is involved with dysregulation during cancer
progression, the biological role of lncRNA-LET in ESCC progression
was investigated in the present study and its clinical significance
was analyzed.
The present study indicated that the expression
level of lncRNA-LET was downregulated in ESCC tissue samples when
compared with adjacent healthy tissue samples. Furthermore, the low
expression level of lncRNA-LET in ESCC tissue samples was
demonstrated to be closely associated with clinicopathological
features. Low expression levels of lncRNA-LET were correlated with
poorly differentiated histology, higher tumor grade and positive
nodal status. Similarly, overexpression of lncRNA-LET expression
was identified to inhibit the migration and invasion of ESCC cells,
and also significantly increased the response of ESCC cells to cell
apoptosis induction.
p53, as a master regulator for gene expression,
directly or indirectly regulates the expression of numerous target
genes, which leads to the suppression of tumor development and
growth by blocking cell proliferation or by activating cell death
programs (36,38). The present study examined whether
lncRNA-LET affects the expression level of p53 protein to further
investigate the underlying mechanisms by which lncRNA-LET induced
cell growth arrest and apoptosis. Overexpression of lncRNA-LET was
identified to significantly increase the level of p53 protein when
compared with that of the controls.
In conclusion, the loss of lncRNA-LET expression was
demonstrated to be a common occurrence underlying EC, suggesting
that lncRNA-LET may perform a key functional role in suppressing
the invasive and metastatic behavior of ESCC cells. These findings
indicate that lncRNA-LET may function as a tumor suppressor and its
deficiency or decreased expression may contribute to ESCC
development. Furthermore, lncRNA-LET may be exploited in a
promising therapeutic approach for the treatment of EC, and
potentially be useful as a novel prognostic marker for EC.
Acknowledgments
The present study was supported by the National
Natural Science Foundation (grant no. 81170158) and the science and
technology project of Jiangsu Provincial Health Department (grant
no. H200821).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Law S and Wong J: Current management of
esophageal cancer. J Gastrointest Surg. 9:291–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hiyama T, Yoshihara M, Tanaka S and
Chayama K: Genetic polymorphisms and esophageal cancer risk. Int J
Cancer. 121:1643–1658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li T, Lu ZM, Chen KN, Guo M, Xing HP, Mei
Q, Yang HH, Lechner JF and Ke Y: Human papillomavirus type 16 is an
important infectious factor in the high incidence of esophageal
cancer in Anyang area of China. Carcinogenesis. 22:929–934. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pohl H and Welch HG: The role of
overdiagnosis and reclassification in the marked increase of
esophageal adenocarcinoma incidence. J Natl Cancer Inst.
97:142–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv XB, Lian GY, Wang HR, Song E, Yao H and
Wang MH: Long noncoding RNA HOTAIR is a prognostic marker for
esophageal squamous cell carcinoma progression and survival. PLoS
One. 8:e635162013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roder JD, Busch R, Stein HJ, Fink U and
Siewert JR: Ratio of invaded to removed lymph nodes as a predictor
of survival in squamous cell carcinoma of the oesophagus. Br J
Surg. 81:410–413. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montesano R, Hollstein M and Hainaut P:
Genetic alterations in esophageal cancer and their relevance to
etiology and pathogenesis: A review. Int J Cancer. 69:225–235.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tripathi V, Shen Z, Chakraborty A, Giri S,
Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A and
Prasanth KV: Long noncoding RNA MALAT1 controls cell cycle
progression by regulating the expression of oncogenic transcription
factor B-MYB. PLoS Genet. 9:e10033682013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aguilo F, Zhou MM and Walsh MJ: Long
noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a
expression. Cancer Res. 71:5365–5369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang F, Huo XS, Yuan SX, Zhang L, Zhou WP,
Wang F and Sun SH: Repression of the long noncoding RNA-LET by
histone deacetylase 3 contributes to hypoxia-mediated metastasis.
Mol Cell. 49:1083–1096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma MZ, Kong X, Weng MZ, Zhang MD, Qin YY,
Gong W, Zhang WJ and Quan ZW: Long non-coding RNA-LET is a positive
prognostic factor and exhibits tumor-suppressive activity in
gallbladder cancer. Mol Carcinog. 54:1397–1406. 2015. View Article : Google Scholar
|
|
18
|
World Health Organization: Obesity:
Preventing and managing the global epidemic. Report of a WHO
consultation. World Health Organ Tech Rep Ser. 894:i–xii. 1–253.
2000.
|
|
19
|
Takeno S, Noguchi T, Takahashi Y, Fumoto
S, Shibata T and Kawahara K: Assessment of clinical outcome in
patients with esophageal squamous cell carcinoma using TNM
classification score and molecular biological classification. Ann
Surg Oncol. 14:1431–1438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang A, Xu M and Mo YY: Role of the
IncRNA-p53 regulatory network in cancer. J Mol Cell Biol.
6:181–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Q, Huang JG, Zhou N, Zhang Z, Zhang A,
Lu Z, Wu F and Mo YY: LncRNA loc285194 is a p53-regulated tumor
suppressor. Nucleic Acids Res. 41:4976–4987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma XY, Wang JH, Wang JL, Ma CX, Wang XC
and Liu FS: Malat1 as an evolutionarily conserved IncRNA, plays a
positive role in regulating proliferation and maintaining
undifferentiated status of early-stage hematopoietic cells. BMC
Genomics. 16:6762015. View Article : Google Scholar
|
|
25
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheetham SW, Gruhl F, Mattick JS and
Dinger ME: Long noncoding RNAs and the genetics of cancer. Br J
Cancer. 108:2419–2425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kotake Y, Nakagawa T, Kitagawa K, Suzuki
S, Liu N, Kitagawa M and Xiong Y: Long non-coding RNA ANRIL is
required for the PRC2 recruitment to and silencing of p15 (INK4B)
tumor suppressor gene. Oncogene. 30:1956–1962. 2011. View Article : Google Scholar :
|
|
28
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large intervening non-coding RNA HOTAIR is associated with
hepatocellular carcinoma progression. J Int Med Res. 39:2119–2128.
2011. View Article : Google Scholar
|
|
29
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niinuma T, Suzuki H, Nojima M, Nosho K,
Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki
Y, et al: Upregulation of miR-196a and HOTAIR drive malignant
character in gastrointestinal stromal tumors. Cancer Res.
72:1126–1136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matouk IJ, DeGroot N, Mezan S, Ayesh S,
Abulail R, Hochberg A and Galun E: The H19 non-coding RNA is
essential for human tumor growth. PLoS One. 2:e8452007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berteaux N, Lottin S, Monté D, Pinte S,
Quatannens B, Coll J, Hondermarck H, Curgy JJ, Dugimont T and
Adriaenssens E: H19 mRNA-like noncoding RNA promotes breast cancer
cell proliferation through positive control by E2F1. J Biol Chem.
280:29625–29636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barsyte-Lovejoy D, Lau SK, Boutros PC,
Khosravi F, Jurisica I, Andrulis IL, Tsao MS and Penn LZ: The c-Myc
oncogene directly induces the H19 noncoding RNA by allele-specific
binding to potentiate tumorigenesis. Cancer Res. 66:5330–5337.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang P, Ren Z and Sun P: Overexpression of
the long non-coding RNA MEG3 impairs in vitro glioma cell
proliferation. J Cell Biochem. 113:1868–1874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anwar SL, Krech T, Hasemeier B, Schipper
E, Schweitzer N, Vogel A, Kreipe H and Lehmann U: Loss of
imprinting and allelic switching at the DLK1-MEG3 locus in human
hepatocellular carcinoma. PLoS One. 7. pp. e494622012, View Article : Google Scholar
|
|
36
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun M, Xia R, Jin F, Xu T, Liu Z, De W and
Liu X: Downregulated long noncoding RNA MEG3 is associated with
poor prognosis and promotes cell proliferation in gastric cancer.
Tumour Biol. 35:1065–1073. 2014. View Article : Google Scholar
|
|
38
|
Vousden KH and Prives C: Blinded by the
Light: The growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|