Introduction
In the United States, breast cancer is the second
most common cause of cancer-associated mortality in women (1). An estimated 60,290 new cases of
breast carcinoma in situ were expected to be diagnosed among
women in the US during 2015, according to the American Cancer
Society (1). Breast cancer
accounts for 7–10% of all malignant tumors, with a 3–4% increase in
the number of new cases occurring each year in China (2).
Overexpression of the human epidermal growth factor
receptor 2 (HER2) tyrosine kinase receptor gene has been identified
as a negative prognostic factor for node-positive early breast
cancer; therefore, understanding the biology of HER2 has
revolutionized the classification, prognosis, and treatment of
breast cancer (3,4). HER2 is known to be overexpressed in
15–20% of breast cancer and has an important role in regulating
cell survival, proliferation, angiogenesis, invasion and metastasis
(5). At present, targeted
anticancer drugs, such as the monoclonal antibody trastuzumab
(Herceptin®) have been proven to be effective in
clinical settings (6). However,
primary resistance to trastuzumab remains a prevalent challenge for
the treatment of patients with HER2-positive breast cancer
(3). Traditional Chinese herbs and
medicines have been reported to be clinically effective in the
treatment of cancer; however, the underlying mechanisms of action
remain largely unknown. We previously reported a high-throughput
in vitro screen of a 10,000 natural product library against
six representative breast cancer cell lines, and assessed the
cytotoxicity of each drug (7). Out
of the eight natural compounds that selectively inhibit the
proliferation of HER2-positive cells, two anthocyanins:
Peonidin-3-glucoside (P3G) and cyanidin-3-glucoside (C3G) were
studied in vitro and in vivo (7). Subsequently, we investigated the
combined antitumor effects of P3G or C3G with trastuzumab on
representative HER2-positive breast cancer cell lines and on a
tumor xenograft model, and demonstrated that the anthocyanins were
able to significantly enhance trastuzumab-induced growth inhibition
(8).
The present study aimed to characterize the
mechanisms underlying the activity of P3G and C3G against
HER2-positive trastuzumab-resistant human breast cancer cell lines.
Elucidation of the mechanisms of action of P3G and C3G may enhance
the understanding of breast cancer and result in identification of
novel treatment strategies.
Materials and methods
Reagents
Unless otherwise stated all chemicals and reagents
(analytical grade) were purchased from Sigma-Aldrich China, Inc.
(Shanghai, China). Trastuzumab was a gift from the Pharmacology
Department of Chengdu Medical College (Chengdu, China), and was
originally purchased from Roche Diagnostics (Shanghai, China) with
>98% purity. P3G and C3G were purchased from Pharmanic (Chengdu,
China) with >98% purity.
Cell lines and culture conditions
Parental cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). MDA-MB-453 cells were
maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 2 mmol/l L-glutamine. BT474 cells were maintained
in DMEM:Ham's F12 medium (1:1 mixture) supplemented with 2 mmol/l
L-glutamine and 5 µg/ml insulin. Resistant lines
(MDA-MB-453R and BT474R) were developed by continuously exposing
cells to trastuzumab (8 µg/ml) until the cells regained
morphology similar to that of the parental line (~3 months)
(9,10). Subsequently, the cells were
maintained in 8 µg/ml trastuzumab. All media were
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. All cells were maintained in an atmosphere
containing 5% CO2 at 37°C. Trastuzumab was removed from
the media for subsequent experiments.
Cell proliferation assay
Cell proliferation assays were performed as
described previously (7). Briefly,
MDA-MB-453, MDA-MB-453R, BT474 and BT474R cells were plated at a
density of 1×103 cells/well in a total volume of 90
µl/well (96-well plates). Cells were allowed to attach to
the bottom of the plates overnight, and were then treated with or
without 10 µl C3G or P3G (concentration range 0.003–50
µM in a 100 µl total volume, 4-fold dilution) for 48
h at 37°C in an atmosphere containing 5% CO2. Aliquots
of Alamar-Blue reagents (20 µl) were added directly to each
well, the plates were incubated at 37°C for an additional 3 h, and
the fluorescent signal was measured at an excitation wavelength of
530 nm and an emission wavelength of 590 nm using a ZS-2 plate
reader (Beijing Hongrunda Technology Development Co., Ltd.,
Beijing, China). Data were normalized as percentage viability
relative to the vehicle control (dimethyl sulfoxide), defined as
100% survival.
Drug treatment for western blotting
MDA-MB-453, MDA-MB-453R, BT474 and BT474R cells were
grown to 70–80% confluence, harvested, and aliquoted into 60 mm
dishes at a density of 1×106 cells/dish. After an
overnight incubation, media were removed and replaced with fresh
media supplemented with or without 5 µl/ml C3G or P3G. The
dishes were incubated for an additional 24 h prior to
harvesting.
Western blot analysis
Western blotting was performed as previously
described (7). Briefly, the cells
were washed with ice-cold phosphate-buffered saline (PBS) and were
scraped into radioimmunoprecipitation assay buffer supplemented
with protease inhibitor and phosphatase inhibitor (Roche
Diagnostics). The homogenates were centrifuged at the maximum force
for 5 min at 4°C. The supernatants were then transferred to fresh
tubes, and protein concentrations were determined using the Bio-Rad
Protein Assay [Bio-Rad Laboratories (Shanghai) Ltd., Shanghai,
China], according to the manufacturer's protocol. Total protein
samples (25 µg) were subjected to electrophoresis [10%
Tris-HCL, 1.0 mm gel; Bio-Rad Laboratories (Shanghai) Ltd.] and
were transferred to polyvinylidene fluoride membranes (Thermo
Fisher Scientific, Shanghai, China). The membranes were then
blocked in Tris-buffered saline containing 0.05% Tween 20 (TBST)
and 5% nonfat milk or 5% bovine serum albumin (BSA) for 1 h at room
temperature. Primary antibodies were diluted to 1:1,000 using
TBST/5% nonfat milk or TBST/5% BSA, and the membranes were
incubated with them for 2 h at room temperature or overnight at
4°C. Following three washes with TBST, the secondary antibodies
were diluted to 1:10,000 using SuperBlock (PBS) blocking buffer
(Thermo Fisher Scientific), and the membranes were incubated with
them for 1 h at room temperature. Protein levels were detected
using the Enhanced Chemiluminescence (ECL) Plus Western Blotting
Detection system (GE Healthcare, Shanghai, China). Following a 5
min incubation with ECL Plus reagents, the membranes were washed
once with TBST, prior to exposure to films. The image was analyzed
using ImageJ 1.49 software (National Institutes of Health,
Bethesda, MD, USA). The following antibodies purchased from Rui
Biological Ltd. (Shanghai, China) were used: Anti-phosphorylated
(p)-HER2 (Tyr1248; cat. no. AB-2387), anti-HER2 (cat. no. AB-5569),
anti-p-AKT (Thr308 or Ser473; cat. no. AB-2864, AB-2865), anti-AKT
(cat. no. AB-2863), anti-p-p42/44 mitogen-activated protein kinase
(MAPK) (cat. no. AB-6534), anti-p42/44MAPK (cat. no. AB-6535),
anti-β-actin (cat. no. AB-0230), horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) (cat. no.
AB-0025), and (HRP)-conjugated goat anti-mouse IgG (cat. no.
AB-0032).
Annexin V-fluorescein isothiocyanate
(FITC) assay
An Annexin V-FITC assay was used to detect early
apoptotic effects, as previously described (7). Briefly, the cells were treated with
or without reagents for 24 h at 37°C. The cells were then stained
with Annexin-V-FITC antibody (cat. no. A13199;Thermo Fisher
Scientific, Inc., Waltham, MA, USA; supplied by the Chengdu Medical
College Flow-Cytometry Core Facility) for 15 min in the dark on
ice. Propidium iodide (PI; 1 g/ml) was added immediately prior to
analysis. The cells were analyzed using CyFlow® ML
(Sysmex Partec GmbH, Görlitz, Germany). Bivariant analysis was used
to define the population of cells, where FITC (−) and PI (−) cells
were designated as viable cells, FITC (+) and PI (−) cells were
designated as apoptotic cells, and FITC (+) and PI (+) cells were
designated as late apoptotic or necrotic cells.
Caspase 3/7 activity assay
The apoptotic effects of drug treatment were
assessed using a caspase 3/7 activity assay as described previously
(7). Following a 48 h drug
treatment, aliquots of Alamar-Blue reagent (20 µl/well) were
added directly to each well, the plates were incubated at 37°C for
3 h and the fluorescent signal was recorded at an excitation
wavelength of 530 nm, and an emission wavelength of 590 nm, using a
ZS-2 plate reader (Beijing Hongrunda Technology Development Co.,
Ltd.). Subsequently, equal volumes (120 µl/well) of caspase
3/7 activity assay reagent (Shanghai Promega Biological Products,
Ltd., Shanghai, China) were added to each well and the luminescence
signal was measured. Caspase 3/7 activities were normalized as
fold-changes of luminescence relative to fluorescence.
Migration and invasion assays
Migration and invasion assays were conducted using a
Transwell (8 µm; Merck Millipore, Beijing, China) double
chamber co-culture system. Briefly, 1×104 cells were
seeded into the upper chamber of 24-well plates, which were coated
with Matrigel (migration assay) or 1 mg/ml type I collagen solution
(invasion assay). The lower chamber was filled with media
supplemented with 10% FBS. The cells were cultured with medium, or
medium supplemented with C3G (1 mg/ml) or P3G (1 mg/ml), for 48 h.
The migrated or invaded cells were fixed and stained with crystal
violet. Five random fields were selected for each experiment, and
were observed under a microscope (SZX16; Olympus Corporation,
Tokyo, Japan). Experiments were performed in triplicate.
Mice xenograft model
In vivo experiment protocols were reviewed
and approved by the Chengdu Medical College Institutional Animal
Care and Use Committee. All in vivo experiments were
conducted under pathogen-free conditions at the animal facility.
BT474R cells were suspended in PBS (2×106 cells/100
µl) and subcutaneously implanted into the flank region of
6–7-week-old female nude mice weighing 18–22 g (Chengdu Medical
College Animal Facility, Chengdu, China). The rats had ad
libitum access to food and water, and were maintained under
controlled lighting (12 h light/dark cycle) and temperature
(20–25°C). Once tumors had reached 50–60 mm3 volume, the
mice were randomly assigned to three groups (n=10), receiving
either i) intraperitoneal (i.p.) injection of 100 µl PBS
(PBS only, twice a week); ii) i.p. injection of C3G (6 mg/kg in 100
µl PBS, twice a week); or iii) i.p. injection of P3G (6
mg/kg in 100 µl PBS, twice a week). Tumors were measured
every 5 days. The mice were euthanized once the tumors reached
1,000 mm3 due to ethical requirements. All animals were
euthanized by an overdose of CO2 at the end of the
experiment. Tumor tissues were extracted for immunostaining and
weighing.
Immunohistochemistry
Spleen, liver and kidney sections from
formalin-fixed, paraffin-embedded tumor xenografts (4 µm)
were subjected to immunohistochemical staining using anti-HER2
(cat. no. AB-5569), anti-Ki67 (cat. no. AB-2368) and anti-caspase 3
(cat. no. AB-2294) (all Rui Biological Ltd.) antibodies. Briefly,
the tissue sections were deparaffinized and rehydrated, followed by
treatment with citrate buffer (pH 6.0) and 3% hydrogen peroxide.
The sections were then incubated with the primary antibodies for
30–60 min at room temperature, followed by an incubation with
HRP-conjugated goat anti-rabbit IgG (cat. no. AB-0025; Rui
Biological Ltd.) for 1 h at room temperature. Nuclei were
counterstained with hematoxylin. The staining was visualized and
recorded using a microscope (SZX16; Olympus Corporation).
Statistical analysis
In vitro data are presented as the mean ±
standard deviation (n≥3). In vivo data are presented as the
mean ± standard error of the mean (n=10). Data were analyzed by
Student's t-test using SigmaPlot version 12.0 (Systat Software,
Inc., San Jose, CA, USA). P≤0.05 was considered to indicate a
statistically significant difference.
Results
Treatment with C3G or P3G sensitizes
trastuzumab-resistant MDA-MB-453R and BT474R cell lines in
vitro
To determine the ability of C3G and P3G to overcome
trastuzumab resistance, two HER2-positive cell lines (MDA-MB-453
and BT474) and their trastuzumab-resistant cell lines (MDA-MB-453R
and BT474R) were treated with 0.003 to 50 µM (4-fold
dilution) C3G or P3G for 48 h. The calculated half maximal
inhibitory concentration (IC50) value for each cell line
was determined (Fig. 1). Treatment
with C3G or P3G significantly inhibited cell growth in the parental
and trastuzumab-resistant cells, as compared with the control cells
(Fig. 1).
Treatment with C3G or P3G inhibits
p-HER2, p-AKT and p-MAPK expression levels in vitro
The expression levels of p-HER2, and its downstream
mediators AKT and MAPK, were assessed by western blotting. The
expression levels of p-HER2, p-AKT (Ser473) and p-p44/42 MAPK were
downregulated in MDA-MB-453, MDA-MB-453R, BT474 and BT474R cells
following 24 h treatment with C3G (5 µg/ml) and P3G (5
µg/ml) (Fig. 2).
Treatment with C3G or P3G induces
apoptosis in trastuzumab-resistant HER2-positive human breast
cancer cells in vitro
The effects of C3G or P3G treatment on apoptosis
were assessed by Annexin V and caspase 3/7 activity assays
(Fig. 3). Following a 24 h
treatment, MDA-MB-453 cells treated with trastuzumab, C3G or P3G
exhibited 2.8±0.50, 3.2±0.45 and 4.0±0.34 fold-changes in the
number of Annexin V-positive cells, as compared with the control
cells, respectively (Fig. 3A). In
addition, following a 24 h treatment, MDA-MB-453R cells treated
with trastuzumab, C3G or P3G exhibited 1.2±0.45, 3.0±0.38 and
3.5±0.44 fold-changes in the number of Annexin V-positive cells, as
compared with the control cells, respectively (Fig. 3A). Following a 24 h treatment,
BT474 cells treated with trastuzumab, C3G or P3G exhibited
2.5±0.32, 4.2±0.25 and 5.5±0.40 fold-changes in the number of
Annexin V-positive cells, as compared with the control cells,
respectively (Fig. 3B).
Furthermore, following a 24 h treatment, BT474R cells treated with
trastuzumab, C3G or P3G exhibited 1.5±0.55, 3.8±0.23 and 5.0±0.32
fold-changes in the number of Annexin V-positive cells, as compared
with the control cells, respectively (Fig. 3B).
Following a 48 h treatment, MDA-MB-453 cells treated
with trastuzumab, C3G or P3G exhibited 3.1±0.40, 12.0±2.10 and
8.9±3.0 fold-changes in caspase 3/7 activity, as compared with the
control cells, respectively (Fig.
3C). In addition, following a 48 h treatment, MDA-MB-453R cells
treated with trastuzumab, C3G or P3G exhibited 1.2±0.31, 10.2±1.80
and 6.8±2.20 fold-changes in caspase 3/7 activity, as compared with
the control cells, respectively (Fig.
3C). Following a 48 h treatment, BT474 cells treated with
trastuzumab, C3G or P3G exhibited 3.0±0.60, 5.8±2.10 and 9.2±1.80
fold-changes in caspase 3/7 activity, as compared with the control
cells, respectively (Fig. 3D).
Furthermore, following a 48 h treatment, BT474R cells treated with
trastuzumab, C3G or P3G exhibited 1.1±0.23, 4.4±1.20 and 8.1±1.80
fold-changes in caspase 3/7 activity, as compared with the control
cells, respectively (Fig. 3D).
Treatment with C3G or P3G inhibits
invasion and migration of trastuzumab-resistant human breast cancer
cells in vitro
As determined by Transwell migration assay, the
number of migrated BT474R cells treated with C3G (0.21±0.11) or P3G
(0.32±0.15) were significantly lower, as compared with the
vehicle-treated control cells (1.0±0.25) (Fig. 4A upper panel and B). In addition, the number of migrated
MDA-MB-435R cells treated with C3G (0.32±0.22) or P3G (0.40±0.15)
were significantly lower, as compared with the vehicle-treated
control cells (1.0±0.12) (Fig. 4C
upper panel and D). As determined
by Transwell invasion assays, the number of invasive BT474R cells
treated with C3G (0.12±0.06) or P3G (0.35±0.12) were significantly
lower, as compared with the vehicle-treated control cells
(1.0±0.18) (Fig. 4A lower panel
and B). Furthermore, the number of
invasive MDA-MB-453R cells treated with C3G (0.25±0.22) or P3G
(0.42±0.15) were significantly lower, as compared with the
vehicle-treated control cells (1.0±0.21) (Fig. 4C lower panel and D).
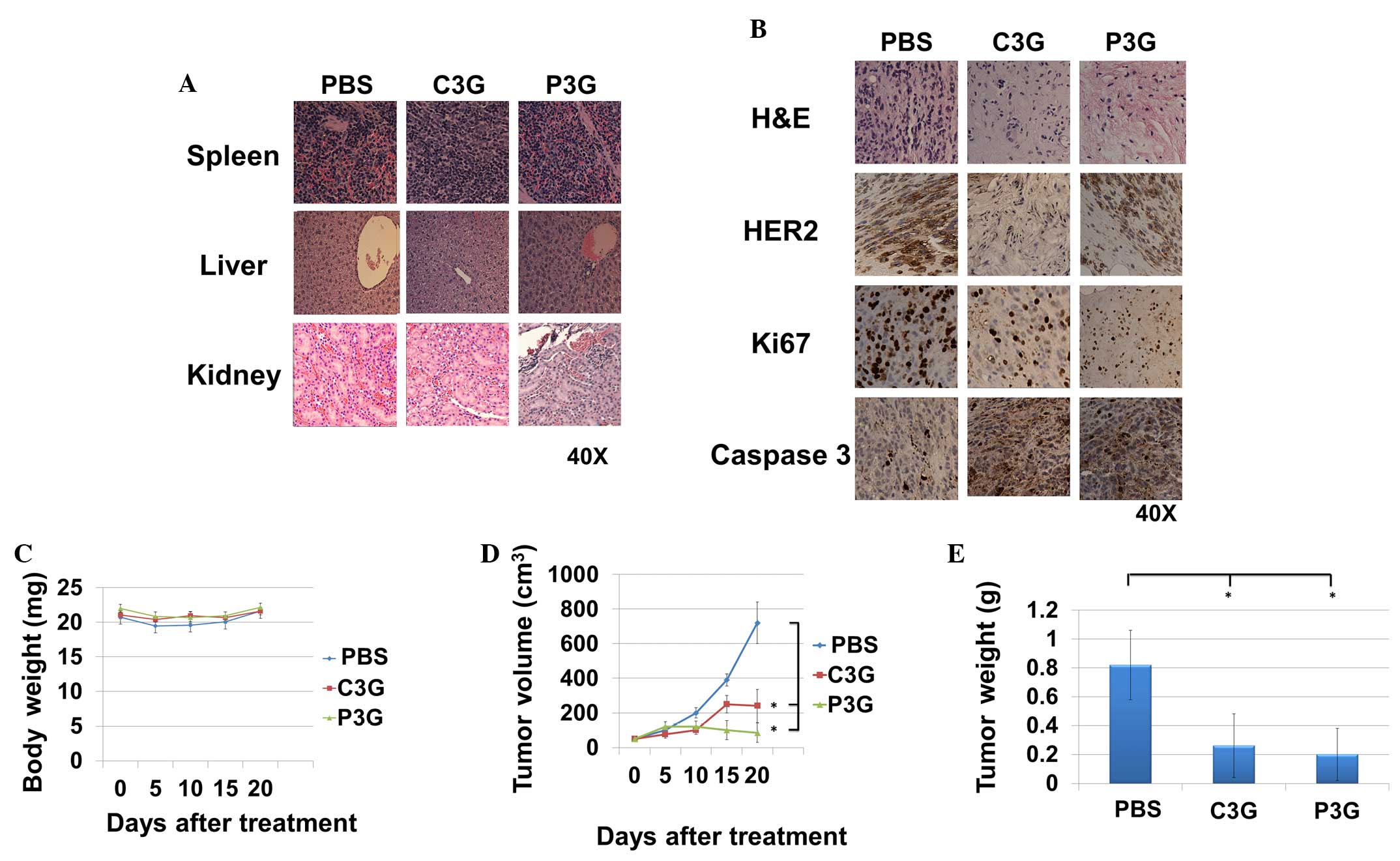
Treatment with C3G or P3G reduces
trastuzumab-resistant cell-mediated tumor growth in vivo
The in vivo anti-trastuzumab-resistant tumor
growth activities of C3G or P3G were determined using BT474R cells.
A pilot study was conducted to determine drug tolerance at the
indicated levels, and no signs of organ damage were observed
(8). After the experiment, the
harvested kidney, liver and spleen samples from each treatment
group were indistinguishable from the control group (Fig. 5A). Histopathological studies
demonstrated that tumors treated with C3G or P3G expressed lower
levels of HER2, as well as the proliferation maker Ki67, as
compared with the control group (Fig.
5B). In addition, tumors treated with C3G or P3G expressed
higher levels of caspase 3, as compared with the control group
(Fig. 5B). During the experiment,
body weight of the mice in the C3G or P3G treatment groups was
indistinguishable from those in control groups (Fig. 5C). Mice treated with C3G or P3G
exhibited a ~67 or ~88% reduction in tumor volume at day 20,
respectively (Fig. 5D), and a ~68
or ~76% reduction in tumor weight at day 20, respectively (Fig. 5E).
Discussion
It has previously been reported that C3G and P3G
inhibit G2/M arrest, downregulate cyclin-dependent
kinase expression, and induce caspase-3 activation, chromatin
condensation and cell death in vitro (11). In addition, mulberry anthocyanins,
cyanidin 3-rutinoside and C3G, have been reported to inhibit the
migration and invasion of human lung cancer cells (12). Numerous in vivo experiments
have also suggested promising anti-tumor activities of
anthocyanins. C3G has been shown to inhibit human lung tumor growth
in xenograft models, including carcinoma cell A549 and Lewis lung
carcinoma cell tumor-bearing models (11,13).
In addition, bilberry-derived C3G was able to inhibit intestinal
adenoma formation in an Apc (Min) mouse model (14). Previous preclinical studies have
also detected the antitumor efficacy of C3G and P3G (7,11–19).
In our previous study, we confirmed that the
pharmacological activity of C3G and P3G alone in HER2-positive cell
lines was dependent on the inhibition of HER2 activity (7). In addition, a series of experiments
were conducted, which were designed to examine the synergism
between C3G and trastuzumab, which demonstrated that anthocyanins
were able to significantly enhance trastuzumab-induced growth
inhibition in representative HER2-positive cell lines, including
MDA-MB-453, BT474 and HCC156 cells in vitro. In addition,
treatment with C3G in combination with trastuzumab resulted in a
more potent inhibition of tumor growth in a BT474 tumor-bearing
mouse model, as compared with the control, C3G or trastuzumab
treatment groups. These data supported the need for further studies
to explore the therapeutic potential of anthocyanin components in
combination with trastuzumab in HER2-positive breast cancer.
The in vitro and in vivo data reported
in the present study indicated that C3G or P3G treatment may
inhibit the activity of HER2, AKT and MAPK in HER2-positive
MDA-MB-453 and BT474 cells, as well as in trastuzumab-resistant
MDA-MB-453R and BT474R cells. Treatment with C3G or P3G induced
apoptosis in the trastuzumab-resistant cells and suppressed the
migration and invasion of these cells. In conclusion, C3G and P3G
were able to significantly inhibit the growth of
trastuzumab-resistant cells in vitro and in vivo.
These results support the requirement for further studies that
explore the therapeutic potential of anthocyanins in the treatment
of patients with trastuzumab-resistant breast cancer. Further
studies with regards to overcoming drug resistance are also
essential.
Acknowledgments
The authors of the present study would like to thank
the Chengdu Medical College flow-cytometry core facility and the
animal core facility for their help conducting in vitro and
in vivo studies. The present study was supported by the
Sichuan Province Health Bureau (grant no. 110465) and the National
Natural Science Foundation of China (grant no. 81273074).
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2015. American Cancer Society; Atlanta, GA: 2015
|
|
2
|
Lv F, Yu Y, Zhang B, Liang D, Li ZM and
You W: Inhibitory effects of mild hyperthermia plus docetaxel
therapy on ER(+/−) breast cancer cells and action mechanisms. J
Huazhong Univ Sci Technolog Med Sci. 33:870–876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bedard PL, Cardoso F and Piccart-Gebhart
MJ: Stemming resistance to HER-2 targeted therapy. J Mammary Gland
Biol Neoplasia. 14:55–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vrbic S, Pejcic I, Filipovic S, Kocic B
and Vrbic M: Current and future anti-HER2 therapy in breast cancer.
J BUON. 18:4–16. 2013.PubMed/NCBI
|
|
7
|
Liu W, Xu J, Wu S, Liu Y, Yu X, Chen J,
Tang X, Wang Z, Zhu X and Li X: Selective anti-proliferation of
HER2-positive breast cancer cells by anthocyanins identified by
high-throughput screening. PLoS One. 8:e815862013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Xu J, Liu Y, Yu X, Tang X, Wang Z
and Li X: Anthocyanins potentiate the activity of trastuzumab in
human epidermal growth factor receptor 2-positive breast cancer
cells in vitro and in vivo. Mol Med Rep. 10:1921–1926.
2014.PubMed/NCBI
|
|
9
|
Nahta R, Takahashi T, Ueno NT, Hung MC and
Esteva FJ: P27(kip1) down-regulation is associated with trastuzumab
resistance in breast cancer cells. Cancer Res. 64:3981–3986. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nahta R and Esteva FJ: In vitro effects of
trastuzumab and vinorelbine in trastuzumab-resistant breast cancer
cells. Cancer Chemother Pharmacol. 53:186–190. 2004. View Article : Google Scholar
|
|
11
|
Chen PN, Chu SC, Chiou HL, Chiang CL, Yang
SF and Hsieh YS: Cyanidin 3-glucoside and peonidin 3-glucoside
inhibit tumor cell growth and induce apoptosis in vitro and
suppress tumor growth in vivo. Nutr Cancer. 53:232–243. 2005.
View Article : Google Scholar
|
|
12
|
Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang
CL and Hsieh YS: Mulberry anthocyanins, cyanidin 3-rutinoside and
cyanidin 3-glucoside, exhibited an inhibitory effect on the
migration and invasion of a human lung cancer cell line. Cancer
Lett. 235:248–259. 2006. View Article : Google Scholar
|
|
13
|
Ding M, Feng R, Wang SY, Bowman L, Lu Y,
Qian Y, Castranova V, Jiang BH and Shi X: Cyanidin-3-glucoside, a
natural product derived from blackberry, exhibits chemopreventive
and chemotherapeutic activity. J Biol Chem. 281:17359–17368. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooke D, Schwarz M, Boocock D,
Winterhalter P, Steward WP, Gescher AJ and Marczylo TH: Effect of
cyanidin-3-glucoside and an anthocyanin mixture from bilberry on
adenoma development in the ApcMin mouse model of intestinal
carcinogenesis - relationship with tissue anthocyanin levels. Int J
Cancer. 119:2213–2220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernandes I, Marques F, de Freitas V and
Mateus N: Antioxidant and antiproliferative properties of
methylated metabolites of anthocyanins. Food Chem. 141:2923–2933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamenickova A, Anzenbacherova E, Pavek P,
Soshilov AA, Denison MS, Zapletalova M, Anzenbacher P and Dvorak Z:
Effects of anthocyanins on the AhR-CYP1A1 signaling pathway in
human hepatocytes and human cancer cell lines. Toxicol Lett.
221:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen XQ, Nagao N, Itani T and Irifune K:
Anti-oxidative analysis and identification, and quantification of
anthocyanin pigments in different coloured rice. Food Chem.
135:2783–2788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fernandes I, Faria A, Azevedo J, Soares S,
Calhau C, De Freitas V and Mateus N: Influence of anthocyanins,
derivative pigments and other catechol and pyrogallol-type
phenolics on breast cancer cell proliferation. J Agric Food Chem.
58:3785–3792. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heinonen M: Antioxidant activity and
antimicrobial effect of berry phenolics - a Finnish perspective.
Mol Nutr Food Res. 51:684–691. 2007. View Article : Google Scholar : PubMed/NCBI
|