Introduction
Pituitary tumors account for between 10 and 25% of
all intracranial neoplasms (1).
They represent the third most common type of cancer within the
central nervous system, and is only less frequent than glioma and
meningioma (1,2). Pituitary tumors are also one of the
most common tumors of the endocrine system (3). With the continuous development of
diagnostic imaging and laboratory diagnosis, the incidence of
pituitary tumors has gradually increased (4). Clinically, the most common
classification methods for pituitary tumors are based on their
endocrine function and immunohistochemistry staining analysis.
Pituitary adenomas are classified based upon anatomical,
histological and functional criteria (5), including lactotrophic adenomas
(prolactinomas), somatotrophic adenomas, corticotrophicadenomas,
gonadotrophicadenomas, thyrotrophic adenomas (rare) and null cell
adenomas (6,7). Lactotrophic adenomas, secreting
prolactin, account for 30%, somatotrophic adenomas account for 15%
and non-secretive null cell adenomas account for 25% of pituitary
adenomas (8,9).
Although several techniques and drugs have been
investigated and used in the treatment of pituitary cancer, there
has been no significant improvement in the local control of
carcinoma development and progression, and there have been no
significant improvements in survival rates or in quality of life of
patients with pituitary cancer (10,11).
Following its rapid development in cancer therapy, immunotherapy
has generally become one of the important treatment strategies
following surgery, radiation therapy and chemotherapy (12,13).
Provenge is a dendritic cell (DC)-based therapeutic cancer vaccine,
which was approved by the Food and Drug Administration in April
2010 (14,15), until which DCs were first approved
for the treatment of advanced prostate cancer. Currently, DC
immunotherapy is broadly used in clinical therapy, and is used in
the treatment of breast cancer, lung cancer, gastrointestinal
cancer, prostate cancer, kidney cancer and malignant melanoma
(16–20). DC immunotherapy has a broad range
of applications, is safe and has no significant adverse reactions
(21,22).
Mucins are a family of high molecular weight,
heavily glycosylated proteins (23). To date, over 20 mucin genes have
been identified and cloned, including membrane-bound Muc1, Muc3,
Muc4 and Muc12, and secreted Muc2, Muc5, Muc6 and Muc9 (24,25).
Muc1 is an epithelial membrane associated glycoprotein
(Episialin), and it is the first mucin to be characterized
and cloned in detail (26). An
increasing number of mucins are being used as important molecular
markers. In the present study, polyinosinic:polycytidylic acid
(poly I:C) was used as an immunostimulatory agent or immune
adjuvant. The present study also aimed to investigate whether Muc1
offers potential as a candidate vaccine antigen to prevent or
control the progression of pituitary tumors in vitro and
in vivo.
Materials and methods
Cell line and reagents
The AtT20 cell line was obtained from American Type
Culture Collection (cat. no. CRL1795), and the cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Thermo Trace, Ltd., Melbourne, Australia)
at 37° C in an atmosphere of 95% air and 5% CO2. Human
recombinant Muc1 protein was obtained from Abnova Corporation
(Tapei City, Taiwan). Mouse anti-human MUC1 (sc-59795) and
anti-human β-actin (sc-69879) monoclonal antibodies (mAbs), and
horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary
antibody (sc-2031) were obtained from Santa Cruz Biotechnology, Inc
(Santa Cruz, CA, USA).
Patients
A total of 42 patients with invasive pituitary
adenoma, and 44 patients with non-invasive pituitary adenoma, at
the Affiliated Hospital of Binzhou Medical College (Binzhou, China)
were enrolled in the present study. Pituitary adenoma specimens
were collected during surgery in order to detect the expression
levels of Muc1 by immunohistochemistry and western blotting. The
samples were maintained at −80° C until further analysis. The
present study was approved by the ethics committee of Affiliated
Hospital of Binzhou Medical College. Written informed consent was
obtained from all patients.
Immunohistochemical analysis
Immunohistochemical staining was performed in order
to detect the expression of Muc1 in the pituitary adenoma
specimens. Paraffin-embedded sections were dewaxed, rehydrated,
blocked and incubated overnight with mouse anti-human MUC1
(1:1,000) and anti-human β-actin (1:1,000) mAbs. Subsequently, the
specimens were washed three times and incubated with HRP-conjugated
goat anti-mouse secondary antibody (1:1,000) for 1 h. The stained
slides were dehydrated, made transparent, mounted in Permount and
visualized using a Nikon Eclipse Ti-E microscope (Nikon
Corporation, Tokyo, Japan). The images were captured with a camera
attached to the microscope at ×400 magnification.
Western blotting
The invasive and non-invasive pituitary adenoma
specimens were lysed using radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Shanghai, China) consisting
of 50 mmol/l Tris, 1% NP-40, 150 mmol/l NaCl, 1.0 mmol/l EDTA, 0.1%
sodium dodecyl sulfate (SDS) and 0.25% SDC. Equal quantities of
protein (10 µg) were separated by 10% SDS-polyacrylamide gel
electrophoresis and blotted onto polyvinylidene difluoride
membranes. After blocking the membranes with 5% bovine serum
albumin (Beyotime Institute of Biotechnology) in phosphate-buffered
saline, the membranes were incubated with mouse anti-MUC1 and
anti-β-actin mAbs (1:1,000) at 4° C overnight. Subsequently, the
membranes were washed three times with PBS containing 0.05%
Tween-20 (PBST), followed by incubation with HRP-conjugated goat
anti-mouse secondary antibody (1:1,000) at room temperature for 40
min. The membranes were visualized using the Pierce ECL Western
Blotting Substrate (cat. no. 32209; Thermo Fisher Scientific,
Inc.), and blot intensities were analyzed using ImageJ software,
version 2 (National Institutes of Health, Bethesda, MA, USA).
Phenotype identification of peripheral
blood mononuclear cell (PBMC)-derived DCs by fluorescence-activated
cell sorting (FACS) analysis
The DCs were generated and cultured from human
PBMCs, as described in a previous study (27). The PBMCs were obtained from blood
samples derived from healthy donors using Ficoll-Hypaque density
gradient centrifugation at 300 x g for 5 min at 37° C. The PBMCs
(5×104 cells/well) were plated into 6-well plates.
Following 5 days of culture, human immature DCs were stimulated
with 50 µg/ml Muc1 and 50 µg/ml poly I:C, or with 50
µg/ml Muc1 alone for 48 h at 37° C. Subsequently, the cells
were collected and washed with PBS for phenotypic analysis. The DCs
were stained with phycoerythrin (PE)-conjugated mAbs against CD40
(cat. no. 313005), CD80 (cat. no. 305207) and CD83 (cat. no.
305307), and fluorescein isothiocyanate (FITC)-conjugated mAbs
against CD14 (cat. no. 325603) and histocompatability locus antigen
(HLA)-DR (cat. no. 307603), as well as allophycocyanin anti-human
CD86 mAb (cat. no. 305411; all BioLegend, San Diego, CA, USA) for
20 min at 4° C (all 1:1,000).
Determination of DC cytokine production
using an enzyme-linked immunosorbent assay (ELISA)
The human immature DCs were adjusted to a density of
5×105 cells/ml and plated into 24-well plates. The cells
were treated with Muc1, Muc1 + poly I:C or lipopolysaccharide (LPS)
for 48 h, and the culture supernatants were harvested at the end of
the experiment at 37° C, and centrifuged at 300 × g for 5 min at
37° C, prior to ELISA assay. The levels of the cytokines tumor
necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 in the
supernatants were measured, according to the manufacturer's
protocol (Neobioscience Technology, Co., Ltd., Beijing, China).
Mice and immunization
A total of 18 C57BL/6 male mice (6–8 weeks old) were
obtained from the Laboratory Animal Center of Peking University
Health Science Center (Beijing, China). The mice were housed in
specific pathogen-free conditions under a 12-h light/dark cycle at
21–25° C, and with ad libitum access to food and water. The
mice were randomly assigned to three groups (6 mice/group), as
follows: i) The Muc1 group; ii) the Muc1 + poly I:C group; and iii)
the control group. The mice were twice injected subcutaneously on
days 1 and 15 with 20 µg Muc1 antigen, 20 µg Muc1
antigen + 50 µg poly I:C or with 100 µl PBS. The mice
were sacrificed by cervical dislocation 7 days following the final
immunization and the abdominal cavity was opened with scissors in
order to obtain the spleens. Splenocyte cell suspensions were
prepared using a sterile syringe core and 200 mesh steel
filter.
Previous studies have reported that tumor cell
lysate (TLA), which is used as a source of tumor-specific antigens,
is able to induce the maturation of dendritic cells; although the
maturation-inducing effect of TLAs was not particularly strong
(11,12). In the present study, the mice were
vaccinated with TLA or TLA + poly I:C, and the protein expression
levels of interferon (IFN)-γ and IL-2 in the splenocytes of the
mice were investigated using the Mouse IFN-γ ELISA (cat. no.
EM0560) and the Mouse IL-2 ELISA kit (cat. no. EM0290),
respectively (both Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China). The TLA was obtained from the AtT20 cells.
Briefly, cells were washed once with PBS, harvested and then
resuspended in PBS at 1×107 cells/ml, prior to 5 cycles
of freezing using methanol with dry ice and thawing at 56° C.
Subsequently, the cells were harvested, and washed in PBS, prior to
centrifugation at 1,700 × g for 5 min. The TLAs were contained in
the supernatant, which were separated and stored at −80° C prior to
vaccination of the mice.
T-cell subtype analysis by FACS
analysis
The T-cell subtype was determined using FACS
analysis. At 10 days following the second immunization (day 15),
blood samples were taken from the venous plexus of the mouse eyes
and 50 µl heparin-treated orbital blood was lysed with red
blood cell lysis buffer (eBioscience, San Diego, CA, USA).
Lymphocytes were stained with 100 µl PBS containing 1% BSA
and 0.1% NaN3, together with 5 µl FITC-conjugated
anti-CD3 mAb (cat. no. 16-0037-81), followed by simultaneous
staining with 5 µl of PE-labeled anti-CD4 (cat. no.
12-0048-41) or anti-CD8 (cat. no. 12-0809-41) mAbs (all
eBioscience). The cells were then incubated for 20 min at 4° C, and
flow cytometry was performed using the BD FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Measurement of Muc1-specific
antibodies
Muc1-specific antibodies in the mouse serum were
measured using an ELISA (28).
Firstly, microtiter plates (Nalge Nunc International, Penfield, NY,
USA) were coated with Muc1 (10 µg/ml) in 50 mM carbonate
buffer (pH 9.6) overnight at 4° C. Following washing with PBST, the
wells were blocked with 100 µl 5% dried milk in PBST for 4 h
at 37° C. To measure IgM, IgG, IgG2a and IgG1, the plates
containing with serum samples were incubated with HRP-conjugated
rabbit anti-mouse IgG for 30 min at 37° C. Following incubation
with substrate buffer, the enzyme reaction was terminated after 5
min by adding 50 µl 2 M H2SO4. The
absorbance at 450 nm was then measured using the Multiskan MK3
microplate reader (Thermo Fisher Scientific, Inc.). All washes were
performed using PBS-T. Serum samples and HRP-conjugated rabbit
anti-mouse IgG (sc-358914; 1:2,000; Santa Cruz Biotechnology, Inc.)
were diluted in PBS-T containing 3% dried milk.
Cytotoxicity assay
Lactate dehydrogenase (LDH) release assays were
performed according to the manufacturer's protocol (KeyGen Biotech
Co., Ltd., Nanjing, China). The C57BL/6 mice were immunized, as
described above and, 10 days following the final immunization,
splenocytes were prepared as effector cells, and AtT20 cells were
used as target cells. Cytolytic T lymphocyte activity was measured
by a LDH release assay in 96-well round-bottom plates. Target cells
(2×104 cells per well) in a volume of 100 µl were
incubated with 100 µl effector cells at 5/1, 10/1 or 20/1
effector:target (E:T) ratios for 24 h at 37° C in phenol red-free
DMEM containing 2% FBS. Following centrifugation at 300 × g for 5
min at 37° C, the supernatants (50 µl per well) were
transferred into 96-well flat-bottom plates for measurement of
absorbance at 490 nm using the Multiskan MK3 microplate reader.
Statistical analysis
Data were analyzed using SPSS software, version 19.0
(IBM SPSS, Armonk, NY, USA). Data are presented as the mean ±
standard deviation. Statistical significance in the different
treatment groups was compared using a log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Positive Muc1 staining is observed in
invasive pituitary adenoma
In the present study, 42 registered cases of
invasive pituitary adenoma, and 44 samples of non-invasive
pituitary adenoma were included from the hospital database. The
expression of Muc1 was detected using immunohistochemistry in the
invasive and non-invasive pituitary adenomas, respectively. As
shown in Fig. 1A, marked positive
expression of Muc1 was observed, with dark brown or brown
particles, which were predominantly located in the cytoplasm and
membrane. By contrast, negative or weak staining of Muc1 was
observed in the non-invasive pituitary adenomas. The positive rates
of Muc1 expression in the invasive and non-invasive pituitary
adenomas were 90.4% (32/42) and 20.5% (9/44), respectively
(Table I). There was a significant
difference between the two groups (P<0.01). The expression
levels of Muc1 were also determined in samples of non-invasive
pituitary tissues and malignant pituitary adenomas by western
blotting, and the results were consistent with those obtained by
the immunohistochemical staining (Fig.
1B).
 | Table IComparison of the expression levels
of mucin 1 in pituitary adenoma tissues. |
Table I
Comparison of the expression levels
of mucin 1 in pituitary adenoma tissues.
| Type of tissue | Negative
expression | Weak positive
expression | Strong positive
expression | Positivity (%) |
|---|
| Invasive pituitary
adenoma | 4 | 6 | 32 | 90.4 |
| Non-invasive
pituitary adenoma | 35 | 9 | 0 | 20.5 |
Production of cytokines in human DCs in
the Muc1 + poly I:C group and poly I:C group
In the present study, DCs were derived from adherent
PBMCs cultured in medium supplemented with human
granulocyte-macrophage colony-stimulating factor and IL-4, and the
DCs are shown in Fig. 2A. As Muc1
was expressed at high levels in the malignant pituitary adenomas,
Muc1 was used as the tumor antigen, and poly I:C was selected as an
immune stimulator agent. Cytokine secretion was examined following
stimulation of the immature PBMC-DCs with Muc1 or different
concentrations of Poly I:C, and the resulting supernatants were
harvested after 48 h. As shown in Fig.
2B–D, poly I:C significantly enhanced the secretion of IL-1β,
TNF-α and IL-6 in a dose-dependent manner, as determined using
ELISA. In this experiment, untreated cells were used as negative
controls and cells treated with LPS were used as positive
controls.
 | Figure 2Cytokine production in human DCs in
the Muc1 + poly I:C group and poly I:C group. (A) Image of human
DCs generated from PBMCs and cultured in medium supplemented with
human granulocyte-macrophage colony-stimulating factor and IL-4 on
day 5 (magnification, ×100). PBMC-derived DCs were stimulated with
different concentrations of poly I:C (5, 25 and 50 µg/ml)
for 24 h, and the levels of (B) TNF-α, (C) IL-6 and (D) IL-1β
cytokines in the supernatants were measured using enzyme-linked
immunosorbent assays. Untreated cells were used as a negative
control, and lipopolysaccharide (20 ng/ml) was used as a positive
control. Data are presented as the mean ± standard deviation.
*P<0.05 and **P<0.01 vs. the untreated
cells. DCs, dendritic cells; Muc1, mucin 1; poly I:C, polyinosinic:
polycytidylic acid; PBMCs, peripheral blood mononuclear cells; LPS,
lipopolysaccharide; IL, interleukin; TNF-α, tumor necrosis
factor-α. |
Phenotypic identification of PBMC-derived
DCs
In order to assess the phenotypic variation of the
human DCs, the expression of surface markers on the PBMC-derived
DCs was examined using FACS analysis. The immature DCs were treated
with either 50 µg/ml poly I:C or with 50 µg/ml poly
I:C + 50 µg/ml Muc1 for 48 h, following which the expression
levels of costimulatory molecules (CD40 and CD80), HLA-DR and the
CD83 maturation marker were measured. As shown in Fig. 3A and B, the results demonstrated
that poly I:C or Muc1-poly I:C induced the phenotypic maturation of
the human DCs, with low expression levels of CD14, moderate
expression levels of CD40, CD80 and CD83, and marked expression of
HLA-DR.
Determination of
CD4+CD3+ T cell subpopulations using FACS
analysis
In order to detect the immune response in
vivo, C57BL/6 mice were used as an animal model. As is already
known, T-cell subsets include T helper cells
(CD4+CD3+ T lymphocytes) and cytotoxic T
cells (CD8+CD3+ T lymphocytes) (29). As shown in Fig. 4A–D, the mice immunized with Muc1 +
poly I:C contained a higher percentage of
CD3+CD4+ T lymphocytes in their peripheral
blood, compared with the mice injected with the negative control,
PBS. However, the percentage of CD8+CD3+ T
lymphocytes in the peripheral blood did not change significantly
(data not shown).
Mice immunized with Muc1 + poly I:C have
increased Muc1-specific serum antibody titers following
vaccination
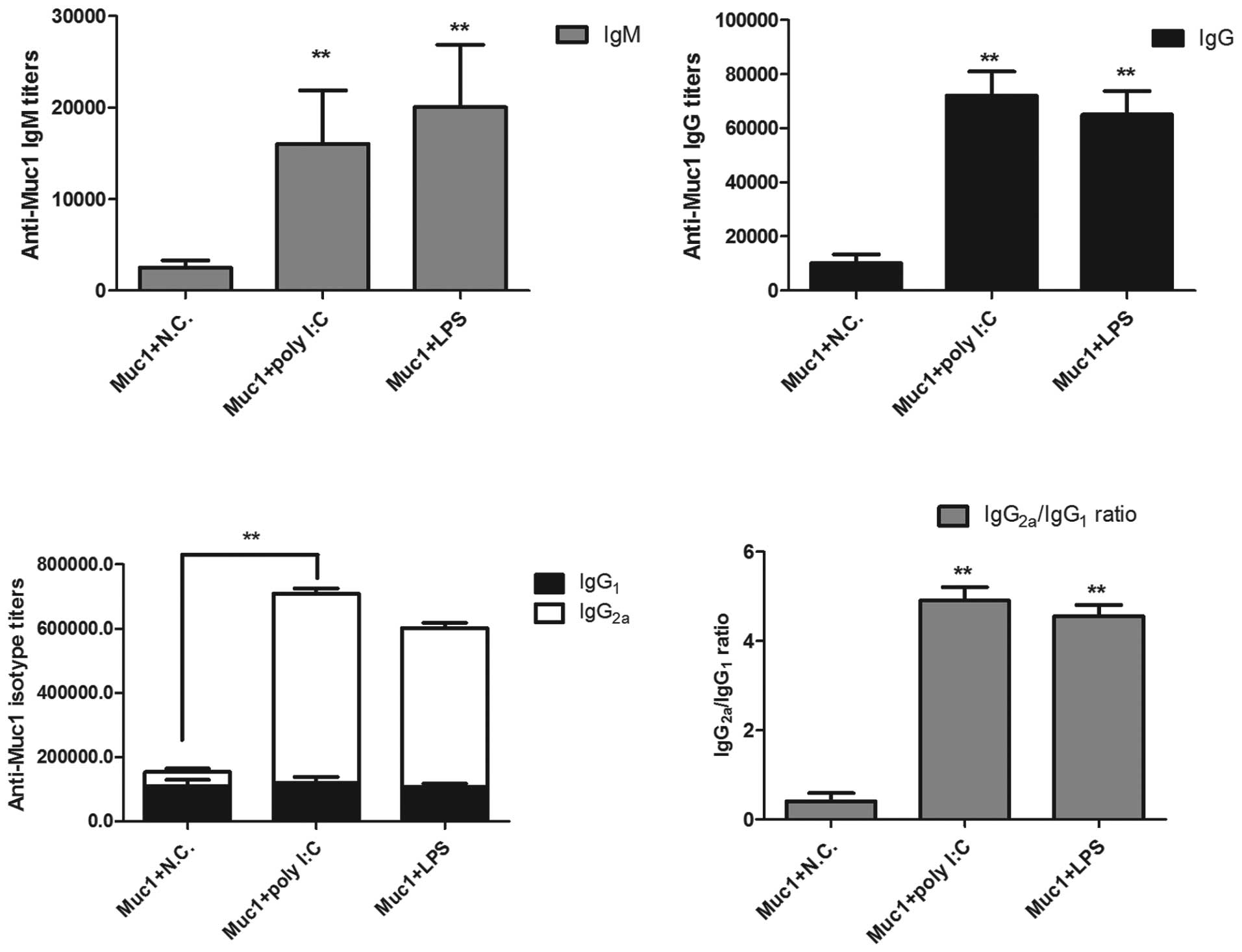
The present study also measured antigen-specific
antibody titers in the immunized mice. Muc1 antigen was injected
subcutaneously into mice, together with poly I:C, with Muc1 antigen
only or with LPS and 10 days following the final immunization, the
levels of IgM, total IgG, IgG2a and IgG1 of the anti-HBs antibodies
were examined. As shown in Fig. 5,
the mice immunized with Muc1 + poly I:C had a higher geometric mean
titer of Muc1 antibodies, compared with the mice immunized with the
antigen alone. In addition, IgM titers in the Muc1 + poly I:C group
increased by 6.4 times, and IgG titers increased by 7.2 times,
compared with the Muc1 group. Of note, ploy I:C altered the balance
of IgG2a and IgG1, inducing more extensive production of IgG2a
antibody, compared with the control group. It has been reported
that IgG2a is directly associated with the T helper (Th)1
cell-mediated immune response, whereas IgG1 is associated with the
Th2 immune response (30). This
suggests that Muc1 + poly I:C may be involved in modulating
cellular immunity, with positive effects in preventing tumor
progression.
IFN-γ and IL-2 cytokines are increased in
mice immunized with Muc1 + poly I:C or TLA + poly I:C
Cytokine patterns can reflect effector populations
of immune cells to a certain degree. Th1 cytokines activate
macrophages, natural killer cells and cellular immunity, whereas
Th2 cytokines tend to promote humoral immune response (31). In the present study, at 10 days
following the final immunization, splenocytes in the different
treatment groups were stimulated with Muc1 at a concentration of 1
µg/ml, and the production of the IFN-γ and IL-2 Th1
cytokines were examined using an ELISA. As shown in Fig. 6A and B, the levels of IFN-γ and
IL-2 in the splenocytes of the mice immunized with Muc1 + ploy I:C
were significantly higher, as compared with those of the mice
immunized with Muc1 alone (P<0.01). The present study also used
TLAs from AtT-20 cells as antigens, and the results demonstrated
that the levels of IFN-γ and IL-2 in the splenocytes of the mice
immunized with TLA + poly I:C were significantly higher, as
compared with those of the mice immunized with TLA alone
(P<0.01).
Cytotoxic T cell (CTL) activity is
increased following immunization with TLA + poly I:C, as compared
with TLA alone
To demonstrate the cytotoxic activity of splenocytes
from the immunized mice, the present study examined the release of
cytosolic LDH into the culture medium by damaged AtT20 cells. At 10
days following the final immunization, damage to the membranes of
the AtT20 cells was evaluated in a 24 h cytotoxicity assay by
measuring LDH release. The LDH release assays were performed with
splenocytes as effector cells and AtT20 cells as target cells. The
E:T cell ratios were 5:1, 10:1 and 20:1. As shown in Fig. 7, the CTL response was significantly
higher in the mice immunized with TLA + poly I:C, as compared with
that in those the mice immunized with TLA alone (P<0.01) or with
PBS (P<0.01), which demonstrated that TLA + poly I:C effectively
induced cytotoxic activity to induce tumor cell death.
 | Figure 7CTL activity was higher in the
TLA-poly I:C group, as compared with the TLA group. C57BL/6 mice
were immunized and, 7 days following the final immunization, damage
to the membranes of AtT-20 cells was evaluated in a 24 h
cytotoxicity assay, by measuring LDH release. LDH release assays
were performed with splenocytes as effector cells and AtT-20 as
target cells. Specific lysis was calculated as follows: Lysis (%) =
[(experimental LDH release) − (spontaneous LDH release)] /
[(maximum LDH release) − (spontaneous LDH release)] × 100. The CTL
activity of the TLA + poly I:C group was significantly higher,
compared with the control group (**P<0.05). All
experiments were repeated twice and the results are expressed as
the mean ± standard error of the mean. CTL, cytotoxic T cell; TLA,
tumor lysate antigen; poly I:C, polyinosinic: polycytidylic acid;
LDH, lactate dehydrogenase; E:T, effector:target. |
Discussion
Cancer can be treated using several techniques,
including surgery, chemotherapy, radiation therapy, immunotherapy
and mAb therapy. Cancer immunotherapy has emerged as one of the
four most commonly used treatment modality, in addition to surgery,
chemotherapy and radiotherapy (32). Vaccines have been investigated for
a long time and have been used for the treatment of infectious
disease or cancer (33–35), however, progression has remained
slower, compared with other forms of immunotherapy (35–37).
This is partly due to the weak immunogenicity of the tumor
antigens. In the present study the, expression levels of Muc1 in
pituitary tumors were examined, and the results demonstrated
increased positive expression of Muc1 in invasive pituitary tumors.
The aim of the present study was to induce potent antitumor T-cell
responses to assist in the therapy of pituitary tumors. Thus, Muc1
was used as the tumor antigen and poly I:C, the ligand of TLR3, was
selected as the adjuvant to prime the antitumor immune response
(37–40). The results of the present study
demonstrated that Muc1 + poly I:C successfully induced effective
antitumor immune responses.
DCs are considered to be important antigen
presenting cells, and they are the most effective cells for antigen
presentation and T cell stimulation (41). The maturation of DCs is important
for the initiation of an adaptive immune response, thus, the
present study detected the presence of certain major
histocompatibility complex class II molecules and T-cell
costimulatory molecules on the surface of PBMC-derived DCs. The
results of the present study revealed that, Muc1 + poly I:C
significantly enhanced the expression levels of CD40, CD80, CD83
and HLA-DR, in which CD83 is considered a relatively specific
marker of mature DCs, and CD14 is a marker of monocytes. Treatment
with Muc1 + poly I:C increased the expression of CD83 and decreased
the expression of CD14, which demonstrated that Muc1 + poly I:C
promoted the maturation of the DCs. Additionally, Muc1 + poly I:C
stimulated the secretion of TNF-α, IL-6 and IL-1β cytokines, which
may assist in attracting neutrophils, monocytes and DCs to
inflammatory sites and induce the adaptive immune response
(42,43).
It has been reported that the Th1-type immune
response is important for protection against viral infections,
tumor cells and several pathogens. Th1 cells can secret cytokines,
including IFN-γ and IL-2, which activate macrophages and are
involved in the generation of CTLs. In the present study, the mice
injected with Muc1 + poly I:C showed higher levels of IFN-γ and
IL-2 in the splenocytes when stimulated with Muc1. The CTL response
following the final immunization was also examined in the different
groups of mice. Supporting the idea that the induction of IFN-γ and
IL-2 suggested polarization towards the Th1 response, higher
specific CTL activity was detected when stimulated with Muc1
antigens in vitro.
In the mouse model, immunization with Muc1 + poly
I:C induced more extensive production of anti-Muc1, compared with
the antigen alone. Notable, activation of the Th1 immune response
was marked, as was the switch in the balance of IgG1 and IgG2a,
which may contribute to the shift between the Th2 and Th1 immune
respons, and was positive for preventing the progression of
pituitary tumors.
In conclusion, Muc1 + poly I:C stimulated the
expression of costimulatory molecules and promoted the secretion of
inflammatory cytokines, IL-6, TNF-α, IL-1β, by human PBMC-derived
DCs. In the mouse model, immunization with Muc1 + poly I:C enhanced
Muc1-specific antibody titers and induced the shift between the Th2
and Th1 immune response. Therefore, it may offer potential as a
useful strategy for the treatment of malignant pituitary
tumors.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81171119).
References
|
1
|
Loyo-Varela M, Herrada-Pineda T,
Revilla-Pacheco F and Manrique-Guzman S: Pituitary tumor surgery:
Review of 3004 cases. World Neurosurg. 79:331–336. 2013. View Article : Google Scholar
|
|
2
|
Salehi F, Kovacs K, Scheithauer BW, Lloyd
RV and Cusimano M: Pituitary tumor-transforming gene in endocrine
and other neoplasms: A review and update. Endocr Relat Cancer.
15:721–743. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boutros C, Cheng-Robles D and Goldenkranz
R: Intestinal neuroendocrine tumor in a patient with pituitary
adenoma. A case report and review of the current screening
recommendations. J Med Case Rep. 1:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sogani J, Yang W, Lavi E, Zimmerman RD and
Gupta A: Sellar collision tumor involving metastatic lung cancer
and pituitary adenoma: Radiologic-pathologic correlation and review
of the literature. Clin Imaging. 38:318–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ironside JW: Best practice No 172:
Pituitary gland pathology. J Clin Pathol. 56:561–568. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nistor RF: Parasellar classification of
pituitary adenomas. Neurosurgery. 35:542–544. 1994.PubMed/NCBI
|
|
7
|
Kovacs K, Horvath E and Vidal S:
Classification of pituitary adenomas. J Neurooncol. 54:121–127.
2001. View Article : Google Scholar
|
|
8
|
Sánchez-Tejada L, Sánchez-Ortiga R,
Moreno-Pérez O, Montañana CF, Niveiro M, Tritos NA and Alfonso AM:
Pituitary tumor transforming gene and insulin-like growth factor 1
receptor expression and immunohistochemical measurement of Ki-67 as
potential prognostic markers of pituitary tumors aggressiveness.
Endocrinol Nutr. 60:358–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuno A, Teramoto A, Takekoshi S, Sanno
N, Osamura RY and Kirino T: Expression of plurihormonal mRNAs in
somatotrophic adenomas detected using a nonisotopic in situ
hybridization method: Comparison with lactotrophic adenomas. Hum
Pathol. 26:272–279. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bergmann-Leitner ES, Duncan EH and Leitner
WW: Identification and targeting of tumor escape mechanisms: A new
hope for cancer therapy? Curr Pharm Des. 9:2009–2023. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmed S, Pahwa P, Fields A,
Chandra-Kanthan S, Iqbal N, Zaidi A, Reeder B, Plaza FA, Zhu T and
Leis A: Predictive factors of the use of systemic therapy in stage
iv colorectal cancer: Who gets chemotherapy? Oncology. 88:289–297.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voloshin T, Voest EE and Shaked Y: The
host immunological response to cancer therapy: An emerging concept
in tumor biology. Exp Cell Res. 319:1687–1695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rini BI, Weinberg V, Fong L, Conry S,
Hershberg RM and Small EJ: Combination immunotherapy with prostatic
acid phosphatase pulsed antigen-presenting cells (provenge) plus
bevacizumab in patients with serologic progression of prostate
cancer after definitive local therapy. Cancer. 107:67–74. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thara E, Dorff TB, Pinski JK and Quinn DI:
Vaccine therapy with sipuleucel-T (Provenge) for prostate cancer.
Maturitas. 69:296–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao Q, Li L, Zhang C, Sun Y, Liu S and Cui
S: Clinical effects of immunotherapy of DC-CIK combined with
chemotherapy in treating patients with metastatic breast cancer.
Pak J Pharm Sci. 28(3 Suppl): 1055–1058. 2015.PubMed/NCBI
|
|
17
|
Shi YJ, Ren HY, Cen XN, Dong YJ, Ma MX,
Zhao YL, Zhu Y and Yu JR: Dendritic cells elicit cellular immune
response by targeting to capture breast cancer cells. Zhonghua
zhong liu za zhi. 30:107–111. 2008.In Chinese. PubMed/NCBI
|
|
18
|
Chen W, Rains N, Young D and Stubbs RS:
Dendritic cell-based cancer immunotherapy: Potential for treatment
of colorectal cancer? J Gastroenterol Hepatol. 15:698–705. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carmi Y, Spitzer MH, Linde IL, Burt BM,
Prestwood TR, Perlman N, Davidson MG, Kenkel JA, Segal E, Pusapati
GV, et al: Allogeneic IgG combined with dendritic cell stimuli
induce antitumour T-cell immunity. Nature. 521:99–104. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmed Ali HA, Di J, Mei W, Zhang YC, Li Y,
Du ZW and Zhang GZ: Antitumor activity of lentivirus-mediated
interleukin -12 gene modified dendritic cells in human lung cancer
in vitro. Asian Pac J Cancer Prev. 15:611–616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bilusic M, Heery C and Madan RA:
Immunotherapy in prostate cancer: Emerging strategies against a
formidable foe. Vaccine. 29:6485–6497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pardoll D and Drake C: Immunotherapy earns
its spot in the ranks of cancer therapy. J Exp Med. 209:201–0209.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Corfield AP: Mucins: A biologically
relevant glycan barrier in mucosal protection. Biochim Biophys
Acta. 1850:236–252. 2015. View Article : Google Scholar
|
|
24
|
Kao CJ, Wurz GT, Monjazeb AM, Vang DP,
Cadman TB, Griffey SM, Wolf M and DeGregorio MW: Antitumor effects
of cisplatin combined with tecemotide immunotherapy in a human MUC1
transgenic lung cancer mouse model. Cancer Immunol Res. 2:581–589.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pérez-Sánchez J, Estensoro I, Redondo MJ,
Calduch-Giner JA, Kaushik S and Sitjà-Bobadilla A: Mucins as
diagnostic and prognostic biomarkers in a fish-parasite model:
Transcriptional and functional analysis. PLoS One. 8:e654572013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asano R, Takemura S, Tsumoto K, Sakurai N,
Teramae A, Ebara S, Katayose Y, Shinoda M, Suzuki M, Imai K, et al:
Functional construction of the anti-mucin core protein (MUC1)
antibody MUSE11 variable regions in a bacterial expression system.
J Biochem. 127:673–679. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao X, Zhang W, Wan T, He L, Chen T, Yuan
Z, Ma S, Yu Y and Chen G: Molecular cloning and characterization of
a novel CXC chemokine macrophage inflammatory protein-2 gamma
chemoattractant for human neutrophils and dendritic cells. J
Immunol. 165:2588–2595. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Costa CI, Delgado IF, de Costa JA, de
Carvalho RF, Mouta Sda S Jr, Vianna CO and de Moraes MT:
Establishment and validation of an ELISA for the quantitation of
HBsAg in recombinant hepatitis B vaccines. J Virol Methods.
172:32–037. 2011. View Article : Google Scholar
|
|
29
|
Jones N, Agrawal D, Elrefaei M, Hanson A,
Novitsky V, Wong JT and Cao H: Evaluation of antigen-specific
responses using in vitro enriched T cells. J Immunol Methods.
274:139–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holdsworth SR, Kitching AR and Tipping PG:
Th1 and Th2 T helper cell subsets affect patterns of injury and
outcomes in glomerulonephritis. Kidney Int. 55:1198–1216. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muraille E and Leo O: Revisiting the
Th1/Th2 paradigm. Scand J Immunol. 47:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mishra J, Drummond J, Quazi SH, Karanki
SS, Shaw JJ, Chen B and Kumar N: Prospective of colon cancer
treatments and scope for combinatorial approach to enhanced cancer
cell apoptosis. Crit Rev Oncol Hematol. 86:232–250. 2013.
View Article : Google Scholar :
|
|
33
|
Kirkwood JM, Moschos S and Wang W:
Strategies for the development of more effective adjuvant therapy
of melanoma: Current and future explorations of antibodies,
cytokines, vaccines, and combinations. Clin Cancer Res. 12(Suppl):
S2331–S2336. 2006. View Article : Google Scholar
|
|
34
|
Kirkwood JM, Strawderman MH, Ernstoff MS,
Smith TJ, Borden EC and Blum RH: Interferon alfa-2b adjuvant
therapy of high-risk resected cutaneous melanoma: The eastern
cooperative oncology group trial EST 1684. J Clin Oncol. 14:7–17.
1996.PubMed/NCBI
|
|
35
|
Terando A, Sabel MS and Sondak VK:
Melanoma: Adjuvant therapy and other treatment options. Curr Treat
Options Oncol. 4:187–199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krishnan L, Deschatelets L, Stark FC,
Gurnani K and Sprott GD: Archaeosome adjuvant overcomes tolerance
to tumor-associated melanoma antigens inducing protective CD8 T
cell responses. Clin Dev Immunol. 2010:5784322010. View Article : Google Scholar
|
|
37
|
Mechl Z and Kopecny J: Current results
with surgery and adjuvant chemotherapy in malignant melanoma. Arch
Geschwulstforsch. 56:367–371. 1986.In German.
|
|
38
|
Inao T, Harashima N, Monma H, Okano S,
Itakura M, Tanaka T, Tajima Y and Harada M: Antitumor effects of
cytoplasmic delivery of an innate adjuvant receptor ligand, poly
(I:C), on human breast cancer. Breast Cancer Res Treat. 134:89–100.
2012. View Article : Google Scholar
|
|
39
|
Huang YK, Zheng Z, Cheng CX, Wang LY, Li
YR and Qiu F: The antitumor effect of the toll-like receptor 3
ligand polyinosinic-cytidylic acid as an adjuvant. Cancer Immunol
Immunother. 62:237–244. 2013. View Article : Google Scholar
|
|
40
|
Martinez-Gil L, Goff PH, Hai R,
Garcia-Sastre A, Shaw ML and Palese P: A Sendai virus-derived RNA
agonist of RIG-I as a virus vaccine adjuvant. J Virol.
87:1290–1300. 2013. View Article : Google Scholar :
|
|
41
|
Ebner S, Ratzinger G, Krosbacher B,
Schmuth M, Weiss A, Reider D, Kroczek RA, Herold M, Heufler C,
Fritsch P and Romani N: Production of IL-12 by human
monocyte-derived dendritic cells is optimal when the stimulus is
given at the onset of maturation, and is further enhanced by IL-4.
J Immunol. 166:633–641. 2001. View Article : Google Scholar
|
|
42
|
Lindstedt M, Johansson-Lindbom B and
Borrebaeck CA: Global reprogramming of dendritic cells in response
to a concerted action of inflammatory mediators. Int Immunol.
14:1203–1213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Y, Ochando J, Yopp A, Bromberg JS and
Ding Y: IL-6 plays a unique role in initiating c-Maf expression
during early stage of CD4 T cell activation. J Immunol.
174:2720–2729. 2005. View Article : Google Scholar : PubMed/NCBI
|