Introduction
Bone marrow mesenchymal stem cells (BMSCs) are a
more easily accessible type of mesenchymal stem cells and have
received increasing attention in induced cellular differentiation
(1,2), tissue engineering (3), cell therapy (4,5) and
additional research areas. However, the number of BMSCs in bone
marrow is relatively low (~0.001–0.01% of nucleated cells), and
BMSCs easily differentiate and age in in vitro culture
(6). Thus, a suitable method for
acquiring sufficient levels of high-purity, high-activity and
low-differentiation state mesenchymal stem cells is required for
associated research.
Currently, the purification of BMSCs from bone
marrow predominantly employs the following four methods: Plastic
adherence (7), density gradient
centrifugation (8), the flow
cytometry separation method (9,10)
and the immunomagnetic separation method (11). The plastic adherence method
isolates mesenchymal stem cells according to the differences in the
adhesion capacity of different cell types. This method is simple,
however cell purity is reduced. The density gradient centrifugation
method is more complicated. Although the extracted cells are of a
higher purity, the cells grow slowly and have low activity, with a
lengthy first fusion time for primary cells (12). The flow cytometry separation method
and the immunomagnetic separation method use specific antibodies to
isolate BMSCs. These methods are costly and are burdened by a lack
of accessibility, and only specific stem cells may be achieved
(9,11). Therefore, a simple and highly
efficient method for isolating BMSCs is required.
The isolation and purification of mesenchymal stem
cells from bone marrow is contaminated by two cell types:
Hematopoietic stem cells and fibroblasts. Of these cells, the
fibroblasts have the strongest adhesion capacity, followed by
BMSCs, and then the hematopoietic stem cells (7). A previous study described a
purification method for microglial and oligo-dendrocyte precursor
cells (OPCs) which involved vigorous agitation (13). Therefore the current study
hypothesized that the mechanical shaking of cells for different
durations and frequencies may aid in the purification of BMSCs due
to hematopoietic stem cells being swept away.
The whole bone marrow adherent method may be used to
purify BMSCs, however it is a lengthy process. In the current
study, the addition of shaking to the whole bone marrow adherent
method was observed to improve the purity of the cells obtained and
reduce the duration of the purification process.
Materials and methods
Animals
Sprague Dawley (SD) rats, 100±10 g in weight, were
provided by the Animal Experimental Center of The Second Military
Medical University (Shanghai, China). The experimental procedures
for the current study were approved by The Second Military Medical
University Animal Ethics Committee.
Isolation and culture of primary
BMSCs
As previously described (14), a total of 36 SD rats were
sacrificed by CO2 asphyxia, and then placed in 75%
ethanol for 5 min. Under sterile conditions, bilateral femurs and
tibias were surgically separated and cleaned. Subsequently, both
ends of the epiphysis were cut and the bone marrow cavity was
flushed from the end of the long bone with Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) using a 5 ml syringe. Following centrifugation (200 × g, 20°C,
5 min), cells were resuspended in complete medium containing 10%
fetal bovine serum (ScienCell Research Laboratories, San Diego, CA,
USA), DF-12 (Gibco; Thermo Fisher Scientific, Inc.) and 1%
streptomycin and penicillin (Gibco; Thermo Fisher Scientific,
Inc.). Cell suspensions were grown in T75 flasks (Corning
Incorporated, Corning, NY, USA) in 10 ml culture medium, with the
cells in each derived from an average of 2 rats, then incubated at
37°C in 5% CO2. The media was refreshed 24 h later and
then every other day. The cells were cultured for 7 days, following
which cell colony formations were observed, and the flask randomly
selected for the subsequent experiments. The medium was refreshed
30 min prior to the shaking.
Verification indices and treatment
methods
Flow cytometry (FAC500; Beckman Coulter, Inc., Brea,
CA, USA) was used to detect the presence of the following cellular
markers: CD29-fluorescein isothiocyanate (FITC),
CD90-R-phycoerythrin (PE), CD45-allophycocyanin, CD31-PE) and the
apoptotic rate using annexin V FITC, propidium iodide (PI) all
supplied by BD Biosciences (Franklin Lakes, NJ, USA) (15–18).
All staining was performed according to the
manufacturer's instructions. Briefly, the attached cells in the
flask were gently washed with 15 ml of phosphate-buffered saline
(PBS). Cells were digested with 0.25% trypsin (pre-warmed to 37°C)
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C for 2–3 min.
Following this, the majority of cells became round in shape and
were free-floating, and 5 ml complete medium was added to terminate
digestion. Following sufficient pipetting to dissociate the cells,
the cells in suspension were centrifuged at 150 x g for 5 min at
20°C, and then the supernatant was discarded. Following three
washes with PBS, the cell pellet was resuspended in PBS,
(1×107cells/ml for verification and
1×106cells/ml for apoptotic tests). Subsequently the
antibodies or PI were added and mixed and incubated for 15 min. All
flow cytometry analyses were complete within 1 h.
Verification of the effects of mechanical
shaking on cell purity
The culture flasks were divided into two groups. One
group was shaken at 37°C on a horizontal shaker at 180 rpm for 1 h
and the second group served as a control. The supernatant was
removed immediately following shaking and then the purity of the
attached cells was detected by flow cytometry (FAC500; Beckman
Coulter, Inc.,).
Selection of the frequency of mechanical
shaking
The culture flasks were shaken at 140, 180 and 220
rpm following the media replacement, with 3 flasks used for each
group. Following 1 h of mechanical shaking, the supernatant and
attached cells were collected for purity analysis. Sufficient
numbers of attached cells were obtained to perform apoptotic
analysis and cell counting (Countess Automated Cell Counter,
Invitrogen; Thermo Fisher Scientific, Inc.).
Determination of the duration of
mechanical shaking
A total of 2 flasks from the same batch were used
for each group, and were shaken at 180 rpm for 0, 0.5, 1, 2, 4, 6
and 8 h. Immediately following mechanical shaking, the supernatant
and attached cells were collected for purity analysis, apoptotic
analysis and cell counting.
Long-term effects of mechanical
shaking
A total of 2 flasks from the same batch were used
for each group, and were shaken at 180 rpm for 0, 0.5, 1, 2, 4, 6
and 8 h. The media was refreshed immediately following shaking and
3 days later. The attached cells were analyzed for purity.
Evaluating the effects of multiple
short-duration shakes
A total of 8 flasks of cells from the same batch
were used. Of these, 4 flasks were mechanically shaken at 180 rpm
for 0.5 h each day for 2 days, and the media replaced with fresh
media following each shake. The remaining 4 flasks were shaken at
180 rpm for 4 h. The duration of shaking was precisely controlled
so that the two groups finished their treatment at the same time.
Subsequently, the attached cells from all 8 flasks were analyzed
for purity.
Evaluation of the in vitro
differentiation potential of isolated BMSCs
Following a 2 h shake at 180 rpm, the attached cells
were seeded on 24-well plates (Corning Incorporated) at a cell
density of 1×105cells/ml, 0.5 ml/well. Following 24 h
incubation, osteogenic and adipogenic differentiation media
(ScienCell Research Laboratories) were added individually. The
media was replaced every 3 days. At 2 weeks later, the cells were
fixed using 4% formaldehyde and were stained with alizarin red or
oil red O (Sigma-Aldrich, St. Louis, MO, USA).
Statistical analysis
Values are presented as the mean ± standard error of
the mean. All experiments were repeated with a minimum of 3
different batches. The differences between groups were analyzed
using one way analysis of variance, and multiple comparisons were
performed using Fisher's Least Significant Difference test or
Student-Newman-Keuls method. Data were analyzed using SPSS
software, version 21.0, (ISM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Verification of the effects of mechanical
shaking on cell purity
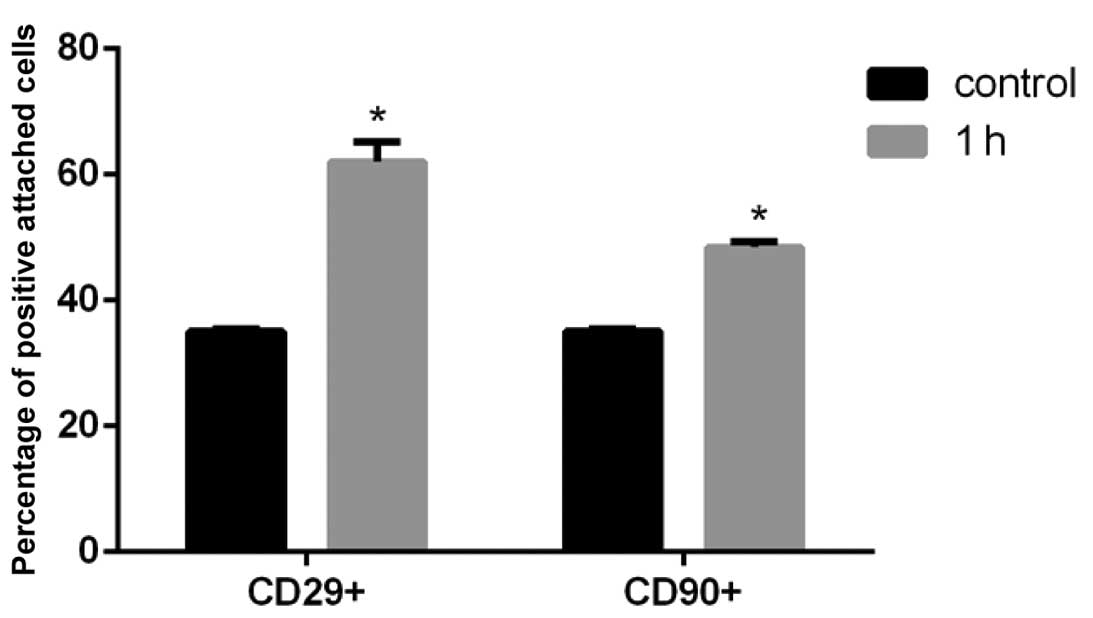
Following a 1 h shake at 180 rpm, the percentage of
CD29 and CD90 positive cells was significantly increased
(P<0.05; Fig. 1). The abundance
of CD45 and CD31 positive cells was reduced, however, the
percentage of these markers was lower than 1% and had minor effects
on the results, therefore this data is not presented.
Selection of the frequency of mechanical
shaking
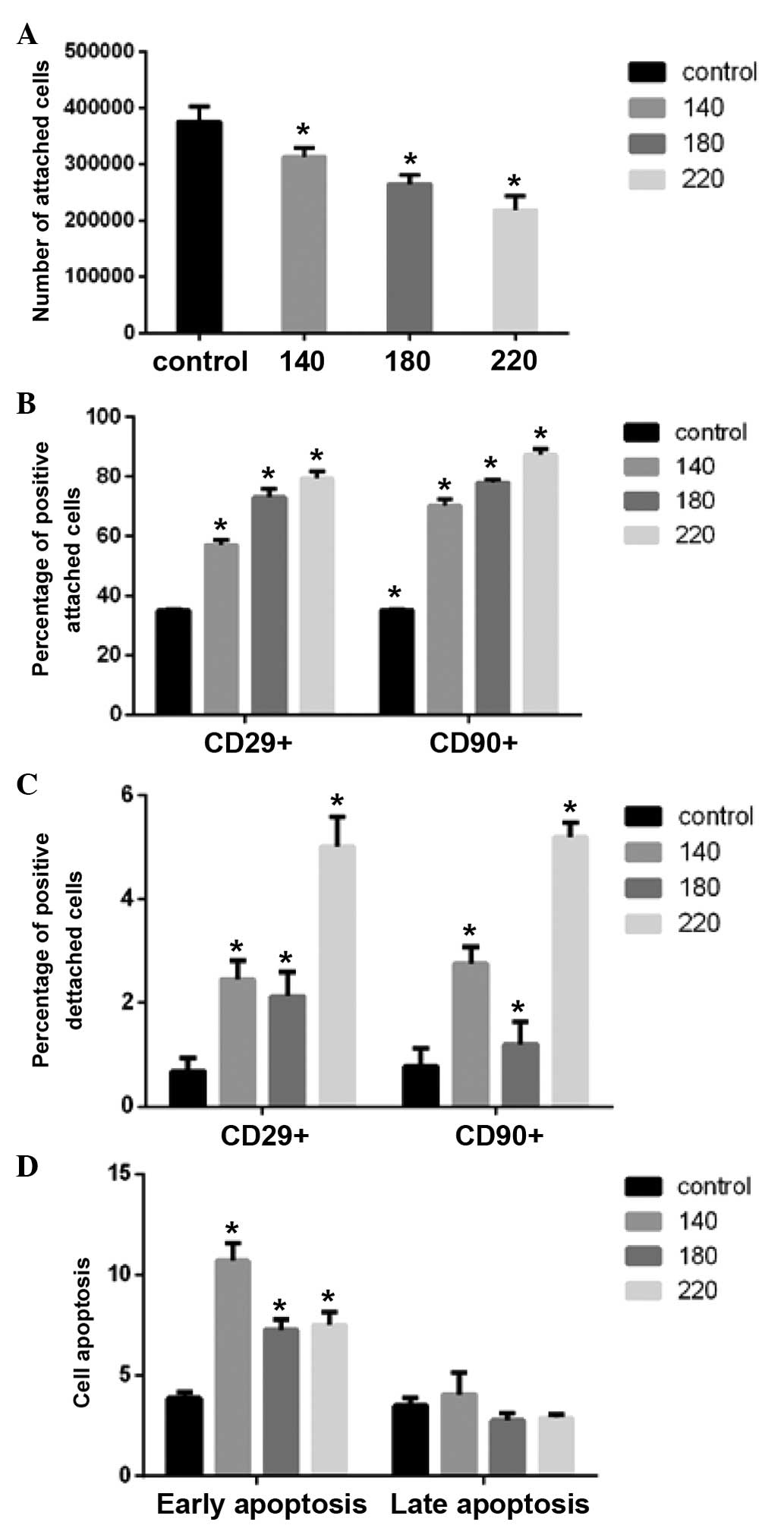
The cell number was reduced following shaking at 220
rpm for 1 h, therefore only ~2×105 cells were cultured
for subsequent tests.
Flow cytometric analyses revealed that with
increasing shaking frequency, the number of attached cells was
reduced and the cell purity was increased (Fig. 2A and B). The purity of the cells
lost in the supernatant was increased, and in addition the purity
increased with increasing frequency. However, the increase was of a
smaller magnitude than that of the attached cells (Fig. 2C).
Apoptosis was observed to increase following
shaking, with the level of apoptotic cells significantly greater at
140 rpm compared with the 180 and 220 rpm groups. However, there
was no significant difference between 180 rpm and 220 rpm
(P<0.05; Fig. 2D).
The determination of the duration of
mechanical shaking
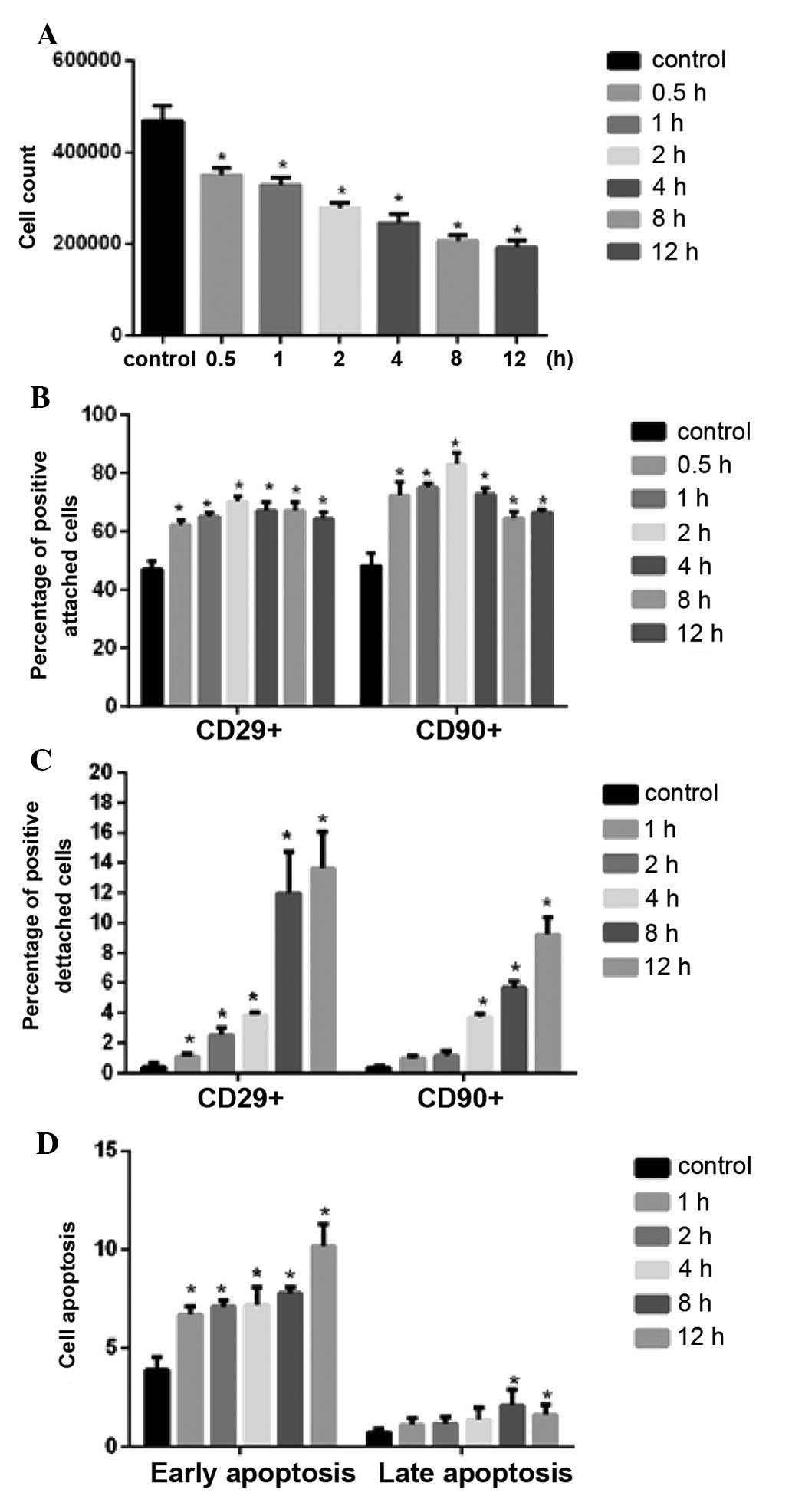
Following 8 h shaking, greater than 3×105
cells were present in culture. The percentage of CD29 and CD90
positive cells increased significantly with shaking durations up to
4 h, however, shaking durations longer than 4 h resulted in a
reduction in CD29 and CD90 positive cells (Fig. 3B). In addition, significant
differences were observed between the different shaking duration
groups (P<0.01). The cells positive for CD29 and CD90 in the
supernatant were increased with the increasing duration of shaking,
with a large increase observed above 4 h shaking (Fig. 3C).
The level of apoptosis in the attached cells was
observed to increase with increasing shaking duration, however, all
values were lower than 12% (Fig.
3D).
Long-term effects of mechanical
shaking
Following 3 days of mechanical shaking, the total
number of cells obtained was high, with greater than
4×106 cells obtained from a single flask. The purity of
the samples was greater compared with the unshaken control
(P<0.01). However, the longer duration of shaking did not yield
superior purity (Fig. 4).
Effect of multiple shorter duration
shakes
Compared with a single 4 h shake at 180 rpm,
multiple 0.5 h shakes prior to replacement of the media resulted in
lower cell purity (P<0.01; Fig.
5).
Evaluation of the in vitro
differentiation potential of isolated BMSCs
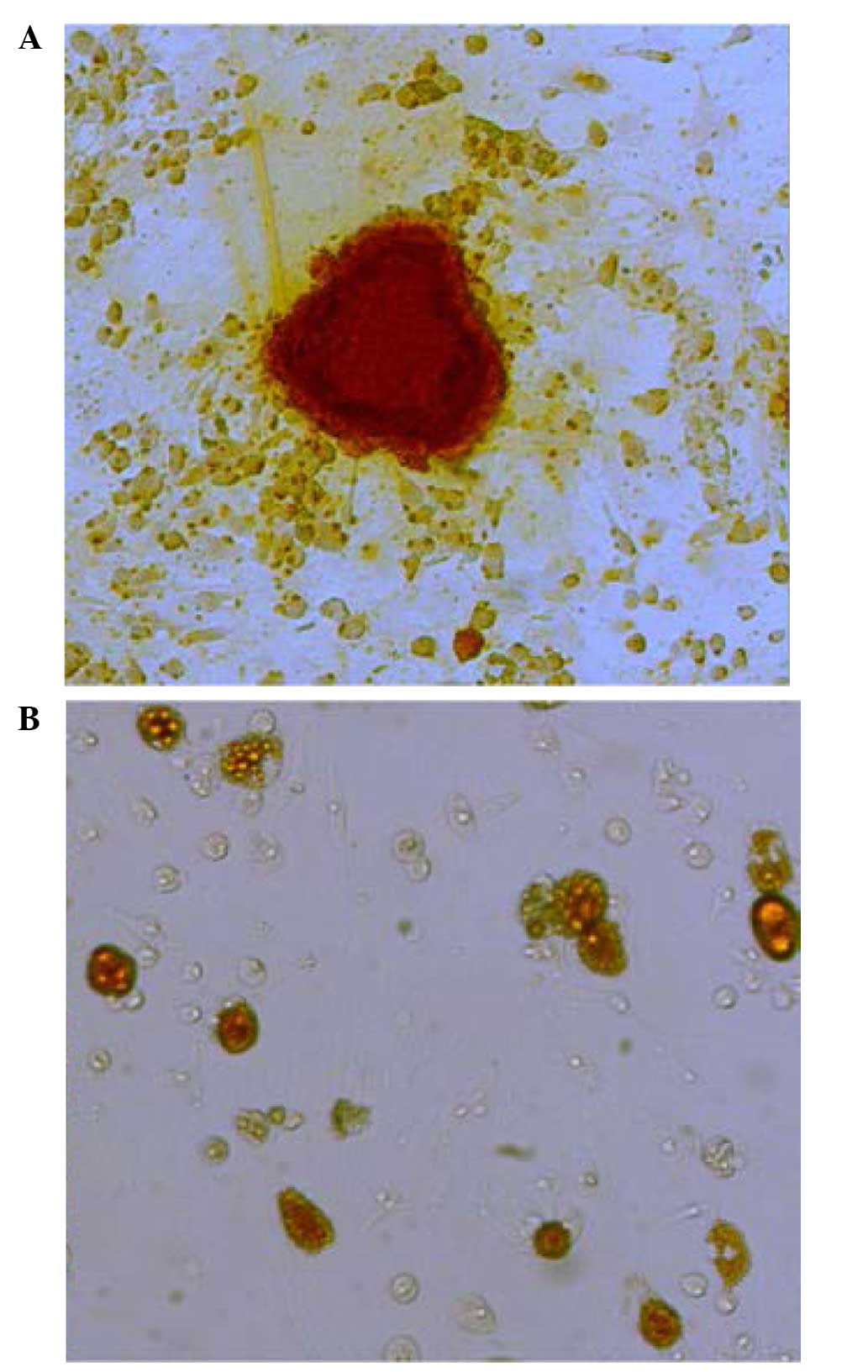
Following 2 weeks of differentiation induction, the
purified BMSCs (passage 1) demonstrated osteogenic and adipogenic
differentiation capacities (Fig.
6). BMSCs were cultured in osteogenic medium for 2 weeks,
following which staining using Alizarin red was performed to detect
the presence of bone nodules. The microscopy images indicated the
presence of calcium nodules. In addition, the BMSCs were cultured
in adipogenic differentiation medium for 2 weeks and the presence
of lipid droplets detected using oil red O. As shown in Fig. 6B, red lipid droplets were observed
in the differentiating BMSCs.
Discussion
During the cell culture process, simply increasing
cell purity is not sufficient as cell numbers and activities must
be taken into consideration. Therefore, a good isolation method
must find a balance between cell purity, cell volume and cell
activity. Thus, in the current study the purity of both the
attached and detached cells was investigated. However, when
evaluating the apoptotic indices, only apoptosis in attached cells
was measured. This measure suggested that as the damage resulting
from shaking accumulated, progressive apoptosis became an important
factor for cell detachment.
A previous study of the isolation of microglial
cells led to the selection of shaking at 180 rpm for 4 h as the
initial testing condition (13).
In the present study, different shaking frequencies were
investigated for their effect on cell purity and apoptosis, and 180
rpm was selected as the experimental frequency. Based on multiple
trials of shaking duration and the analysis of shaking-dependent
cell apoptosis, 4 h was determined to be the most suitable time
duration for isolation.
The present study indicated that shaking played a
significant role in BMSC purification. BMSCs demonstrated a strong
adhesion capacity, indicating that it is feasible and effective to
isolate BMSCs using a physical method. Isolation using a horizontal
shaker is achieved through liquid movement induced by the
horizontal shaking to wash the flask bottom. Different shaking
frequencies suggest different movement strengths of the liquid in
the culture flasks, and thus, different amounts of shear stress on
the cells (19). The present study
indicated that isolation may be achieved through the selection of
the appropriate shaking frequency and duration.
Within 1 h of shaking, cell purity increased with
increased shaking frequency, however, the attached cell numbers
were reduced and the apoptotic rate was altered with the different
shaking frequencies. Although a shaking frequency of 220 rpm
demonstrated superior effects on cell purity, the number of
attached cells was markedly reduced compared with 180 rpm. Notably,
the overall proportion of apoptotic cells was greatest for cells
shaken at 140 rpm. This may be due to the lower level of physical
force exerted on the cells, which is unable to detach the cells
undergoing apoptosis. In general, shaking at 180 rpm for 1 h was
advantageous, and resulted in a higher cell purity and reduced rate
of apoptosis. Therefore, 180 rpm was selected as the shaking
frequency.
The present study indicated that lengthy separation
times may lead to reduced cell activity and even apoptosis.
However, a shaking duration of 1 h was not necessarily the best
option. In the preliminary testing, shaking for greater than 4 h
did not result in a significant improvement in cell purity. In
addition, the number of cells was greatly reduced following 12 h of
shaking, resulting in a shortage in the number of cells available
for flow cytometric analysis. Therefore, the shaking duration was
limited to 4 h, and the results indicated that 2 h may be the
optimal shaking duration.
Regarding cell apoptosis, the apoptosis of attached
cells under fluid impact is a dynamic balance of continuous
apoptosis and continuous detachment, and the cell populations
maintain relatively stable proportions at different stages
(20) Apoptotic cells, in
particular those in the late stage, have a low adherence capacity
and detach easily (21). In the
present study, shaking led to apoptosis in the attached cells,
which may result in the detachment of these cells from the flask
wall and reduce the overall rate of apoptosis measured. However,
with higher frequencies and the accumulation of damage from long
shaking, there was a higher proportion of cells undergoing
apoptosis. At the higher frequencies, the increased proportion of
apoptotic cells and the subsequent detachment of apoptotic cells
from the flask walls may result in reduced rates of apoptosis being
measured in the 180 and 220 rpm groups.
The present study investigated the long-term effects
of shaking following the isolation of cells, which indicated that
the impact of isolation itself on cell apoptosis may not be
significant. However, following the formation of cell colonies, the
growth rate of the cells rapidly increased. Therefore, the cell
purity indicated by the flow cytometry analysis was high. However,
the high cell density may lead to stem cell differentiation
(22,23). Therefore, although cell purity was
high following cell growth for 3 days after shaking treatment,
amplifying cells using this method is not recommended.
The results from the multiple short shakes indicated
that shaking at 180 rpm for 0.5 h per day for 2 days was not
sufficient to remove the loosely attached cells. However, it may
interfere with fast-dividing BMSCs, leading to poor purity.
In summary, in a mixed culture system, increasing
the purity of a certain cell type may be achieved because either
the cell type's faster proliferation rate leads to a “relative”
increase in purity or the reductions in the numbers of other cell
types leads to an “absolute” increase in purity. The high
proliferation capacity of stem cells is the foundation of the
multiple-passage purification strategy in the whole bone marrow
adhesion method. The mechanism of this physical separation method
may occur as a result of the different physical attributes of the
different cell types or by induced apoptosis due to differential
resistance to physical stimulation. Therefore, the reason why BMSCs
may be purified through physical separation may be due to their
relatively strong adhesion capacity and their resistance to
physical stimulation.
The addition of shaking to the whole bone marrow
adhesion method is a simple and feasible method for BMSC
purification. Following physical separation by shaking on a
horizontal shaker, cell purity may be significantly increased.
Compared with existing purification methods, this modified whole
bone marrow adhesion method is simple, effective and inexpensive.
This method may result in the enrichment of highly pure, highly
active mesenchymal stem cells at low differentiation states and
early stages.
Acknowledgments
The present study was translated by American Journal
Experts. The current study was funded by the National Natural
Science Foundation of China (grant no. 81472071), and the Shanghai
Committee of Science and Technology (grant no. 12ZR1454500).
References
|
1
|
Polisetti N, Chaitanya VG, Babu PP and
Vemuganti GK: Isolation, characterization and differentiation
potential of rat bone marrow stromal cells. Neurol India.
58:201–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hofstetter CP, Schwarz EJ, Hess D,
Widenfalk J, El Manira A, Prockop DJ and Olson L: Marrow stromal
cells form guiding strands in the injured spinal cord and promote
recovery. Proc Natl Acad Sci USA. 99:2199–2204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dezawa M, Takahashi I, Esaki M, Takano M
and Sawada H: Sciatic nerve regeneration in rats induced by
transplantation of in vitro differentiated bone-marrow stromal
cells. Eur J Neurosci. 14:1771–1776. 2001. View Article : Google Scholar
|
|
5
|
Yoshimura H, Muneta T, Nimura A, Yokoyama
A, Koga H and Sekiya I: Comparison of rat mesenchymal stem cells
derived from bone marrow, synovium, periosteum, adipose tissue, and
muscle. Cell Tissue Res. 327:449–462. 2007. View Article : Google Scholar
|
|
6
|
Friedenstein AJ, Deriglasova UF, Kulagina
NN, Panasuk AF, Rudakowa SF, Luriá EA and Ruadkow IA: Precursors
for fibroblasts in different populations of hematopoietic cells as
detected by the in vitro colony assay method. Exp Hematol. 2:83–92.
1974.PubMed/NCBI
|
|
7
|
Dexter TM, Testa NG, Allen TD, Rutherford
T and Scolnick E: Molecular and cell biologic aspects of
erythropoiesis in long-term bone marrow cultures. Blood.
58:699–707. 1981.PubMed/NCBI
|
|
8
|
Chen ZZ, Van Bockstaele DR, Buyssens N,
Hendrics D, De Meester I, Vanhoof G, Scharpé SL, Peetermans ME and
Berneman ZN: Stromal populations and fibrosis in human long-term
bone marrow cultures. Leukemia. 5:772–781. 1991.PubMed/NCBI
|
|
9
|
Dezawa M, Kanno H, Hoshino M, Cho H,
Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H,
et al: Specific induction of neuronal cells from bone marrow
stromal cells and application for autologous transplantation. J
Clin Invest. 113:1701–1710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia L, Young MF, Powell J, Yang L, Ho NC,
Hotchkiss R, Robey PG and Francomano CA: Gene expression profile of
human bone marrow stromal cells: High-throughput expressed sequence
tag sequencing analysis. Genomics. 79:7–17. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nadri S and Soleimani M: Isolation murine
mesenchymal stem cells by positive selection. In Vitro Cell Dev
Biol Anim. 43:276–282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rouger K, Fornasari B, Armengol V, Jouvion
G, Leroux I, Dubreil L, Feron M, Guevel L and Cherel Y: Progenitor
cell isolation from muscle-derived cells based on adhesion
properties. J Histochem Cytochem. 55:607–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giulian D and Baker TJ: Characterization
of ameboid microglia isolated from developing mammalian brain. J
Neurosci. 6:2163–2178. 1986.PubMed/NCBI
|
|
14
|
Schwarz EJ, Alexander GM, Prockop DJ and
Azizi SA: Multipotential marrow stromal cells transduced to produce
L-DOPA: Engraftment in a rat model of Parkinson disease. Hum Gene
Ther. 10:2539–2549. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barry FP, Boynton RE, Haynesworth S,
Murphy JM and Zaia J: The monoclonal antibody SH-2, raised against
human mesenchymal stem cells, recognizes an epitope on endoglin
(CD105). Biochem Biophys Res Commun. 265:134–139. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: Biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Guo Z, Guo B, Xie Y, Yang J and Wang
A: Inhibition of the endogenous CSE/H2S system
contributes to hypoxia and serum deprivation-induced apoptosis in
mesenchymal stem cells. Mol Med Rep. 9:2467–2472. 2014.PubMed/NCBI
|
|
18
|
De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary
A, Zhu M, Ashjian P, Benhaim P, Hedrick MH and Fraser JK:
Differential expression of stem cell mobilization-associated
molecules on multi-lineage cells from adipose tissue and bone
marrow. Immunol Lett. 89:267–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feugier P, Black RA, Hunt JA and How TV:
Attachment, morphology and adherence of human endothelial cells to
vascular prosthesis materials under the action of shear stress.
Biomaterials. 26:1457–1466. 2005. View Article : Google Scholar
|
|
20
|
Chen R, Wang J, Jiang B, Wan X, Liu H, Liu
H, Yang X, Wu X, Zou Q and Yang W: Study of cell apoptosis in the
hippocampus and thalamencephalon in a ventricular fluid impact
model. Exp Ther Med. 6:1463–1468. 2013.PubMed/NCBI
|
|
21
|
Jiang ZL, Fletcher NM, Diamond MP,
Abu-Soud HM and Saed GM: S-nitrosylation of caspase-3 is the
mechanism by which adhesion fibroblasts manifest lower apoptosis.
Wound Repair Regen. 17:224–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Majd H, Wipff PJ, Buscemi L, Bueno M,
Vonwil D, Quinn TM and Hinz B: A novel method of dynamic culture
surface expansion improves mesenchymal stem cell proliferation and
phenotype. Stem Cells. 27:200–209. 2009. View Article : Google Scholar
|
|
23
|
Engler AJ, Sen S, Sweeney HL and Discher
DE: Matrix elasticity directs stem cell lineage specification.
Cell. 126:677–689. 2006. View Article : Google Scholar : PubMed/NCBI
|