Introduction
Alzheimer's disease (AD) is a degenerative disorder
of the nervous system of elderly individuals, and the most common
type of dementia (1). AD is
predominantly characterized by the progressive loss or decline of
cognition and memory function (1).
The pathological characteristics of AD include the formation of
intracellular neurofibrillary tangles (NFTs) and extracellular
neuritic plaques containing amyloid-β (Aβ) peptide (2). The association of NFTs and Aβ is yet
to be fully elucidated, however one hypothesis is that Aβ may
trigger the hyper-phosphorylation of the tau protein, leading to
the impairment of axonal transport and destabilization of
microtubules, resulting in neuronal apoptosis (3). Based on the above, it was suggested
that the phosphorylation of tau may act as an important process in
the pathogenesis of AD.
A previous study identified the dominant mutations
in the amyloid precursor protein (APP) gene, which was also
discovered in the presenilin 1 gene (PSEN1) and presenilin 2 gene
(PSEN2) (4). Aβ1-40 and Aβ1-42
peptides are generated following the sequential cleavage of APP.
Aβ1-40 and Aβ1-42 accumulate to form the amyloid plaques, one of
the major characteristics of AD (2,3).
Previous studies investigated the role of APP in AD and suggested
various hypotheses, however its function remains elusive (5,6). In
addition, the mechanisms of activation of the pathways involved in
the process of APP, in normal and AD-ageing remain to be fully
clarified. A previous study on APP identified that APP was
modulated by phosphorylation and phosphorylation-dependent
pathways, directly and indirectly (7). Kojro and Fahrenholz (8) reported that the processing of APP
occurs via two alternative pathways, the amyloidogenic and
nonamyloidogenic pathways, which serve a role in the activation of
β-secretase and α-secretase, respectively.
Sirtuins (SIRTs) or silent information regulators
were firstly discovered and extracted in yeast (9). SIRT are grouped as class III histone
deacetylases, that function by removing acetyl groups from lysines
through consumption of nicotinamide adenine dinucleotide (NAD)
(9). There are seven homologs of
SIRTs (1–7) in humans displaying various enzymatic
activities and functions (10).
SIRT1, 2 and 3 have higher deacetylase activities compared with
SIRT4, 5 and 6 (11–14). SIRTs are located in different cell
components, such as the nucleus (SIRT1, SIRT6 and SIRT7), cytoplasm
(SIRT2) and mitochondria (SIRT3, SIRT4 and SIRT5) (15). SIRTs are highly conserved
NAD+-dependent enzymes that have beneficial effects on
certain age-associated diseases (12,13).
Numerous studies investigated the effects of SIRTs on AD in
numerous mouse models in vivo and cell models in
vitro (16–18), concluding that the SIRT1
overexpression displayed a protective effect on the AD phenotype,
with SIRT1 being the only SIRT studied in AD animal or cell models.
Thus, a therapeutic strategy for AD was designed based on the SIRT1
activity.
Fuzhisan (FZS) is a Chinese herbal complex
prescription, which contains the Scutellaria baicalensis
Georgi (Labiatae family), Ginseng root
(Araliaceae family), Glycyrrhiza uralensis
(Leguminosae family) and Anemone altaica
(Araceae family) (19). FZS
has been used in the clinical therapy for senile dementia for over
fifteen years (19,20). Previous studies indicated that FZS
increased the cognitive function of patients with AD or AD animal
models (21). In addition, other
effects or functions of FZS have been identified, including
neurotrophic effects, neuroprotective functions. FZS regulates cell
apoptosis, therefore, it may prevent the toxicity in SH-SY5Y
neuroblastoma cells resulting from Aβ25-35 accumulation (22). Shirong et al (23) demonstrated that FZS increased the
hippocampal acetylcholine levels and enhanced the spatial learning
capability. Furthermore, FZS improved glucose metabolism in the
brain, and blood flow in the frontal and temporal lobes of patients
with AD. However, the specific effects of FZS on tau
phosphorylation remain to be identified. In addition, the potential
signaling pathways used or the mechanisms for neurotrophic and
neuroprotective properties of FZS are elusive.
Therefore, the present study investigated the
effects and mechanism of FZS and donepezil on the SIRT1 pathway and
APP metabolism in PC12 cells, to identify whether FZS attenuates
the Aβ25-35-induced toxicity in the cultured PC12 cells, and the
effect underlying the signaling mechanisms.
Materials and methods
Cell culture
The neuronal cell line PC12 was purchased from the
Cell Resource Center of Shanghai Institutes, Academy of Sciences
(Shanghai, China). The PC12 cells were cultured and grown as a
mono-layer of cells in the Roswell Park Memorial Institute 1640
(RPMI-1640) medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), 60 µg/ml penicillin
(Sigma-Aldrich, St. Louis, MO, USA) and 100 µg/ml
streptomycin (Sigma-Aldrich) in 5% CO2 at 37°C.
FZS preparation
The specimen, extraction methods and the effects of
evaluation and analysis of FZS were performed as previously
described (19,20). The component of FZS, including the
Ginseng root, Anemone altaica, Glycyrrhiza uralensis
and Scutellaria baicalensis Georgi, was obtained from the
Harbin Pharmaceutical Company (Harbin, China). The four components
were mixed in proportions of 2:1:1:1, respectively, and macerated
for 40 min in 8 volumes (v/w) of distilled water, and then decocted
for 1 h. The filtrate was collected and the residue was decocted
for another 1 h with 6 volumes (v/w) of distilled water. The
filtrate was pooled and lypholized (crude extract). Finally, the
crude extract of FZS was dissolved in water at a final
concentration of 0.5 g/ml (crude drug), and stored at −20°C for
further experimental use.
Aβ25-35 peptide preparation
The synthetic Aβ25-35 peptide (purity ≥97%,
high-performance liquid chromatography) solution was prepared as
previously described (24).
Briefly, the Aβ25-35 peptide was dissolved in sterile deionized
water at a final concentration of 1.0 mM, and then incubated at
37°C for 3 days to allow for aggregation.
Neurotoxic cell model establishment
The PC12 cell line was cultured in RPMI-1640,
supplemented with 10% FBS at 37°C in a humidified atmosphere
supplemented with 5% CO2. RPMI-1640 medium was added to
the cells for 3 days, and then replaced with new medium for another
3 days. In order to prepare the experiments, cells were seeded into
24-well plates (2×104 cells/cm2), and after
24 h, Aβ25-35 (10, 20 or 40 µM) was added to the medium.
Cells were evaluated and observed under a microscope (CKX-31;
Olympus Corporation, Tokyo, Japan) at 24 and 48 h following Aβ25-35
incubation.
Effect of FZS and donepezil on cultured
cells
PC12 cells were seeded in 24-well plates and divided
into two groups for Aβ25-35 treatment as follows: i) Donepezil
group, treatment with 20 mM donepezil; ii) FZS group, treatment
with 2.5, 5, 15, 45, 90, 135 or 270 µg/ml FZS. Cells that
were not treated with the therapeutic agents were designated as the
control group. Following incubation for 24 or 48 h, cells were
cultured, harvested and subjected to the different experiments.
Protective effect of FZS and donepezil on
cells treated with Aβ25-35
PC12 cells were seeded in 24-well plates and divided
into three groups for Aβ25-35 treatment as follows: i) Aβ25-35
injury group, 20 mM Aβ25-35 treatment; ii) donepezil
(Sigma-Aldrich) protection group, 20 µM donepezil were added
to 1 ml culture medium 2 h prior to Aβ25-35 injury; iii) FZS
protection group, 2.5, 5, 15, 45, 90, 135 or 270 µg/ml FZS
were added to 1 ml culture medium 2 h prior to Aβ25-35 injury.
Following incubation for 24 or 48 h, cells were cultured, harvested
and subjected to the different experiments.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
PC12 cells were cultured in 96-well plates and
treated with 20 µM Aβ25-35, FZS or donepezil for 24 h. MTT
(5 mg/ml; Sigma-Aldrich) was added into each well and incubated at
37°C for 4 h. The MTT reaction was terminated by removing the
supernatant and dimethyl sulfoxide (Sigma-Aldrich) was added to
dissolve formazan products. Finally, the 24-well plates were
assessed at the wavelength of 405 nm on a 550 Bio-Rad microELISA
plate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each
experiment was repeated a minimum of three times.
Detection of apoptosis
Apoptosis was detected according to the alterations
in nuclear morphology. The nuclei were stained with
4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) fluorescent
DNA dye (Tiangen Biotech Co., Ltd., Beijing, China). Briefly, PC12
cells were cultured in RPMI-1640 and treated with FZS (0, 5, 15, 45
and 90 µg/ml) for 24 h. Following treatment with FZS and
permeabilization, cells were incubated with 2 mg/ml DAPI in
methanol at 37°C for 30 min. A fluorescence microscope (IX70;
Olympus Corporation) was used to observe cell apoptosis at 300–500
nm UV excitation.
Microscopy
The morphological alterations of the PC12 cell
models were observed using a light microscope (Olympus
Corporation). During the experimental period, cell morphology was
observed and evaluated under the CKX-31 light microscope at various
time points.
Enzyme-linked immunosorbent assay
(ELISA)
The capture antibody, mouse monoclonal anti-human
anti-p-Shc (cat. no. sc-81520; 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), was coated at the final concentration of 2
g/ml in the antibody coating buffer solution in 96-well plates at
4°C for 24 h. Following incubation with the antibody, cells were
washed 4 times with Tris-buffered saline Tween-20 (TTBS; Tiangen
Biotech Co., Ltd.) for 5 min, and then blocked with TBS starting
block buffer (Pierce Biotechnology, Inc., Rockford, IL, USA) at
room temperature for 1 h. Aβ40, Aβ42, sAPPα, sAPPβ, SIRT1, forkhead
box O (FoxO) standards and biotinylated 4G8 (reporter antibody, at
0.5 g/ml in 20% Pierce Biotechnology, Inc. SuperBlock; 1:1,000;
BioLegend, Inc., San Diego, CA, USA; cat. no. SIG-39240-500) were
added to the cells, and incubated at 20°C for 2 h. Cells were then
washed with TTBS and incubated with streptavidin-horseradish
peroxidase (Santa Cruz Biotechnology, Inc.) at 20°C for 1 h.
Subsequently, the fluorogenic substrate Amplex Ultra Red (Molecular
Probes; Thermo Fisher Scientific, Inc.) was added to the cells and
incubated in RPMI-1640 for 15 min. The reaction products were
quantified and examined using the Tecan Genios Pro plate reader
(Tecan Group, Ltd., Männedorf, Switzerland) at the wavelength of
450 nm excitation and 535 nm emission.
Western blotting
The PC12 cells were harvested and lysed with the
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Lysates were extracted and protein
concentration was determined using the Bicinchoninic Acid Assay kit
(Bio-Rad Laboratories, Inc.). Proteins were separated by 15% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto nitrocellulose membranes. Following blocking with 5% non-fat
milk in phosphate-buffered saline and Tween 20 (PBST; pH 7.6) at
4°C overnight, the membranes were incubated with polyclonal rabbit
anti-human SIRT1 (1:1,000; cat. no. sc-15404), polyclonal mouse
anti-human APP (1:2,000; cat. no. sc-117075), goat polyclonal
anti-human Aβ40 (1:1,000; cat. no. sc-7496), rabbit polyclonal
anti-human Aβ42 (1:1,000; sc-134426), monoclonal mouse anti-human
against sAPPα and anti-sAPPβ (1:1,000; cat. no. sc-69796), rabbit
polyclonal anti-human A disintegrin and metalloproteinase
domain-containing protein (ADAM)10 (1:1,000; cat. no. sc-25578) and
anti-FoxO polyclonal antibodies (1:1,000; Abcam, Cambridge, MA,
USA; cat. no. ab195977). All the antibodies were obtained from
Santa Cruz Biotechnology, Inc. and incubated overnight at 4°C.
Subsequently, the membranes were incubated with goat anti-rabbit
polyclonal antibody (1:1,000; OriGene Technologies, Inc., Beijing,
China) or rabbit anti–goat polyclonal antibody (1:1,000; Abcam;
cat. no. ab39594) for 1 h at room temperature. Finally, the
membranes were stained with enhanced chemiluminescence reagent (EMD
Millipore, Billerica, MA, USA). Western blot bands were analyzed
with the Quantity One software, version 2.0 (Bio-Rad Laboratories,
Inc.) to evaluate protein expression.
Statistical analysis
Quantitative and statistical analysis of immuno-blot
bands were performed using GraphPad Prism software, version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Briefly, the blots
images were scanned with Typhoon (Pharmacia; GE Healthcare Life
Sciences, Uppsala, Sweden), digitalized and saved as a TIF format.
The relative protein expression of each blot was determined. Data
are presented as the mean ± standard deviation of at least three
independent experiments. Statistical analysis was performed using
the t-test, and the differences among two groups or more were
determined using one-way or two-way analysis of variance,
respectively. P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated for a minimum
of three times.
Results
Neurotoxic cell model is successfully
established following treatment with Aβ25-35
Following Aβ25-35 administration, the cell viability
of PC12 cells was evaluated using the MTT assay. The results
demonstrated that upon 20 µM Aβ25-35 treatment the cell
viability was reduced by ~40% compared with the control group
(P<0.01; Fig. 1). The results
indicated that the neurotoxic cell model of PC12 cells was
successfully established.
FZS and donepezil have a protective
effect on PC12 cells
Donepezil (20 mM) and FZS (90 µg/ml)
treatments had a protective effect on PC12 cells compared with the
control group (P<0.01; Fig. 2).
Following an increase in the FZS concentration (135 or 270
µg/ml) a toxic effect was observed compared with the control
group (P<0.01; Fig. 2).
Protective effect of FZS and donepezil on
PC12 cells treated with Aβ25-35
Upon the establishment of the neurotoxic cell model
of PC12 cells, donepezil (20 mM) and FZS (2.5, 5, 15, 45, 90, 135
and 270 µg/ml) were used to evaluate the protective effect
against Aβ25-35-induced neurotoxicity. The results indicated that
FZS and donepezil had protective effects on cell models (Fig. 3).
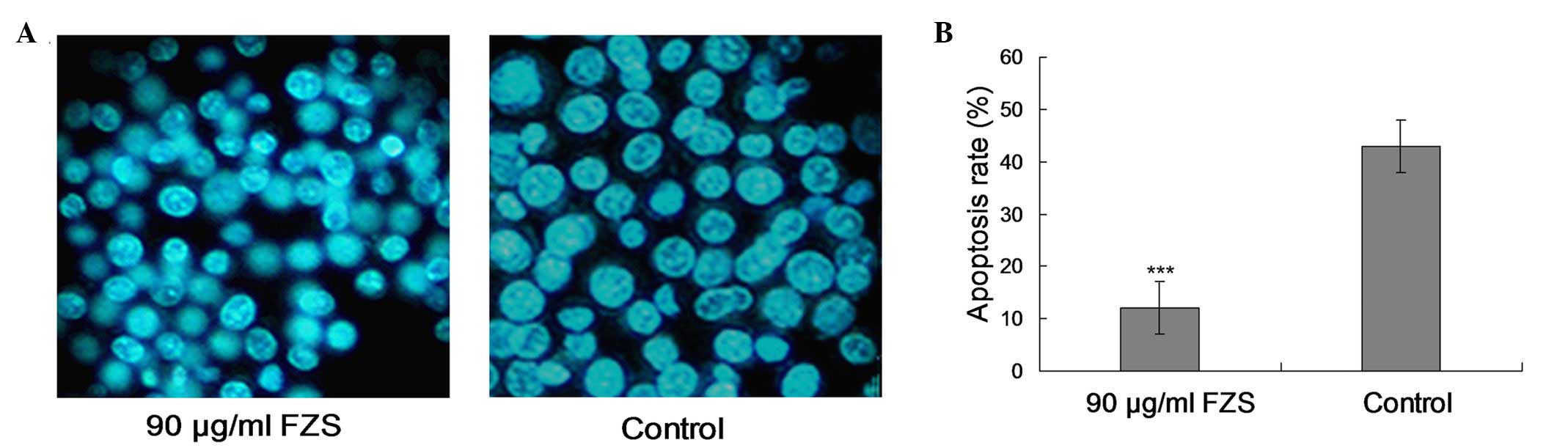
FZS inhibits apoptosis in PC12 cells
treated with Aβ25-35
PC12 cells were incubated with Aβ25-35 (20
µM) for 24 h and FZS (90 µg/ml), and the
anti-apoptotic effect of FZS was evaluated using DAPI staining
(Fig. 4A). Compared with the
control group, 90 µg/ml FZS treatment protected the cells
from apoptosis (P<0.01; Fig.
4B) and resulted in observable morphological alterations. The
morphological alterations illustrated typical apoptotic
characteristics, including chromatin condensation, DNA
fragmentation, apoptotic body formation cell shrinkage and
chromatin crescent formation/margination (21,22).
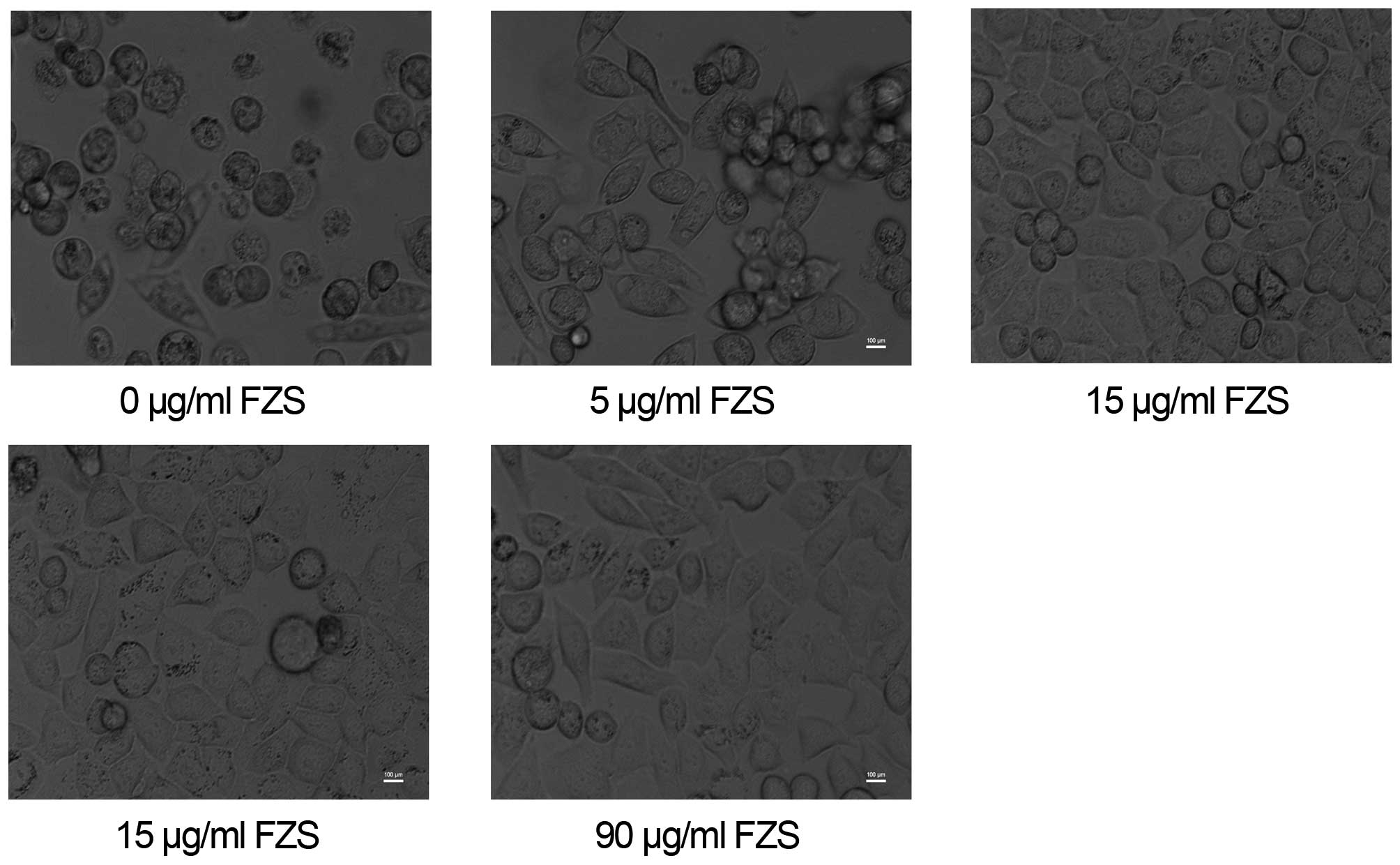
Apoptotic morphology
After a 24-h treatment with 0, 5, 15, 45 or 90
µg/ml FZS, the typical apoptosis morphological changes of
cells were observed in the cells (Fig.
5). The alterations included plasmic budding, nuclear chromatin
condensation and fragmentation, forming of apoptotic body and
phagocytosis of the extruded.
FZS prevents Aβ25-35-induced APP/Aβ
processing
Upon verification of the FZS protective effect on
PC12 cells, the relevant protein expression levels were determined
using western blot and ELISA assays. The results indicated that FZS
served a role in the APP/Aβ processing. The Aβ40, Aβ42 and sAPPβ
levels were downregulated. The level of sAPPα was upregulated in
this study (Fig. 6).
 | Figure 6FZS prevents Aβ25-35-induced APP/Aβ
processing. PC12 cells were treated with FZS (5, 15, 45 or 90
µg/ml) for 24 h. (A) Protein expression levels of APP, Aβ40,
Aβ42, sAPPα and sAPPβ. β-actin served as a loading control. (B)
Enzyme-linked immunosorbent assay analysis on APP, Aβ40, Aβ42,
sAPPα, and sAPPβ in the supernatant of nutrient solution. FZS,
fuzhisan; Aβ, amyloid β; APP, amyloid precursor protein; sAPPα,
soluble APPα peptide. |
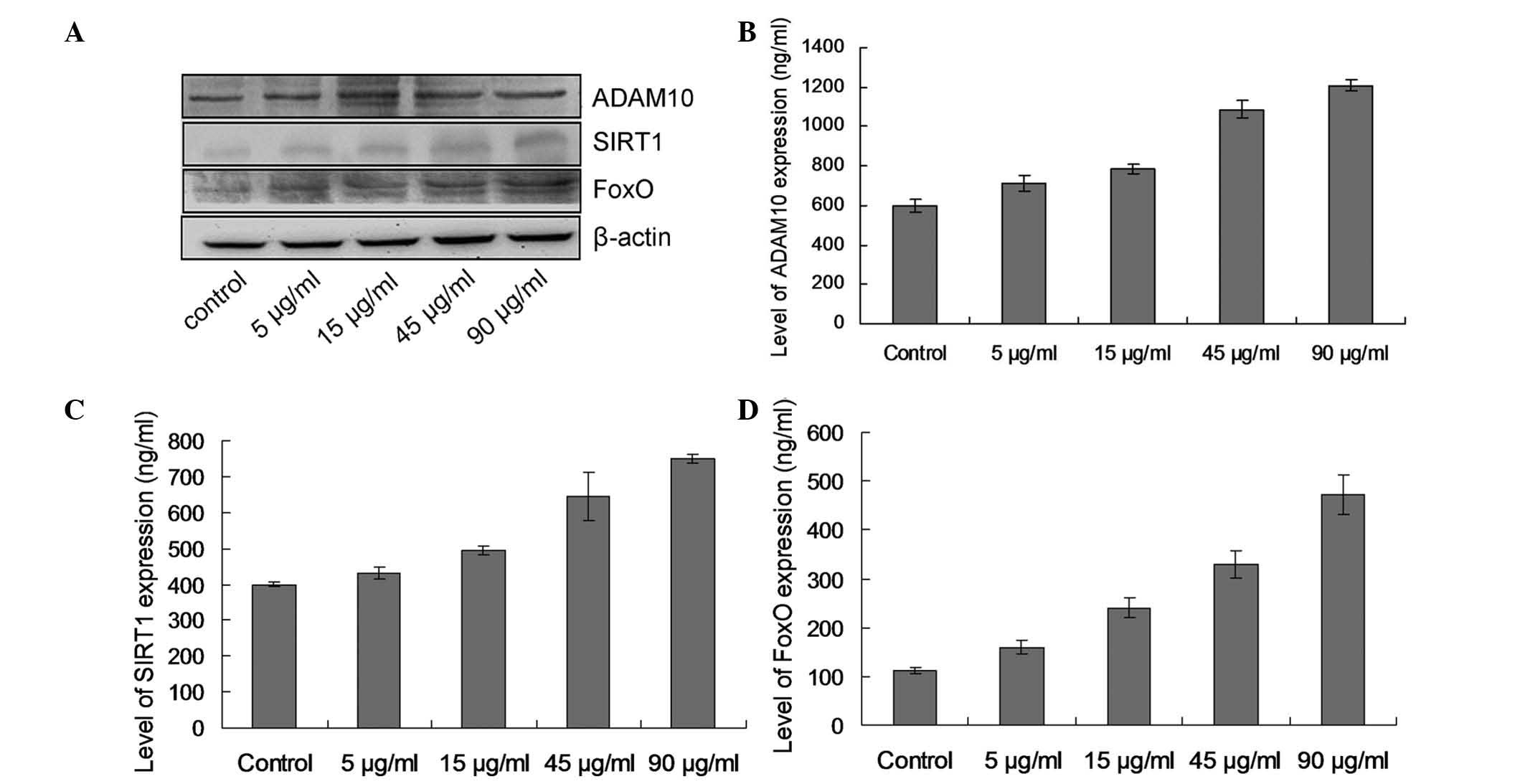
FZS promotes the expression of ADAM10 and
regulates the SIRT1-FoxO signaling pathway
The observation that ADAM10 levels are markedly
increased in PC12 cells treated with FZS (Fig. 7; P<0.05). The results suggested
that ADAM10 is involved in the basal unstimulated processing of the
APP. SIRT1 and FoxO protein expression levels demonstrated an
increasing tendency (Fig. 7),
indicating that FZS may serve a role in promoting the expression of
ADAM10 and regulating the SIRT1-FoxO signaling pathway.
Discussion
The results of the present study indicated that FZS
may prevent Aβ25-35-induced neurotoxicity as demonstrated by the
increased apoptosis and reduced cell viability. Furthermore, FZS
serves an important role in preventing the Aβ25-35-induced APP/Aβ
accumulation or processing in the AD model, through promotion of
ADAM10 expression and regulation of the SIRT1-FoxO signaling
pathway.
In the present study, the cell viability of the PC12
cells was observed to be significantly reduced following treatment
with the Aβ25-35 peptide (20 µM). The results demonstrated
that upon Aβ25-35 (20 µM) treatment, cell viability was
reduced to ~40%, indicating successful establishment of the
neurotoxic cell model. However, the FZS could prevent the cell
neurotoxicity induced by the Aβ25-35 peptide treatment in a
concentration-dependent manner. These results were consistent with
a previous study which indicated that FZS protected the
neuroblastoma cell line SH-SY5Y from the Aβ-induced cell apoptosis
(20). In addition, another study
indicated that FZS protected cortical neurons from Aβ-induced
apoptosis (22).
Upon confirmation of the protective effect of FZS on
the cell model, relevant protein expression levels were determined
using the western blotting and the ELISA assays. The results
indicated that FZS had a role in the APP/Aβ processing in AD.
Compared with the control group, the expression levels of APP
remained stable, where as Aβ40, Aβ42 and sAPPβ expression levels
were downregulated and sAPPα was upregulated.
APP is an important component of the amyloid cascade
and AD (4). APP is processed or
cleaved by numerous pathways, including α, β or γ cleavage forms
(4). Among the above cleavage
form, the most important physiological route involving cleavage is
the cleavage by α-secretase along with the secretory pathway, which
ranges from Golgi to plasma membrane (4). A previous study demonstrated that the
α-secretase cleavage mainly appears within the localization of Aβ
in APP (4). Furthermore, cleavage
of α-secretase leads to the secretion of soluble extracellular APP,
named sAPPα (6). Although numerous
cells possess a basal level of α-secretase activity, the
proteolysis of APP by the cleavage of α-secretase is increased by
diverse intracellular pathways, for example, the activation of the
protein kinase C (PKC) (5). The
activation or reactivation of other membrane receptors coupled to
PKC was indicated to enhance the APP cleavage by α-secretase.
Furthermore, the experimental findings illustrated that the sAPPα
may have a role in the neuroprotective function in the processes of
memory and learning capability (6). Therefore, it was hypothesized that
the metabolism of APP or its regulation via α-secretase pathway may
be correlated with the mechanism of AD pathogenesis. Hartmann et
al (25) demonstrated that the
ADAMs are capable of cleaving the APP in different cell systems, at
the α-cleavage domains. At present, the most commonly suggested
ADAMs for candidate α-secretases include ADAM10, ADAM9 and ADAM17
(25). These ADAMs illustrate the
identified structures and are all sensitive to the peptide
hydroxamates, however, a previous study demonstrated that ADAM17
does not possess inducible α-secretase activity (25). Another study indicated that ADAM10
has a PKC-stimulated α-secretase activity and other classical
characteristics, excluding the APP proteolytic processing (26). Evaluation of the α-secretase
activity is critical in patients with AD, and may be significant
for acknowledging the role of α-secretase in AD pathogenesis and
progression (27).
The current study demonstrated that the levels of
ADAM10 were significantly increased in PC12 cells treated with FZS.
In addition, ADAM10 was involved in the basal unstimulated APP
processing, and may be involved in the progression of AD (28). In the present study, the reduction
or increase of ADAM10 were hypothesized to trigger the β-secretase
amyloidogenic cleavage of APP. This hypothesis was verified by a
previous study, which demonstrated that increased sAPPα release and
α-secretase activity subsequent to reduction of cholesterol in
neuronal cell lines may lead to the reductive secretion of Aβ and
sAPPβ (29).
The precise biochemical mechanism for the sAPPα and
Aβ formation remains unclear, although numerous studies have
indicated the abnormal formations of these two parameters in
patients with AD (30). The
production or release of APP from platelets is associated with two
critical intracellular signaling pathways, the PKC activation
pathway and the cyclooxygenase pathway. Multiple intracellular
signaling pathways may have an effect on the reduced
thrombin-induced αAPP release in patients with AD (31). Therefore, the current study
suggested that the reduced ADAM10 levels and the modified
intracellular cascade may regulate the processing and trafficking
of APP.
Patel et al (32) identified the protective effect of
SIRT1 on AD, and demonstrated that calorie restriction reduced the
Aβ levels and plaque formation in transgenic AD mouse brains. In
addition, a reduction in Aβ has been demonstrated in the cortex of
starved squirrel monkeys, and is inversely correlated with SIRT1
(32). The above studies suggest
that SIRT1 has a neuroprotective effect on AD progression.
Furthermore, previous studies demonstrated that SIRT1 activation
reduced brain atrophy and neuronal apoptosis induced by the
progression of AD (33,34). SIRT1 deficiency was associated with
the enhanced phosphorylated-tau levels in neurons and the number of
NFTs in the AD brain (32,33).
The SIRT1 molecule primarily targets the two AD
pathological biomarkers, tau protein and Aβ peptide. The
phosphorylated tau degradation reduces the neuronal apoptosis and
improves the cognitive function in AD mice. However, the tau
breakdown is suppressed upon acetylation of the tau protein by the
histone acetyltransferase p300. During the process, SIRT1
deacetylates the acetylated tau, and subsequently decreases the tau
levels. Furthermore, SIRT1 inhibition may result in the opposite
effect, increasing the tau levels and exacerbating the accumulation
of the phosphorylated-tau (35).
Furthermore, previous studies indicated that
resveratrol administration and overexpression of SIRT1 may reduce
the Aβ levels in vitro and in vivo (9,32–34).
The Aβ peptide is generated from APP, a physiological protein, and
overexpression of SIRT1 stimulates the α-secretase production in
neurons and mice models (32,34).
SIRT1 regulation has an effect on activation of the retinoic acid
receptor pathway and inhibition of the rho-associated,
coiled-coil-containing protein kinase 1 (10). Furthermore, SIRT1 inhibits the
NF-κB signaling transduction pathway and reduces the Aβ peptide
levels (11). The above
observations indicated that SIRT1 may be a protective biomarker of
AD progression through multiple pathways and mechanisms, including
the degradation of tau protein and the decrease of Aβ peptide
levels. In the current study, SIRT1 and FoxO levels demonstrated an
increasing tendency, indicating that FZS has a role in the
regulation of the SIRT1-FoxO signaling pathway.
SIRT1 is used to determine the association between
the aging-associated signaling cascades (9,32).
Furthermore, SIRT1 is a selective activator of the FoxO signaling
pathway, and acts as a selective inhibitor of the NF-κB signaling
pathway (36). SIRT1 increases the
FoxO-dependent longevity functions, however, it inhibits the
NF-κB-dependent processes of inflammation in aging (9,34).
Brunet et al (37)
demonstrated that the FoxO/Daf-16 and SIRT/Sir2 longevity genes
share certain similar functions in C. elegans and human
mammalian system. For example, the interaction between the FoxOs
and the SIRT1 enhanced the effects against the oxidative stress and
increased the cell-cycle arrest (37). A previous study indicated that the
SIRTs affect the FoxO-dependent longevity via another mechanism.
For example, SIRT1 increases the efficiency of the nuclear
translocation and the trapping of FoxO1, which may enhance the
targeted gene-specific transcription (38).
The components of FZS that promote the
neuron-protective functions remain to be elucidated (39). Ginseng, an important component of
FZS, was demonstrated to alleviate numerous ailments, particularly
those in patients associated with increased age and memory
deterioration (40). A previous
study demonstrated that the ginsenoside Rb1 blocked the Aβ25-35
peptide-induced tau phosphorylation via inhibition of the Cdk5
activity (40). Thus, ginseng may
inhibit the Aβ-induced neurotoxicity. Other components of FZS, such
as anemone altaica, scutellaria baicalensis and
glycyrrhiza uralensis, will need further investigation as
they may contribute to the its function.
In conclusion, FZS inhibits the Aβ25-35-induced
neurotoxicity. Induction of ADAM10 and SIRT1-FoxO pathway may serve
a role in the neuroprotective effects of FZS and its pathogenic
mechanism. The results of the present study demonstrated novel
insights into the neuroprotective function of FZS against
Aβ-triggered neurotoxicity. Furthermore, FZS may act as a
therapeutic drug for the AD progression and pathology.
References
|
1
|
Xing S, Shen D, Chen C, Wang J and Yu Z:
Early induction of oxidative stress in a mouse model of Alzheimer's
disease with heme oxygenase activity. Mol Med Rep. 10:599–604.
2014.PubMed/NCBI
|
|
2
|
Ferri CP, Prince M, Brayne C, Brodaty H,
Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y,
et al Alzheimer's Disease International: Global prevalence of
dementia: A Delphi consensus study. Lancet. 366:2112–2117. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walsh DM and Selkoe DJ: Deciphering the
molecular basis of memory failure in Alzheimer's disease. Neuron.
44:181–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hardy J and Selkoe DJ: The amyloid
hypothesis of Alzheimer's disease: Progress and problems on the
road to therapeutics. Science. 297:353–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanzi RE and Bertram L: Twenty years of
the Alzheimer's disease amyloid hypothesis: A genetic perspective.
Cell. 120:545–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Postina R, Schroeder A, Dewachter I, Bohl
J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M,
et al: A disintegrin-metalloproteinase prevents amyloid plaque
formation and hippocampal defects in an Alzheimer's disease mouse
model. J Clin Invest. 113:1456–1464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Strooper B: Loss-of-function presenilin
mutations in Alzheimer disease. Talking Point on the role of
presenilin mutations in Alzheimer disease. EMBO Rep. 8:141–146.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kojro E and Fahrenholz F: The
non-amyloidogenic pathway: Structure and function of α-secretases.
Subcell Biochem. 38:105–127. 2005. View Article : Google Scholar
|
|
9
|
Sinclair DA and Guarente L:
Extrachromosomal rDNA circles - a cause of aging in yeast. Cell.
91:1033–1042. 1997. View Article : Google Scholar
|
|
10
|
Chen J, Zhou Y, Mueller-Steiner S, Chen
LF, Kwon H, Yi S, Mucke L and Gan L: SIRT1 protects against
microglia-dependent amyloid-beta toxicity through inhibiting
NF-kappaB signaling. J Biol Chem. 280:40364–40374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen
L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al: Neuronal
SIRT1 activation as a novel mechanism underlying the prevention of
Alzheimer disease amyloid neuropathology by calorie restriction. J
Biol Chem. 281:21745–21754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim D, Nguyen MD, Dobbin MM, Fischer A,
Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et
al: SIRT1 deacetylase protects against neurodegeneration in models
for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J.
26:3169–3179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Julien C, Tremblay C, Emond V, Lebbadi M,
Salem N Jr, Bennett DA and Calon F: Sirtuin 1 reduction parallels
the accumulation of tau in Alzheimer disease. J Neuropathol Exp
Neurol. 68:48–58. 2009. View Article : Google Scholar
|
|
14
|
Donmez G and Guarente L: Aging and
disease: Connections to sirtuins. Aging Cell. 9:285–290. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Min SW, Cho SH, Zhou Y, Schroeder S,
Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C,
et al: Acetylation of tau inhibits its degradation and contributes
to tauopathy. Neuron. 67:953–966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Donmez G: The neurobiology of sirtuins and
their role in neurodegeneration. Trends Pharmacol Sci. 33:494–501.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donmez G, Wang D, Cohen DE and Guarente L:
SIRT1 suppresses beta-amyloid production by activating the
alpha-secretase gene ADAM10. Cell. 142:320–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haigis MC and Sinclair DA: Mammalian
sirtuins: Biological insights and disease relevance. Annu Rev
Pathol. 5:253–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XL, Wang S, Zhao BQ, Li Q, Qu HY, Zhang
T, Zhou JP and Sun MJ: Effects of Chinese herbal medicine fuzhisan
on aged rats. Exp Gerontol. 43:853–858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Wang D, Duan S, Wang J, Bai J and
Li W: Analysis of fuzhisan and quantitation of baicalin and
ginsenoside Rb(1) by HPLC-DAD-ELSD. Arch Pharm Res. 32:989–996.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gang BZ and Wang CL: The efficacy of
Fuzhisan in patients with Alzheimer's disease. Chin J Apoplexy Nerv
Dis. 22:527–529. 2005.
|
|
22
|
Wen SR, Wang DS and Zhang JY: Effect of
Fuzhisan on the area of neurosome and the length of axon. Chin J
Clin Rehabil. 9:241–243. 2005.
|
|
23
|
Shirong W, Desheng W and Jingyan Z: The
effect of FZS on the cellular function of SH-SY5Y. J Harbin Med
Univ. 37:383–388. 2003.
|
|
24
|
Sul D, Kim HS, Lee D, Joo SS, Hwang KW and
Park SY: Protective effect of caffeic acid against
beta-amyloid-induced neurotoxicity by the inhibition of calcium
influx and tau phosphorylation. Life Sci. 84:257–262. 2009.
View Article : Google Scholar
|
|
25
|
Hartmann D, Tournoy J, Saftig P, Annaert W
and De Strooper B: Implication of APP secretases in notch
signaling. J Mol Neurosci. 17:171–181. 2001. View Article : Google Scholar
|
|
26
|
Vieira SI, Rebelo S and Domingues SC: da
Cruz e Silva EF and da Cruz e Silva OA. S655 phosphorylation
enhances APP secretory traffic. Mol Cell Biochem. 9:8–17. 2009.
|
|
27
|
Goodman AB: Retinoid receptors,
transporters, and metabolizers as therapeutic targets in late onset
Alzheimer disease. J Cell Physiol. 209:598–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Costa RM, Drew C and Silva AJ: Notch to
remember. Trends Neurosci. 28:429–435. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corcoran JPT, So PL and Maden M:
Disruption of the retinoid signalling pathway causes a deposition
of amyloid β in the adult rat brain. Eur J Neurosci. 20:896–902.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Firestein R, Blander G, Michan S,
Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S,
de Cabo R, Fuchs C, et al: The SIRT1 deacetylase suppresses
intestinal tumorigenesis and colon cancer growth. PLoS One.
3:e20202008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon K and Gaiano N: Notch signaling in
the mammalian central nervous system: Insights from mouse mutants.
Nat Neurosci. 8:709–715. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Patel NV, Gordon MN, Connor KE, Good RA,
Engelman RW, Mason J, Morgan DG, Morgan TE and Finch CE: Caloric
restriction attenuates Abeta-deposition in Alzheimer transgenic
models. Neurobiol Aging. 26:995–1000. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sydow A, Van der Jeugd A, Zheng F, Ahmed
T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E,
et al: Tau-induced defects in synaptic plasticity, learning, and
memory are reversible in transgenic mice after switching off the
toxic Tau mutant. J Neurosci. 31:2511–2525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santacruz K, Lewis J, Spires T, Paulson J,
Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan
E, et al: Tau suppression in a neurodegenerative mouse model
improves memory function. Science. 309:476–481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Julien C, Tremblay C, Emond V, Lebbadi M,
Salem N Jr, Bennett DA and Calon F: Sirtuin 1 reduction parallels
the accumulation of tau in Alzheimer disease. J Neuropathol Exp
Neurol. 68:48–58. 2009. View Article : Google Scholar
|
|
36
|
Frescas D, Valenti L and Accili D: Nuclear
trapping of the forkhead transcription factor FoxO1 via
Sirt-dependent deacetylation promotes expression of glucogenetic
genes. J Biol Chem. 280:20589–20595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brunet A, Sweeney LB, Sturgill JF, Chua
KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et
al: Stress-dependent regulation of FOXO transcription factors by
the SIRT1 deacetylase. Science. 303:2011–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giannakou ME and Partridge L: The
interaction between FOXO and SIRT1: Tipping the balance towards
survival. Trends Cell Biol. 14:408–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen X, Huang T, Zhang J, Song J, Chen L
and Zhu Y: Involvement of calpain and p25 of CDK5 pathway in
ginsenoside Rb1's attenuation of beta-amyloid peptide25–35-induced
tau hyperphosphorylation in cortical neurons. Brain Res.
1200:99–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee CH, Kim JM, Kim DH, Park SJ, Liu X,
Cai M, Hong JG, Park JH and Ryu JH: Effects of Sun ginseng on
memory enhancement and hippocampal neurogenesis. Phytother Res.
27:1293–1299. 2013. View Article : Google Scholar
|