Introduction
Oxazolidinones are synthetic heterocyclic compounds
demonstrating antimicrobial properties against resistant
Gram-positive pathogenic bacteria and Mycobacterium
tuberculosis. Furazolidone 1 (Fig.
1), a synthetic nitrofuran oxazolidinone was the first
described member of this class of compounds with antimicrobial
activity targeting bacterial DNA. However, several studies on other
oxazolidinone derivatives have also presented data indicating
diverse biological activities, such as anticancer, anticoagulant,
antithyroid and central nervous system effects, as well as
inhibition of monoamine oxidases (1–4). A
recent study by Sun et al (5), reported that furazolidone induced S
phase cell cycle arrest, suppressed cell growth, increased
phosphorylated p38 (p-p38) activity and decreased the activity of
phosphorylated c-Jun N-terminal protein kinase in HepG2 human
hepatoblastoma cells. Furthermore, another study (2) demonstrated antileukemic properties of
furazolidone in acute myeloid leukemia cells in vitro,
through increased stability of tumor suppressor p53 protein.
Furazolidone has also been reported to induce genotoxicity and
carcinogenicity in certain cell types (6). In addition, other oxazolidinone
derivatives (Fig. 1), namely 2
(BAY a 5830) (7), the
2-nitro-1H-imidazol-1-yl oxazolidinone 3 (1), and chloroquinoline oxazolidinone 4
have been shown to exhibit anticancer properties (8). More recently, Naresh et al
(9) reported the activity of
cytoxazone-linezolid hybrid oxazolidinone derivatives exemplified
by 5 (Fig. 1) against DU145
prostate and A549 lung cancer cell lines.
Linezolid 6 (Fig.
1) is a totally synthetic oxazolidinone with efficacy against
Gram-positive bacteria, including multidrug-resistant strains,
namely methicillin-resistant Staphylococcus aureus (MRSA),
penicillin-resistant Streptococcus pneumoniae (PRSP) and
vancomycin-resistant enteroccoci (VRE) (10,11).
Generally, oxazolidinones, including linezolid, inhibit bacterial
ribosomal protein biosynthesis with virtually no effect on DNA and
RNA synthesis (12,13). Other studies (14,15)
have shown that this class of compounds bind to the 50S ribosomal
subunit, in particular to the A-site. As a consequence,
oxazolidinones have been suggested to possess the ability to bind
mitochondrial ribosomes, leading to inhibition of mammalian
mitochondrial protein synthesis, which could translate to drug
toxicity in humans (16,17). In addition, antibacterial agents of
the oxazolidinone class also cause undesirable side effects, such
as bone marrow suppression (18,19),
inhibition of monoamine oxidases (20), linezolid-induced neuropathy
(21) and linezolid-associated
toxic optic neuropathy (22)
usually during prolonged courses of linezolid treatment. The
mechanism of neuronal side effects has been suggested to be due to
the impairment of mitochondrial protein synthesis. Furthermore,
lactic acidosis and pancytopenia have also been reported in the
clinic during linezolid treatment in a number of patients (23–25).
Despite these effects, linezolid is deemed to be a clinically
successful antibacterial drug for treating infections due to
multi-drug resistant bacterial pathogens. Overcoming these
challenges and other pharmacokinetic issues continues to be a focus
of pharmaceutical scientists who are exploiting the oxazoldinone
pharmacophore as a basic nucleus for further drug discovery and
development for the treatment of human diseases.
In this regard, the present study and other studies
(1,26-28)
have evaluated several structural analogs of linezolid in search of
newer derivatives with improved antibacterial activity. In view of
the diverse biological activities exhibited by substituted
oxazolidinone derivatives, the present study evaluated the effects
of selected optionally substituted oxazolidinone derivatives,
namely, the 5-heptanamidomethyl-(PH-97) and
5-(1H-1,2,3-triazolylmethyl) oxazolidinone derivatives 7
(Fig. 1) and linezolid 6 (10) on the growth of several cancerous
human cell lines. This study aimed to determine their potential
alternative use as anticancer agents, and the specificity of their
antimicrobial activity.
Materials and methods
Chemicals
The chemical structures of the oxazolidinone
derivatives utilized in this study are presented in (Fig. 1). Linezolid and 8 previously
reported oxazolidinone derivatives PH13, PH23, PH27, PH68, PH80,
PH97, PH117 and PH188 were synthesized following published
protocols (10,26-28).
The compounds were purified and characterized using appropriate
spectroscopic (NMR, IR MS) and analytical (CHN analysis) methods.
The purified compounds were dissolved in dimethyl sulfoxide (DMSO)
and stored at −20°C until use (within two weeks).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reagent was obtained from Promega Corporation (Madison, WI, USA).
The phycoerythrin (PE) Annexin V apoptosis detection kit I was
purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Cell culture
MCF7 [estrogen receptor (ER)+] and MDA231
(ER−) human breast cancer cell lines were derived from
the American Type Culture Collection (Manassas, VA, USA) and
routinely maintained at 37°C in a humidified atmosphere of 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 600 mg/ml
L-glutamine, 100 U/ml penicillin-100 mg/ml streptomycin and 6 ml
100× non-essential amino acids/500 ml (all purchased from
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell motility assay
Cells were grown to ~90% confluency in 24-well
plates. Guided by a ruler, a fine scratch was made with a yellow
Eppendorf pipette tip through the center of the monolayer. Detached
cells were removed by gentle washing with phosphate-buffered saline
and fresh medium was added. The width of the scratch was recorded
from photographs taken under phase-contrast light microscopy (DM IL
LED; Leica Microsystems Gmbh, Wetzlar, Germany) by an attached
camera (Leica DFC500; Leica Microsystems GmbH) immediately, and
after 24 h, to determine the extent of migration of cells to
re-fill the space.
Agarose invasion assay
After melting in PBS, ultra-pure agarose
(Invitrogen, Thermo Fisher Scientific Inc.) was supplemented with
DMEM with 5% FBS to give a final 0.5% solution, and allowed to
solidify in individual wells of 6-well dishes at room temperature.
Once set, 1–3 sample chambers (3.5 mm in diameter) were created in
the gel, 2.5 mm apart in a horizontal line, by insertion of a
metallic mold made for the present study. Cells (4×104)
were re-suspended in complete DMEM and loaded into formed chambers.
Plates were incubated at 37°C in a 5% CO2 humidified
atmosphere. After 24 h, cells that had penetrated the agarose were
manually counted by visual microscopic examination (DM IL LED).
Random cell invasion was determined as the total number of cells
that moved in both lateral directions out of the well into the
surrounding agarose (29).
Cell proliferation assay
Approximately 200 µl MCF7 or MDA231 cells
re-suspended in DMEM at 5×103 cells/ml were seeded in
quadruplicates into 96-well plates and allowed to attach overnight.
Either vehicle only (DMSO) or oxazolidinone compounds (100 nM-10
µM) were then added to the cells. Growth was assessed by an
MTT assay after 4 days of incubation. Briefly, 100 µl MTT
(0.5 mg/ml) was added to each well and plates incubated at 37°C for
30 min followed by the addition of 100 µl of acidified
isopropanol (Sigma-Aldrich) and vigorous re-suspension of the
converted blue dye crystals. Absorbance of the suspension was
measured at 595 nm using a Thermo Scientific Multiskan Spectrum
plate reader (Thermo Fisher Scientific, Inc.) with background
subtraction at 650 nm. The effect of the compounds was compared
with the untreated control cells (taken as 100%). Each experiment
was repeated a minimum of three times (with quadruplicates) with
different batches of cells.
Flow cytometry
MCF7 cells were incubated in 6-well plates with
PH80, PH68 and PH117 at 100 µM in triplicate for 3 days.
Cell monolayers were trypsinized (Invitrogen; Thermo Fisher
Scientific, Inc.), pelleted by centrifugation at 1,000 × g for 3
min and washed twice by re-suspension and centrifugation in
ice-cold PBS at 1,000 × g at 3°C and once in Annexin-V binding
buffer (10 mM HEPES/NaOH, pH 7.4; 0.14 M NaCl; and 2.5 mM
CaCl2). The final cell pellet was re-suspended in 100
µl of Annexin-V binding buffer at 5×106 cells/ml
and processed for FACS analysis on an FC500 flow cytometer (Beckman
Coulter, Inc., Brea, CA, USA) using the phycoerythrin (PE) Annexin
V apoptosis detection kit I. Cells were stained in the following
manner: (A), cells only (negative control); (B), with 10 µl
Annexin V-PE; (C), with 20 µl 7AAD; and (D), with 10
µl Annexin V-PE plus 20 µl 7AAD. All incubations were
performed in the dark at room temperature for 15 min.
Statistical analysis
Differences between the mean values of control
compared with treated groups were analyzed by Student's t-test and
one-way analysis of variance for multiple comparisons (different
doses of the therapeutic agents) using GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of oxazolidinone derivatives on
the proliferation of MCF7 breast cancer cells
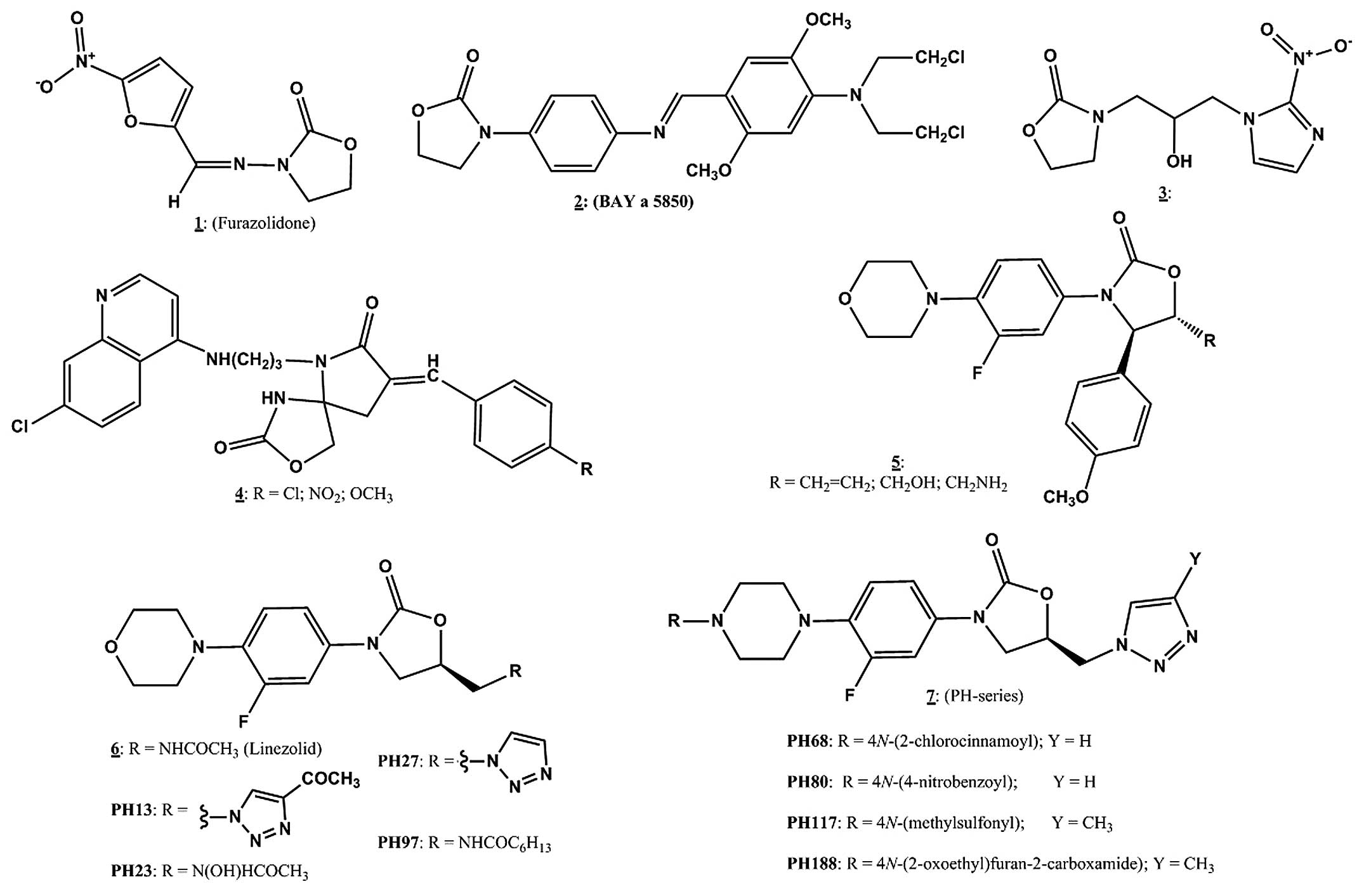
The effects of linezolid and 8 oxazolidinone
derivatives on the growth of MCF7 cells are shown in (Fig. 2). Linezolid and PH27 which have
potent MIC values against bacteria (Table I) and PH13, which does not, showed
no significant inhibition at any concentration, indicating that
neither the 5-acetamido nor the 5-triazolyl substitution on the
morpholino derivatives resulted in any observable antiproliferative
effect. Conversely, the oxazolidinone derivatives containing
piperazino-5-(1H-1,2,3-triazolyl) methyl (PH68, PH80 and
PH117) and the morpholino-5-heptanoyl) methyl (PH97) groups, which
have potent antibacterial activity, showed significant
dose-dependent activity towards MCF7 cells. At 100 µM they
caused 50–60% inhibition of cell proliferation. However, the
morpholino-(5-heptanoyl) methyl derivative, PH97, exhibited a
biphasic effect, causing growth stimulation at 1 µM and
inhibition at 50–100 µM. The piperazino-5-hydroxamic acid
derivative, PH23, which has poor antibacterial activity actually
stimulated growth significantly at 10–100 µM. The
N-(furan-2-carboxamide) glycinyl piperazinyl-5-
(1H-1,2,3-triazol-1-yl)methyl) oxazolidinone derivative,
PH188, also stimulated growth but only significantly at the lower
concentrations of 10 nM-1µM.
 | Table IMIC values of oxazolidinone
derivatives. |
Table I
MIC values of oxazolidinone
derivatives.
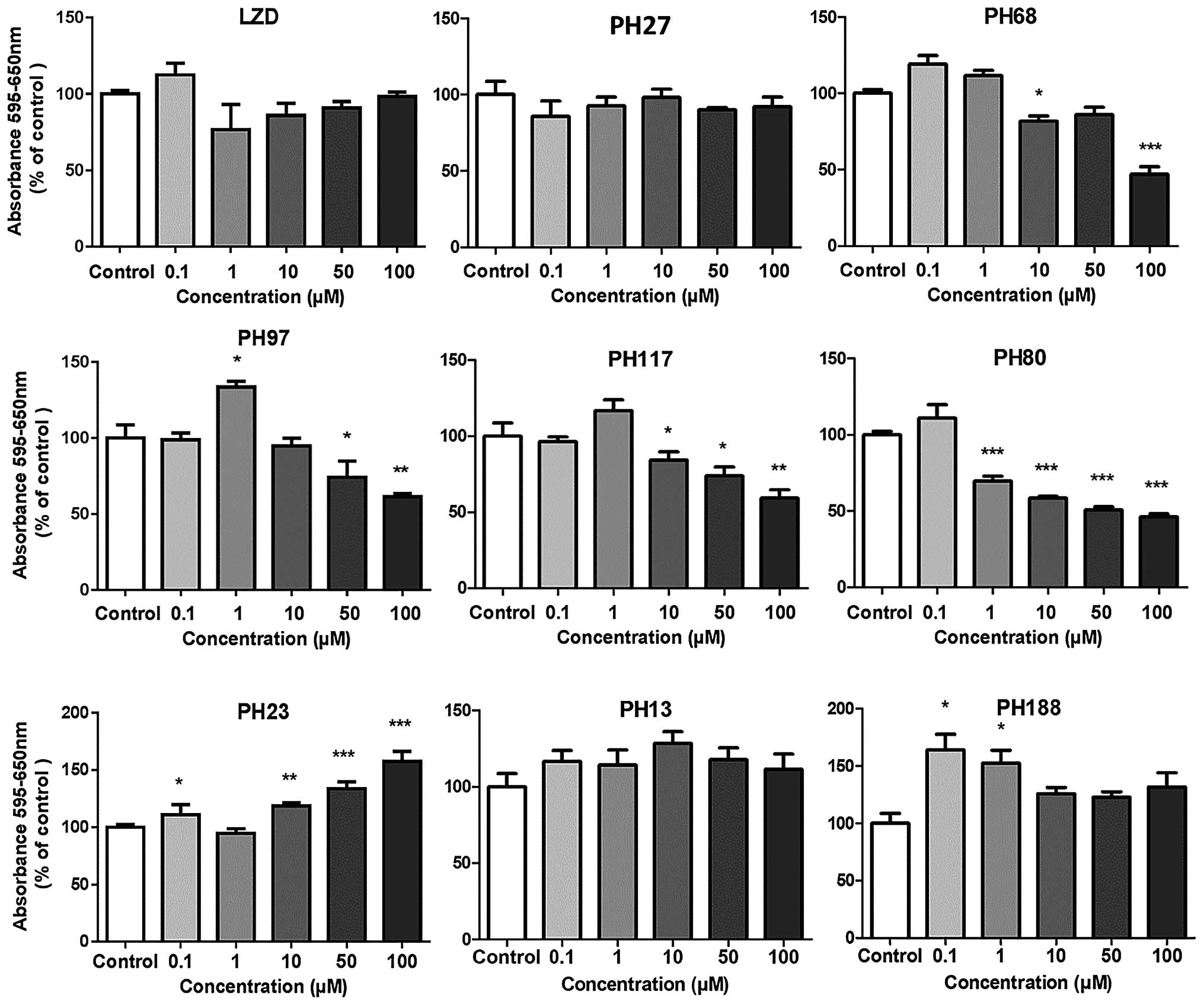
Effect of PH68, PH80 and PH117 on
proliferation of breast cancer cells
Based on the initial observations with MCF7 cells,
the piperazino-5 (1H-1,2,3-triazolyl) methyl derivatives
containing N-(2-chlorocinnamoyl) (PH68),
N-(4-nitrobenzoyl) (PH80) and N-(methylsulfonyl)
(PH117) groups were selected for further assessment. These were
tested for their effect on the growth of a more aggressive breast
cancer cell line, MDA231. The results (Fig. 3) were similar for the two cell
lines and consistent with their previously observed effect on MCF7
cells. The most potent compound was the
N-piperazinyl-(2-chlorocinnamoyl) derivative PH68 with
marked inhibition from 10 µM and reaching 80% inhibition at
100 µM, with the others being less effective (Fig. 3). PH117 exhibited ~50% inhibition
from 50 µM and PH80 at 100 µM. However, all the three
compounds showed pronounced (150%) stimulation of growth at lower
concentrations of 100 nM. The same phenomenon was observed in MCF7
cells but the magnitude of the effect was insufficient to be of
statistical significance.
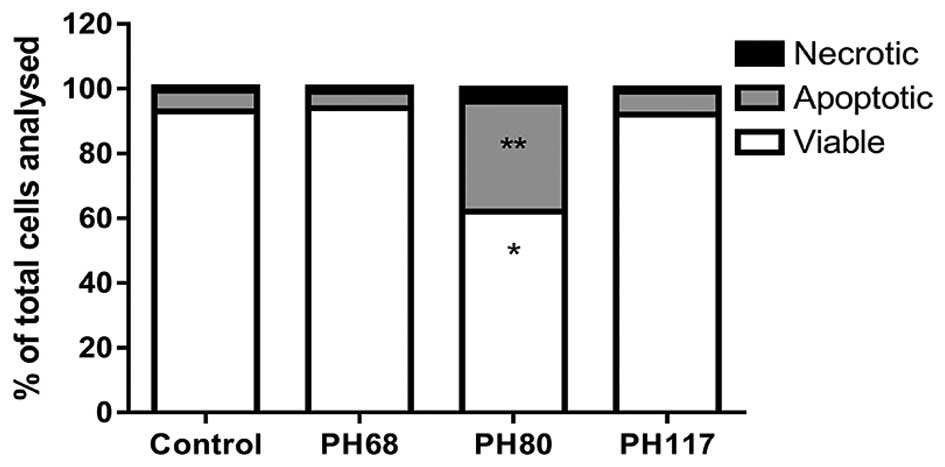
Assessment of cytotoxicity
Flow cytometry was used to determine the extent of
apoptosis induced by PH68, PH80 and PH117 (Fig. 4). Neither PH68 nor PH117 affected
the viability of MCF7 cells, whilst PH80 induced a significant
reduction of the viable population of cells by 36%, and a 69%
increase in apoptosis.
Cell motility
The wound closure assay (30) was used to determine the effects of
PH68, PH117 and PH80 on the motility of MDA231 cells. At
concentrations of 50 and 100 µM, PH68 and PH80 inhibited
wound closure by ~50% as compared with cells treated with vehicle
alone (DMSO). Conversely, at a concentration of 100 nM, they
actually stimulated cell motility and closed the wound to a
significantly greater extent than observed for control vehicle
treated cells (Fig. 5). In the
case of cells treated with PH117 at either a low or high dose,
there was no significant difference from vehicle treatment.
Cell invasion assay
The invasion assay described previously (29) was used to determine the effect of
PH68, PH80 and PH117 on the ability of MDA231 cells to penetrate
agarose gel. As shown in (Fig. 6),
at high doses (PH68 50 µM; PH117 50 µM; PH80 100
µM) all three drugs produced significant inhibition of
invasion by ~50%. At low doses (100 nM), only PH68 exhibited
significant inhibition of invasion.
Discussion
Antibiotics active solely against prokaryotic
organisms have become indispensable to human health. Moreover,
onset of bacterial resistance to these drugs continues to pose a
serious threat that has led to extensive efforts to overcome this
by developing novel potent agents. Linezolid is an antibiotic that
belongs to the oxazolidinone class of compounds, which are often
the last resort when other antibiotic therapies have failed. The
great success of antibiotics has been due to the fact that their
mode of action specifically targets the characteristics that
distinguish prokaryotic from eukaryotic mechanisms, preventing
unwanted side effects. It is important that novel agents do not
exhibit any effects on eukaryotic cells. Certain oxazolidinones
have been shown not only to exhibit antimicrobial properties but
also to affect human cells. It is therefore essential to
distinguish these agents to prevent potential adverse effects. At
the same time, their dual action could be put to good use. Several
studies (1–4) have suggested that application of
oxazolidinones may provide significant therapeutic benefits in
cases of HIV, cancer and epilepsy. In this study, linezolid and
eight other oxazolidinone derivatives bearing 5-hydroxamate-,
5-heptanoyl- and 5-(1H-1,2,3-triazolyl)-methyl moieties were
initially screened for possible inhibitory effects on eukaryotic
cells. The ER+ MCF7 breast cancer line is well studied,
and is an example of a commonly used eukaryotic cell line, for the
initial evaluation. Significant differences were observed between
these compounds which had no direct correlation with their
antibacterial activity. The prototype compound linezolid, and its
5-(1H-1,2,3-triazolyl) methyl derivatives PH27 and PH13 had
no significant effect on MCF7 cell proliferation and were not
investigated further. These are considered to be safe antibacterial
agents. Mixed effects were observed with the remaining 6 compounds.
The compounds PH23 and PH188 appear to be unsuitable as
anti-microbial agents given their MIC values (>95 and 24
respectively) in a range where they actually stimulate eukaryotic
cell growth. This would be particularly problematic if administered
to cancer patients. The precise mechanism for the stimulation is
not clear at this time. By contrast, PH68, PH117 and PH80 all
exhibited dose-dependent inhibition of MCF7, MDA231 and HBL100
proliferation, which may equally compromise their anti-bacterial
use. However, in another context, these compounds may be of use as
anticancer agents instead. Notably, however, they display the
phenomenon of 'hormesis', a term widely used by toxicologists to
describe a biphasic dose response to a drug, with stimulatory
effects at low dose and inhibition at higher doses; thought to be
an adaptive compensatory response to initial disruption of cellular
homeostasis (31,32). At low doses a drug could block an
endogenous inhibitory factor, resulting in stimulation, before it
starts to exert its own inhibitory effect on other pathways at
higher concentration. Furthermore, to distinguish between their
cytostatic and potential cytotoxic activity, MCF7 cells treated
with these 3 compounds were analyzed for evidence of apoptosis by
flow cytometry using Annexin/7-AAD labeling. The results indicated
that only the N-piperazino-4-nitrobenzoyl-5
(1H-1,2,3-triazolyl) methyl derivative PH80 induced cell
death. It is notable that this compound has a terminal nitro-group,
which may be responsible for the cytotoxicity. Nitro groups
attached to aromatic rings may be metabolically converted to
potentially lethal reactive intermediates. This type of conversion
(by the P450 enzymes) has been reported for the nitro-imidazole
group in the antibiotic nitrofurantoin, and has been suggested to
be responsible for its anticancer effects (33).
Much of the difficulty in the treatment of solid
cancers is in the control of disseminated disease, and thus
preventing its spread would greatly enhance treatment. In this
regard, the ability of these drugs to block tumor cell motility and
invasion was determined. Of the three compounds that effectively
reduced proliferation, only PH68 and PH80 (at 50 and 100 µM,
respectively) significantly retarded cell movement. Notably, at a
lower dose of 100 nM, PH68 and PH80 actually stimulated cell
migration, paralleling the phenomenon observed with proliferation
(although in that case it was not identified to be significant).
With respect to invasion of MDA231 cells, all three compounds had
an inhibitory effect at the higher doses, with only PH68 also
exhibiting the same effect at the lower dose. As these are complex
processes involving multiple pathways, it is quite likely that the
oxazolidinone derivatives may have selective as well as common
cellular targets, accounting for the differences. Further studies
are on-going in our laboratories on these compounds.
The observations suggest that linezolid, PH13, PH23
and PH27 lack anticancer activity against breast cancer cell lines
and are suitable as antibacterial agents from that perspective.
However, the triazolyl derivatives PH68, PH80 and PH117 exhibit
activity against breast cancer cells and also inhibited cancer cell
motility and invasion. Therefore these three compounds merit
further investigation as potential anticancer agents particular as
they affect several stages of cancer progression. Due to the
limited number of compounds investigated in this study it was not
possible to establish a meaningful structure-activity relationship
for this class of compounds.
Acknowledgments
This study was supported by the Research
Administration, Kuwait University Research Grant SRUL/0230
(Research Core Facility, Health Science Centers).
References
|
1
|
Pandit N, Singla RK and Shrivastava B:
Current updates on oxazolidinone and its significance. Int J Med
Chem. 2012:1592852012.PubMed/NCBI
|
|
2
|
Jiang X, Sun L, Qui JJ, Sun X, Li S, Wang
X, So CW and Dong S: A novel application of furazolidone:
Anti-luekemic activity in acute myeloid leukemia. PLOS ONE.
8:e723352013. View Article : Google Scholar
|
|
3
|
Reck F, Zhou F, Girardot M, Kern G,
Eyermann CJ, Hales NJ, Ramsay RR and Gravestock MB: Identification
of 4-substituted 1,2,3-triazoles as novel oxazolidinone
antibacterial agents with reduced activity against monoamine
oxidase A. J Med Chem. 48:499–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kombian SB and Phillips OA: In vitro
electrophysiological investigations of the acute effects of
linezolid and novel oxazolidinones on central nervous system
neurons. Neurosci. 180:53–63. 2011. View Article : Google Scholar
|
|
5
|
Sun Y, Tang S, Jin X, Zhang C, Zhao W and
Xiao X: Opposite effects of JNK and p38 MAPK signaling pathways on
furazolidinone-stimulated S phase cell cycle arrest of human
hepatoblastoma cell line. Mutat Res. 775:24–29. 2013. View Article : Google Scholar
|
|
6
|
Auro A, Sumano H, Ocampo L and Barragán A:
Evaluation of the carcinogenic effects of furazolidone and its
metabolites in two fish species. The Pharmacogenomics J. 4:24–28.
2004. View Article : Google Scholar
|
|
7
|
Artico M, De Martino G and Giuliano R:
Research on compounds with antiblastic activity. XL Synthesis of
3-p-(2′, 5′-dimethoxy-4′-(N,
N-bis-(-chloroethyl)-amino)benzylideneamino) phenyl-2-oxazolidinone
(GEA 29; BAY a 5850) and its analogues. Farmaco Sci. 26:771–783.
1971.PubMed/NCBI
|
|
8
|
Devi K, Asmat Y, Agrawal M, Sharma S and
Dwived J: Synthesis and evaluation of some novel precursors of
oxazolidinone analogues of chloroquinoline for their antimicrobial
and cytotoxic potential. J Chem Sci. 125:1093–1101. 2013.
View Article : Google Scholar
|
|
9
|
Naresh A, Venkateswara Rao MV, Kotapalli
SS, Ummanni R and Venkateswara Rao B: Oxazolidinone derivatives:
Cytoxazone-linezolid hybrids induces apoptosis and senescence in
DU145 prostate cancer cell. Eur J Med Chem. 80:295–307. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brickner SJ, Hutchinson DK, Barbachyn MR,
Manninen PR, Ulanowicz DA, Garmon SA, Grega KC, Hendges SK, Toops
DS, Ford CW and Zurenko GE: Synthesis and antibacterial activity of
U-100592 and U-100766, two oxazolidinone antibacterial agents for
the potential treatment of multidrug-resistant gram-positive
bacterial infections. J Med Chem. 39:673–679. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilcox MH: Update on linezolid: The first
oxazolidinone antibiotic. Expert Opin Pharmacother. 6:2315–2326.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eustice DC, Feldman PA, Zajac I and Slee
AM: Mechanism of action of DuP 721: Inhibition of an early event
during initiation of protein synthesis. Antimicrob Agents
Chemother. 32:1218–1222. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalia V, Miglani R, Purnapatre KP, Mathur
T, Singhal S, Khan S, Voleti SR, Upadhyay DJ, Saini KS, Rattan A
and Raj VS: Mode of action of Ranbezolid against staphylococci and
structural modeling studies of its interaction with ribosomes.
Antimicrob Agents Chemother. 53:1427–1433. 2009. View Article : Google Scholar :
|
|
14
|
Zhou CC, Swaney SM, Shinabarger DL and
Stockman BJ: 1H nuclear magnetic resonance study of oxazolidinone
binding to bacterial ribosomes. Antimicrob Agents Chemother.
46:625–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ippolito JA, Kanyo ZF, Wang D, Franceschi
FJ, Moore PB, Steitz TA and Duffy EM: Crystal structure of the
oxazolidinone antibiotic linezolid bound to the 50S ribosomal
subunit. J Med Chem. 51:3353–3356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McKee EE, Ferguson M, Bentley AT and Marks
TA: Inhibition of mammalian mitochondrial protein synthesis by
oxazolidinones. Antimicrob Agents Chemother. 50:2042–2049. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Vriese AS, Coster RV, Smet J, Seneca S,
Lovering A, Van Haute LL, Vanopdenbosch LJ, Martin JJ, Groote CC,
Vandecasteele S and Boelaert JR: Linezolid-Induced Inhibition of
Mitochondrial Protein Synthesis. Clin Infect Dis. 42:1111–1117.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuter DJ and Tillotson GS: Hematologic
effects of antimicrobials: Focus on the oxazolidinone linezolid.
Pharmacotherapy. 21:1010–1013. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerson SL, Kaplan SL, Bruss JB, Le V,
Arellano FM, Hafkin B and Kuter DJ: Hematologic effects of
linezolid: Summary of clinical experience. Antimicrob Agents
Chemother. 46:2723–2726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leach KL, Brickner SJ, Noe MC and Miller
PF: Linezolid, the first oxazolidinone antibacterial agent. Ann N Y
Acad Sci. 1222:49–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Corallo CE and Paull AE: Linezolid-induced
neuropathy. Med J Aust. 177:3322002.PubMed/NCBI
|
|
22
|
Rucker JC, Hamilton SR, Bardenstein D,
Isada CM and Lee MS: Linezolid-associated toxic optic neuropathy.
Neurology. 66:595–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kraleti S and Soultanova I: Pancytopenia
and lactic acidosis associated with linezolid use in a patient with
empyema. J Ark Med Soc. 110:62–63. 2013.PubMed/NCBI
|
|
24
|
Narita M, Tsuji BT and Yu VL:
Linezolid-associated peripheral and optic neuropathy, lactic
acidosis and serotonin syndrome. Pharmacotherapy. 27:1189–1197.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su E, Crowley K, Carcillo JA and Michaels
MG: Linezolid and lactic acidosis: A role for lactate monitoring
with long-term linezolid use in children. Pediatr Infect Dis J.
30:804–806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phillips OA, Udo EE, Ali AA and Al-Hassawi
N: Synthesis and antibacterial activity of 5-substituted
oxazolidinones. Bioorg Med Chem. 11:35–41. 2003. View Article : Google Scholar
|
|
27
|
Phillips OA, Udo EE, Ali AAM and Samuel
SM: Structure-antibacterial activity of arylcarbonyl- and
arylsulfonyl-piperazine 5-triazolylmethyl oxazolidinones. Eur J Med
Chem. 42:214–225. 2007. View Article : Google Scholar
|
|
28
|
Phillips OA, Udo EE, Abdel-Hamid ME and
Varghese R: Synthesis and antibacterial activities of
N-substituted-gylcinyl 1H-1,2,3-triazolyl oxazolidinones. Eur J Med
Chem. 66:246–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khajah MA, Al Saleh S, Mathew PM and
Luqmani YA: Differential Effect of Growth Factors on Invasion and
Proliferation of endocrine resistant breast cancer cells. PLoS One.
7:e418472012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang C, Park A and Guan J: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoco. 2:329–333. 2007. View Article : Google Scholar
|
|
31
|
Calabrese EJ and Blain R: The occurrence
of hormetic dose responses in the toxicological literature, the
hormesis database: An overview. Toxicol Appl Pharmacol.
202:289–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nascarella M, Stanek E, Hoffmann GR and
Calabrese EJ: Quantification of hormesis in anticancer-agent
dose-responses. Dose-Response. 72:160–171. 2009.
|
|
33
|
Wang Y, Gray JP, Mishin V, Heck DE, Laskin
DL and Laskin JD: Role of cytochrome P450 reductase in
nitrofurantoin-induced redox cycling and cytotoxicity. Free Radical
Bio Med. 44:1169–1179. 2008. View Article : Google Scholar
|