Introduction
Age-related macular degeneration (AMD) is the
leading cause of irreversible blindness in developed countries and
retinal pigment epithelium (RPE) is the primary affected tissue
(1–3). Premature senescence has been
implicated as a potentially important pathophysiologic mediator of
RPE dysfunction (2,4–6).
In vivo and in vitro studies have shown that primary
human RPE from older patients or RPE cells exposed to oxidative
stress exhibited senescence phenotypes, including hypertrophy,
senescence-associated β-galactosidase (SA β-gal) activity, growth
arrest and cell cycle arrest in the G1 phase (3,4).
However, the etiology and pathogenesis of AMD remain poorly
understood.
Apoptosis related protein 3 (Apr3) was identified to
be differentially expressed in HL-60 cells following treatment with
all-trans retinoic acid (ATRA) compared with untreated cells
as determined by a polymerase chain reaction (PCR)-based
subtractive hybridization method (7). A previous study demonstrated that
Apr3 overexpression arrested cells at the G1/S phase via inhibiting
the transcriptional activity of Cyclin D1 (8). Zou et al (9) demonstrated that NELL-1 significantly
inhibited osteoblast proliferation partly through interacting with
Apr3, which resulted in the downregulation of Cyclin D1. However,
the molecular mechanism by which Apr3 affects the cell cycle
remains largely unknown. Based on previous studies, it was
hypothesized that Apr3 may participate in cellular activities that
are closely associated with the cell cycle, such as cell
senescence, apoptosis and differentiation.
Our preliminary data revealed elevated Apr3
expression in the heart, lung, liver and kidney tissues of aged
mice. To the best of our knowledge, the present study demonstrates
for the first time that Apr3 levels were significantly increased in
aged mouse RPE and prematurely senescent RPE cells induced by
oxidative stress. Moreover, Apr3 overexpression in human RPE cells
accelerated cellular senescence, which was abrogated by truncated
Apr3. Thus, targeting Apr3 may represent a novel therapeutic
strategy for delaying or inhibiting the progressive effects of
senescence on AMD.
Materials and methods
Isolation of primary mouse RPE
All animal experiments were performed with the
approval of the Institutional Animal Care and Use Committee at the
Capital Medical University (Beijing, China). C57BL/6 mice were
purchased from Capital Medical University. Mice of different ages
(5 mice per group; age, 1, 6, 12 and 18 months) were maintained in
a constant environment of 21±2°C, with a humidity of 50±10% and a
12-h light/dark cycle. Food and water were available ad
libitum. The mice were anesthetized with 2–3% isoflurane
(Halocarbon Products Corporation, Peachtree Corners, GA, USA). Mice
were sacrificed by cervical dislocation and the eyes were
enucleated and washed with Hanks' balanced salt solution (HBSS;
Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA, USA). The
anterior segments were removed and the eyecups were incubated in 1
mg/ml hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA) in HBSS for
1 h at 37°C. The neural retina was peeled off and the RPE
monolayers were cut into 2×2 mm sections for RNA and protein
extraction.
ARPE-19 cell culture
ARPE-19 represents a human RPE cell line that is
widely used as a reproducible model of RPE cell biology and
function. ARPE-19 cells were obtained from CoBioer Biosciences Co.,
Ltd. (Shanghai, China) and were routinely grown in F-12/Dulbecco's
modified Eagle's medium (DMEM; 1:1, Invitrogen; Thermo Fisher
Scientific Inc.) containing 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific Inc.), 2 mM glutamine, 30
µg/ml penicillin and 50 µg/ml streptomycin
(Sigma-Aldrich). ARPE-19 cells were passaged every 3–4 days.
Oxidative stress treatment
ARPE-19 cells were grown to 95% confluence and were
treated with various concentrations of H2O2
(0, 25, 50, 100, 150, 200, 250, 300 and 400 µM) diluted in
ARPE medium for 2 h. Then cells were washed with phosphate-buffered
saline (PBS), and cultured in F12/DMEM for 22 h. Similarly, ARPE-19
cells were treated with tert-butylhydroperoxide (tert-BHP,
Sigma-Aldrich) at 0, 10, 20, 30, 40, 50, 100, 200 and 300
µM.
Lactose dehydrogenase (LDH) viability
assay
ARPE-19 cells were seeded onto a 96-well microplate
(2×103 cells/well). After 24 h, cells were treated with
various concentrations of H2O2 for 2 h,
washed once with PBS and cultured in normal medium for 22 h. Cell
viability was assessed by monitoring LDH release into the culture
medium with an LDH cytotoxicity assay kit (Beyotime Institute of
Biotechnology, Shanghai, China) according to the manufacturer's
instructions. Cell viability was measured spectrophotometrically at
490 nm on a microplate reader (ELx800; Bio-Tek, Winooski, VT, USA).
Data are presented as the mean ± standard error of the mean of
three replicates.
[3H]-thymidine incorporation
assay
ARPE-19 cells (2×104) were seeded onto
24-well plates and underwent oxidative stress as described above.
[3H]-thymidine (1 mCi/well) was added 4 h prior to cell
harvesting and thymidine incorporation was measured by
scintillation counting (PerkinElmer, Waltham, MA, USA).
Constructs
PcDNA3.1-hApr3 and pcDNA3.1-hApr3-N were provided by
Dr Yu (Fourth Military Medical University, Xi'an, China) and
subcloned into the pLVTHM-green fluorescent protein (GFP)
lentivirus expression vector (Addgene, Inc., Cambridge, MA, USA)
using Mlu1 and Cla1 restriction enzymes (Takara Bio
Inc., Otsu, Japan) to generate pLVTHM-hApr3 and pLVTHM-hApr3-N.
293FT cells were obtained from CoBioer Biosciences Co., Ltd) and
maintained in DMEM containing 10% FBS, 2 mM glutamine, 30
µg/ml penicillin and 50 µg/ml streptomycin
(Sigma-Aldrich). pLVTHM-expressing plasmids with other helper
vectors were co-transfected into 293FT cells and the lentiviruses
were titrated by Cytomics FC 500 flow cytometry (Beckman Coulter,
Inc., Brea, CA, USA). ARPE-19 cells (2×106) were seeded
onto a 10-cm plate and 100 µl of Apr3-expressing
lentiviruses were transduced. After 3 days, ARPE-19 cells were
collected and GFP-positive cells were sorted by Cytomics FC 500
flow cytometry. The sorted cells were Apr3-overexpressing ARPE-19
stable cells and denoted as ARPE-Apr3.
SA β-gal activity assay
ARPE-Apr3 and the parent cells [ARPE-control (CTL)]
were fixed in 4% paraformaldehyde (Beyotime Institute of
Biotechnology) for 20 min and washed twice with PBS. Cells were
then incubated overnight at 37°C with freshly prepared SA β-gal
staining solution (1 mg/ml X-Gal, 40 mM citric acid-sodium
phosphate, pH 6.0; 5 mM potassium ferrocyanide, 5 mM potassium
ferricyanide, 150 mM NaCl and 2 mM MgCl2). Cells were
washed and visualized under a light microscope (CKX31; Olympus
Corporation, Tokyo, Japan). The percentage of blue cells per 100
cells was calculated.
Reverse transcription (RT)-quantitative
(q)PCR
Cells with indicated treatment were harvested for
isolation of RNA using TRIzol reagent (Invitrogen, Thermo Fisher
Scientific Inc.) according to the manufacturer's instructions.
First-strand cDNA synthesis was performed using 2 µg of RNA,
random primers and catalyzed by M-MLV reverse transcriptase. qPCR
was performed with the SYBR Premix Ex TaqTM (Takara Bio Inc.)
containing DNA polymerase in 25 µl reactions in 96-well PCR
microplates (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using ABI PRISM 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and StepOne software v2.0 (Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR conditions were as
follows: 95°C for 5 min followed by 35 cycles of 95°C for 20 sec
and 60°C for 20 sec. Primers sequences were as follows: Forward:
5′-tca gct gca gac tct gatac-3′ and reverse: 5′-gcc agt aat tgt caa
cgaag-3′ for Apr3; and forward: 5′-cgc ggt tct att ttg ttggt-3′ and
reverse: 5′-agt cgg cat cgt tta tggtc-3′ for glyceraldehyde
3-phosphate dehydrogenase (GAPDH). Apr3 mRNA relative abundance was
determined by normalizing to GAPDH using the ΔCq method,
where Cq is the quantification cycle (10). The wells without a cDNA template
were used as negative controls and GAPDH was used as a RT control.
The experiment was repeated three times.
Western blot analysis
Cells were harvested and lysed in a
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing 50 mM Tris-HC (pH 7.5), 150 mM NaCl, 1% NP-40,
0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate and 50 mM
NaF. One tablet of protease inhibitor mixture (Complete Mini, Roche
Applied Science, Indianapolis, IN, USA) was added prior to
analysis. Protein concentration was measured using a Bradford
reagent (Bio-Rad, Hercules, CA, USA). Then, 100 µg protein
lysates were separated by 12% SDS-polyacrylamide gel (Invitrogen;
Thermo Fisher Scientific, Inc.) electrophoresis and transferred
onto nitrocellulose membranes (Beyotime Institute of
Biotechnology). Following blocking in a 5% non-fat dried milk
solution in washing buffer containing 10 mmol/l Tris (pH 7.5), 50
mmol/l NaCl and 0.02% Tween 20 (TBST), membranes were incubated
overnight at 4°C with rabbit polyclonal anti-Apr3 (1:2,000,
Sigma-Aldrich; cat. no. SAB2100295), rabbit polyclonal
anti-α-tubulin (1:1,000, Santa Cruz Biotechnology Inc., Dallas, TX,
USA; cat. no. sc-5546) and two senescence markers, rabbit
polyclonal anti-p21 (1:1,000; Santa Cruz Biotechnology, Inc.; cat.
no. sc-756) and rabbit polyclonal anti-p53 (1:1,000; Santa Cruz
Biotechnology, Inc.; cat. no. sc-6243). After washing three times
with TBST, membranes were incubated for 1 h with goat anti-rabbit
IgG horseradish peroxidase-coupled secondary antibodies (1:1,000,
Santa Cruz Biotechnology Inc.; cat. no. sc-2004) at room
temperature. Signals were detected with an enhanced
chemiluminescence kit (Thermo Fisher Scientific Inc., Beijing,
China). The scanned images were quantified using Kodak Digital
Science one-dimensional software (Eastman Kodak Co., New Haven, CT,
USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Unpaired Student's t-test was used for statistical
analysis with GraphPad Prism version 5.02 (GraphPad Software, Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Elevated Apr3 level in the RPE from aged
mice
Our preliminary data showed a significant increase
in the Apr3 expression level in aged mouse tissues, including
heart, lung, liver and kidney tissue (data not shown), which
suggested that Apr3 may function in the aging process. Current
evidence indicates that senescent RPE cells accumulate with age
in vivo (4,5,11).
In order to assess whether Apr3 exhibits a role in RPE cell
senescence, the endogenous expression of Apr3 was detected in RPE
cells from young and aged mice. As expected, the mRNA level of Apr3
gradually increased with age (Fig.
1A). Consistently, the Apr3 protein level in RPE cells showed a
similar trend and its level in 18-month-old mice was enhanced by
7.94-fold compared with that in one-month-old mice (Fig. 1B and C).
Apr3 is upregulated in oxidative
stress-induced senescent human ARPE-19 cells
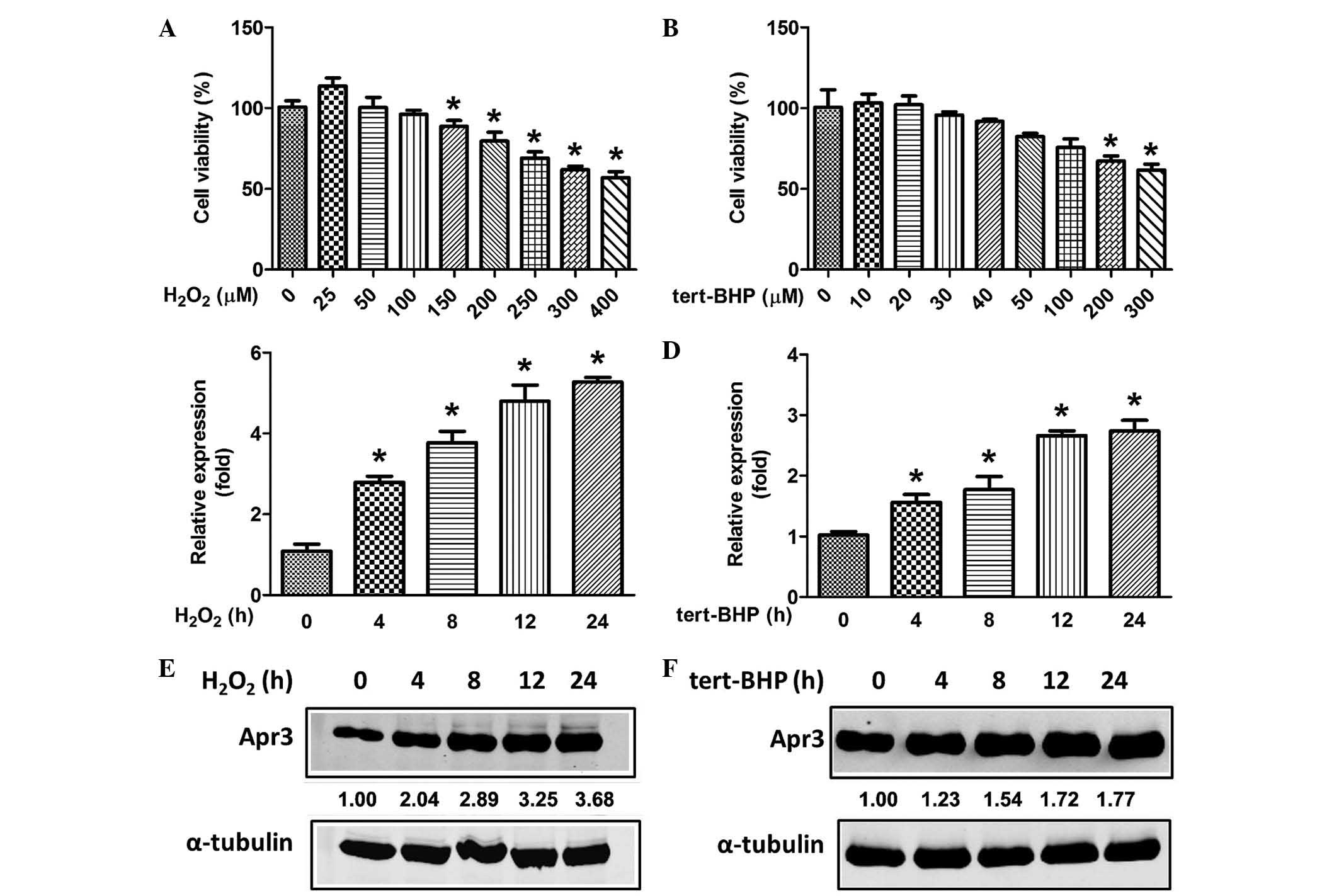
In AMD, chronic low level oxidative stress is
hypothesized to accelerate RPE cell senescence, thus promoting
disease pathogenesis (4,5). The present study examined whether
Apr3 expression was induced in premature senescence caused by
oxidative stress in ARPE-19 cells (a non-transformed human RPE cell
line). Initially, culture conditions that induced ARPE-19
senescence without causing cell death were investigated using an
LDH activity assay. ARPE-19 cells were treated with various
concentrations of H2O2 for 2 h and their
viability was assayed after 22 h. It was demonstrated that
H2O2 at ≤100 µM was not cytotoxic,
whereas concentrations of ≥150 µM were (Fig. 2A). Similarly, different
concentrations of tert-BHP were applied. It was demonstrated that
tert-BHP at ≤40 µM was not cytotoxic (Fig. 2B). Thus, H2O2
at 100 µM and tert-BHP at 40 µM were selected as the
optimal oxidative stress doses to treat RPE cells in the following
experiments. As expected, Apr3 mRNA levels were enhanced in a
time-dependent manner following treatment with
H2O2 and tert-BHP (Fig. 2C and D). Consistently,
H2O2 and tert-BHP resulted in a 3.68- and
1.77-fold increase in Apr3 protein levels following 24 h of
treatment, respectively (Fig. 2E and
F). Thus, the results suggest that elevated endogenous Apr3
expression may be involved in RPE cell senescence.
Apr3 overexpression accelerates
senescence of ARPE-19 cells
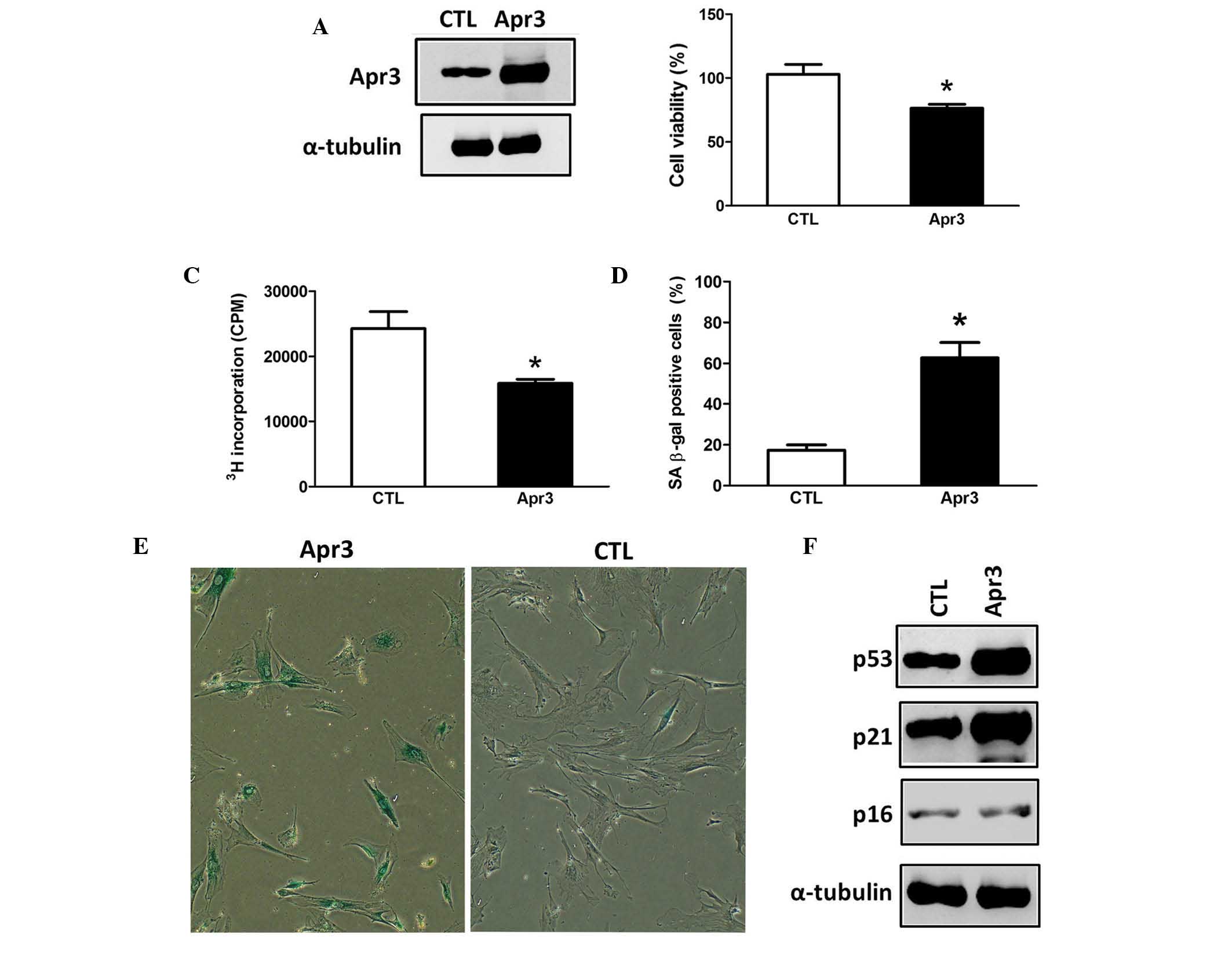
To investigate the putative role of Apr3 in cell
senescence, Apr3-expressing lentivirus was engineered and
transfected into ARPE-19 cells. Subsequently, stably elevated Apr3
protein levels were confirmed in ARPE-19 cells (Fig. 3A). Cell viability was then
determined using an LDH activity assay and it was demonstrated
cells overexpressing Apr3 exhibited decreased cell viability
compared with the parent cells (Fig.
3B, 76.4 vs. 101.9%). Cellular senescence is a stress-response
phenomenon where cells lose the ability to proliferate (2,3).
Thus, it was then assessed whether Apr3 influenced the
proliferative properties of ARPE-19 cells. As shown in Fig. 3C, Apr3 overexpression moderately
inhibited cell proliferation by 34.5% compared with the parent
cells as determined by a 3[H]-thymidine incorporation
assay.
Following 7 days of culture, SA β-gal staining
demonstrated that ~62.7% of cells overexpressing Apr3 were positive
for SA β-gal, compared with 17.3% of the parent cells (Fig. 3D and E). The expression levels of
senescence markers p21 and p53 in Apr3 overexpressed cells were
elevated by 4.3- and 3.5-fold compared with the parent cells,
respectively (Fig. 3F). However,
p16 level was barely altered upon Apr3 overexpres-sion. The above
data demonstrated that Apr3 overexpression produced an accelerated
prematurely senescent phenotype in ARPE-19 cells.
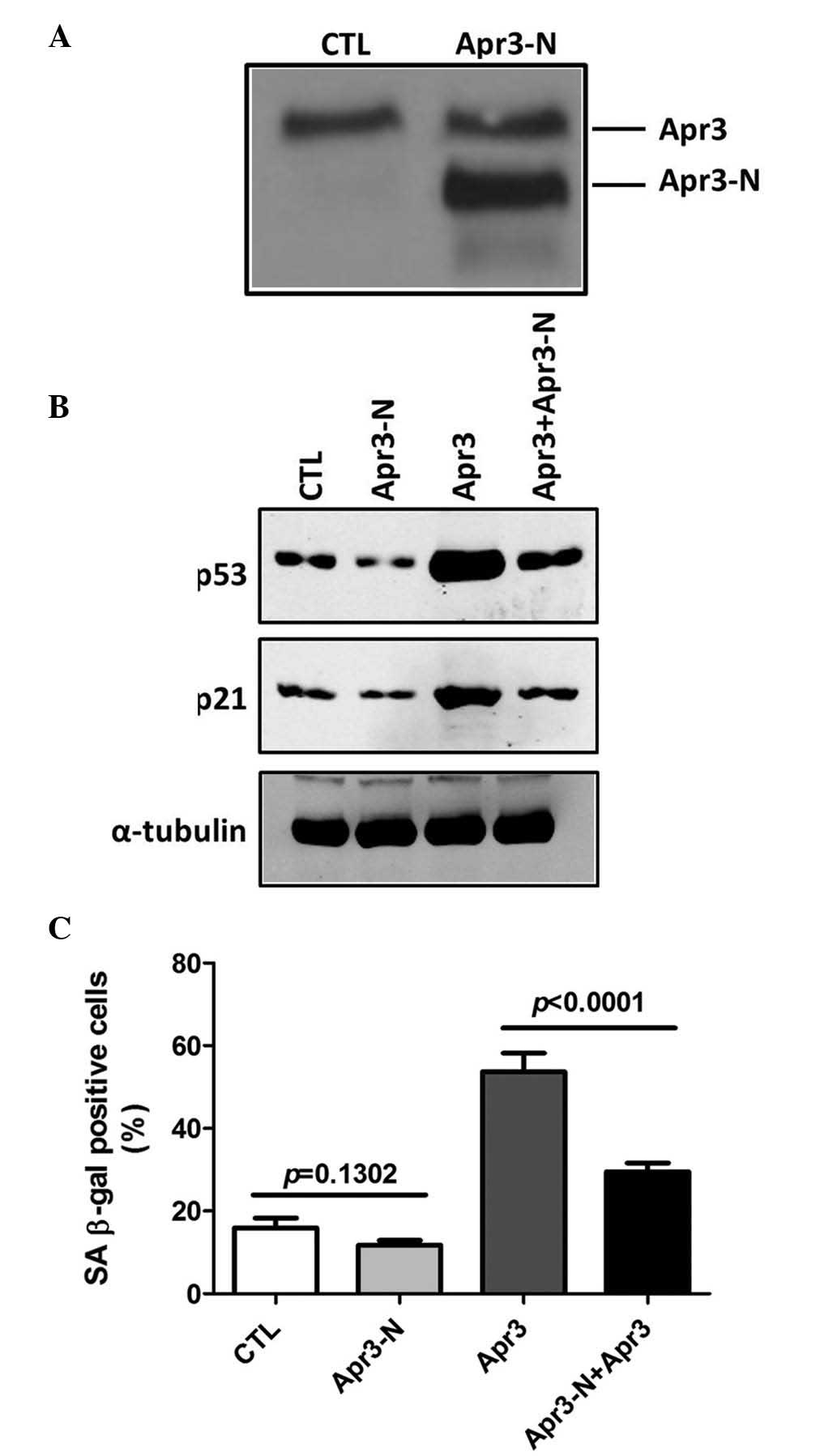
Truncated Apr3 abolished Apr3-induced
senescence
A previous study showed that Apr3-N, a truncated
counterpart of Apr3, strongly antagonized Apr3-induced cell cycle
arrest (8). To further confirm
Apr3-caused senescent phenotype, Apr3-N lentivirus was transduced
into cells overexpressing Apr3 and parent cells (Fig. 4A), and cell senescence was
evaluated. Fig. 4B showed that
Apr3-N overexpression in parent cells resulted in a marginal
decrease of SA β-gal positive cells compared with parent cells
(15.9 vs. 12.3%, P=0.1302). However, in Apr3 overexpressing cells,
the percentage of SA β-gal positive cells was markedly decreased
from 53.7 to 29.5% following Apr3-N overexpression (Fig. 4B, P<0.0001). Although Apr3-N
overexpression alone inhibited p21 and p53 expression in parent
cells (lane 2 vs. lane 1), both protein levels were markedly
decreased by Apr3-N plus Apr3 overexpression (Fig. 4C, lane 4 vs. lane 3). Altogether,
the data indicated that Apr3-N could abrogate the Apr3-induced
cellular senescence phenotype.
Discussion
A previous study demonstrated that Apr3
overexpression arrested the cell cycle at the G1/S phase by
inhibiting Cyclin D1 transcription (8). However, it remains to be determined
whether Apr3 affects cell behaviors other than cell proliferation.
To the best of our knowledge, the present study provided the first
evidence that the Apr3 level was increased in aged mouse RPE cells
and that oxidative stress-induced prematurely senescent ARPE-19
cells, as well as the fact that Apr3 overexpression markedly
promoted ARPE-19 senescence. Further investigation is required to
explore the molecular mechanisms responsible for the Apr3-induced
senescent phenotype.
Bioinformatics analysis demonstrated that Apr3
protein shares 46% homology with plasma membrane-localized Notch
ligands (unpublished data). Yu et al (8) showed that Apr3 was a membrane protein
in breast cancer cells. Kammula et al (12) identified that Apr3 was a novel
membrane-localized interaction partner of Nef. Therefore, Apr3 may
function as a membrane protein to transduce extracellular signals
into cells. This hypothesis was partially supported by a study by
Yu et al (8), which
demonstrated that Apr-N, truncated Apr3 with deletion of its
intracellular region, displayed classical cellular distribution of
secretory proteins and markedly antagonized Apr3 induced cell cycle
arrest (8). Similarly, it was
demonstrated that ARPE-19 cells overexpressing Apr3-N exhibited a
moderate decrease in the level of senescence compared with cells
overexpressing Apr3, and Apr3-N over-expression abrogated
Apr3-induced senescence of ARPE-19 cells. Since Apr3-N
overexpression did not alter the endogenous Apr3 level, it was
hypothesized that it may neutralize putative extracellular proteins
that interact with Apr3 or interfere with the interaction between
Apr3 and its partners, thus partially or completely blocking
Apr3-mediated signal transduction and its subsequent function.
Therefore, it will be interesting to examine whether Apr3 is
localized in the membrane of RPE cells, or whether Apr3
translocates between the cell membrane and the cytoplasm during
senescence.
Dysregulation of growth factor expression in RPE
cells has been implicated as an important pathological mechanism in
AMD (1,5). Increased expression of vascular
endothelial growth factor (VEGF) and pigment epithelial-derived
factor (PEDF) by RPE were identified in AMD and are effective
therapeutic targets for AMD (3,13,14).
However, little is known regarding the growth factor
microenvironment mediating pathological changes in AMD (4). Accumulating evidence demonstrates
that ATRA markedly increased VEGF and PEDF expression, and ATRA
emerged as the most potent inducer among the various RAs (15,16).
Apr3 levels were markedly increased in ATRA-treated HL-60 cells
(7), therefore, it was
hypothesized that ATRA may regulate Apr3 expression in RPE cells
and result in the development of AMD.
Several signal transduction pathways are involved in
the process of cellular senescence, including p53/p21 pathway,
p16/Rb pathway and insulin/insulin-like growth factor-1 (IGF1)
signal pathway (17–19). In the present study, it was
demonstrated that Apr3 induced increases in p53 and p21 expression,
whereas the p16 level was not altered. Collectively, it was
hypothesized that the Apr3-induced phenotype may be partly achieved
through the p53/p21 signaling pathway. Elucidating the molecular
mechanism by which Apr3 acts on senescence may be beneficial for
the understanding of its function.
In conclusion, the results from the present study
demonstrate, for the first time, that Apr3 overexpression in human
RPE cells accelerated cellular senescence. The role of cellular
senescence in a variety of age-associated pathologies is becoming
increasingly accepted. Current findings may aid in investigating
the function of Apr3 in other senescence-associated diseases, such
as cancer, Alzheimer's disease, muscle atrophy and cardiovascular
disease.
Acknowledgments
The authors would like to thank Dr Fang Yu at the
Fourth Military Medical University (Xi'an, China) for providing the
pcDNA3.1-hApr3 and pcDNA3.1-hApr3-N vectors.
References
|
1
|
de Jong PT: Age-related macular
degeneration. N Engl J Med. 355:1474–1485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plafker SM, O'Mealey GB and Szweda LI:
Mechanisms for countering oxidative stress and damage in retinal
pigment epithelium. Int Rev Cell Mol Biol. 298:135–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Makarev E, Cantor C, Zhavoronkov A, Buzdin
A, Aliper A and Csoka AB: Pathway activation profiling reveals new
insights into age-related macular degeneration and provides avenues
for therapeutic interventions. Aging (Albany NY). 6:1064–1075.
2014. View Article : Google Scholar
|
|
4
|
Cao S, Walker GB, Wang X, Cui JZ and
Matsubara JA: Altered cytokine profiles of human retinal pigment
epithelium: Oxidant injury and replicative senescence. Mol Vis.
19:718–728. 2013.PubMed/NCBI
|
|
5
|
Sharma K, Sharma NK and Anand A: Why AMD
is a disease of ageing and not of development: Mechanisms and
insights. Front Aging Neurosci. 6:1512014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hjelmeland LM, Cristofolo VJ, Funk W,
Rakoczy E and Katz ML: Senescence of the retinal pigment
epithelium. Mol Vis. 5:331999.PubMed/NCBI
|
|
7
|
Zhu F, Yan W, Zhao ZL, Chai YB, Lu F, Wang
Q, Peng WD, Yang AG and Wang CJ: Improved PCR-based subtractive
hybridization strategy for cloning differentially expressed genes.
Biotechniques. 29:310–313. 2000.PubMed/NCBI
|
|
8
|
Yu F, Yang G, Zhao Z, Ji L, Cao Y, Bai L,
Lu F, Fu H, Huang B, Li H, et al: Apoptosis related protein 3, an
ATRA-upregulated membrane protein arrests the cell cycle at G1/S
phase by decreasing the expression of cyclin D1. Biochem Biophys
Res Commun. 358:1041–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou X, Shen J, Chen F, Ting K, Zheng Z,
Pang S, Zara JN, Adams JS, Soo C and Zhang X: NELL-1 binds to APR3
affecting human osteoblast proliferation and differentiation. FEBS
Lett. 585:2410–2418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
Jeyapalan JC, Ferreira M, Sedivy JM and
Herbig U: Accumulation of senescent cells in mitotic tissue of
aging primates. Mech Ageing Dev. 128:36–44. 2007. View Article : Google Scholar
|
|
12
|
Kammula EC, Mötter J, Gorgels A, Jonas E,
Hoffmann S and Willbold D: Brain transcriptome-wide screen for
HIV-1 Nef protein interaction partners reveals various
membrane-associated proteins. PLoS One. 7:e515782012. View Article : Google Scholar
|
|
13
|
He Y, Leung KW, Ren Y, Pei J, Ge J and
Tombran-Tink J: PEDF improves mitochondrial function in RPE cells
during oxidative stress. Invest Ophthalmol Vis Sci. 55:6742–6755.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ablonczy Z, Dahrouj M and Marneros AG:
Progressive dysfunction of the retinal pigment epithelium and
retina due to increased VEGF-A levels. FASEB J. 28:2369–2379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tombran-Tink J, Lara N, Apricio SE,
Potluri P, Gee S, Ma JX, Chader G and Barnstable CJ: Retinoic acid
and dexamethasone regulate the expression of PEDF in retinal and
endothelial cells. Exp Eye Res. 78:945–955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JT, Liang JB, Chou CL, Shyu RC and Lu
DW: Retinoic acid induces VEGF gene expression in human retinal
pigment epithelial cells (ARPE-19). J Ocul Pharmacol Ther.
21:413–419. 2005. View Article : Google Scholar
|
|
17
|
Bartke A: Impact of reduced insulin-like
growth factor-1/insulin signaling on aging in mammals: Novel
findings. Aging Cell. 7:285–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dvashi Z, Green Y and Pollack A: TAK1
inhibition accelerates cellular senescence of retinal pigment
epithelial cells. Invest Ophthalmol Vis Sci. 55:5679–5686. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salama R, Sadaie M, Hoare M and Narita M:
Cellular senescence and its effector programs. Genes Dev.
28:99–114. 2014. View Article : Google Scholar : PubMed/NCBI
|