Introduction
Parkinson's disease is the second most common
neurode-generative disease and affects nearly 1% of the worldwide
population over 65 (1,2). Traditionally, PD is regarded as the
most common movement disorder due to the fact that the majority of
patients with PD present with predominantly motor symptoms
including tremor, rigidity, slow movements and gait problems
(3). However, non-motor symptoms
are also very common in PD. For example, sleep problems, the most
frequent non-motor symptoms, affect 65–95% of patients (4–7).
Furthermore, the non-motor symptoms may equally or more adversely
affect the quality life of patients with PD.
Although the etiology of PD is not clear, it is
generally believed that both genetic susceptibility and
environmental factors contribute to the pathogenesis of PD.
Mutations in leucine-rich repeat kinase 2 (LRRK2) gene is the most
common genetic cause of familial and sporadic PD (8–11).
Similar to sporadic late-onset PD, sleep problems are the major
complaints of patients with PD who have LRRK2 mutations. It has
been reported that sleep disruptions are present in 60–98% of
patients with LRRK2-associated PD (12). Thus, studying the role of LRRK2 in
sleep disturbances may generate new insights into the
pathophysiology of PD, providing therapeutic options for the
management of sleep disorders in LRRK2-associated PD.
The fruit fly, Drosophila melanogaster, has
been increasingly used to model neurologic diseases due to the
similarity in the nervous system between fruit flies and humans
(13–17). Several LRRK2 transgenic flies have
been created to study the role of LRRK2 mutations (18,19).
These LRRK2 transgenic flies recapitulate several key features of
human PD including locomotor dysfunction and selective loss of
dopaminergic neurons (20).
However, little is known about sleep disorders in these LRRK2
transgenic flies. Sleep in Drosophila is regulated by the
MBs. MB output regulates the sleep through different downstream
neurons, while ablation of MB output leads to sleep disturbances
(21,22).
Clinically, sleep problems are more difficult to
treat than motor symptoms in PD due to the multifactorial causes of
sleep disturbances. Furthermore, current treatments for PD sleep
disorders are often associated with undesirable adverse effects.
Melatonin is an endogenous regulator of the sleep/wake cycle.
Melatonin secretion patterns change in PD patients, and treatment
with melatonin replenishes the inadequate melatonin levels,
relieving the sleep symptoms (23). In addition to its sleep-promoting
effects, melatonin is an effective antioxidant. The neuroprotective
activity of melatonin has been well documented in a range of models
of PD (24,25). However, these beneficial effects of
melatonin have not been explored in LRRK2-associated PD. In the
present study, transgenic flies were generated to express human
(h)LRRK2 in the MBs. Transgenic flies expressing hLRRK2 were
observed to exhibit sleep problems such as sleep fragmentation
during the night. Additionally, hLRRK2 was observed to disrupt the
presynaptic function in the Kenyon cells (KCs) in the MB and
increase bouton density. Furthermore, administration of melatonin
significantly attenuated the sleep problems and rescues the
presynaptic dysfunction in the hLRRK2 transgenic flies.
Materials and methods
Generation of human LRRK2 transgenic
flies
The transgenic flies were constructed to bear the
different hLRRK2 constructs [wild type (WT) and G2019S] under the
control of a yeast upstream activating sequence (UAS). A green
fluorescent protein (GFP) XbaI-EcoRI fragment was
first ligated into the pUAST vector to generate a UAS-GFP
construct. Flag tagged hLRRK2 was then inserted between the
KpnI and BglII sites of the UAS-GFP vector. The
constructs were injected into w1118 embryos (Bloomington
Drosophila Stock Center, Bloomington, IN, USA; n=6 in each group)
to obtain transformant lines. Two transgenic lines each were
generated for UAS-GFP-hLRRK2 and UAS-GFP-hLRRK2-G2019S. The hLRRK2
expression levels were examined by western blot analysis using
anti-Flag staining. OK107-GAL4 was used to selectively express
UAS-GFP-hLRRK2 and UAS-GFP-hLRRK2-G2019S in the MBs. The phenotypic
characterization was conducted on hLRRK2-WT and hLRRK2-G2019S
lines. w1118 served as a negative control.
Drosophila were grown on standard cornmeal medium at 25°C
under 12/12 h light/dark (L/D) conditions. For melatonin treatment,
flies were transferred to regular fly food containing 4 mM
melatonin.
Western blotting
The heads of adult flies (3–7 days post eclosion)
were collected and homogenized in radioimmunoprecipitation assay
lysis buffer (EMD Millipore, Billerica, MA, USA) containing a
protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim,
Germany). Following centrifugation at 8,000 × g for 15 min at 4°C,
the supernatants were subjected to Bradford protein assays
(Beyotime Institute of Biotechnology, Haimen, China) to ensure
equal protein loading (50 µg) and resolved on 8% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis gel, and then
transferred onto polyvinylidene difluoride membranes (0.45 mm; EMD
Millipore). The membranes were blocked in Tris-buffered saline-0.1%
Tween 20 containing 5% nonfat milk for 1 h at room temperature and
then probed with primary antibodies overnight at 4°C and secondary
antibodies for 1 h at room temperature. The primary antibodies used
were as follows: a mouse monoclonal anti-Flag-tag antibody
(1:2,000; Sigma-Aldrich, St. Louis, MO, USA; cat. no. F3165) and
mouse monoclonal anti-β-actin (1:3,000; ProteinTech Group, Inc.,
Chicago, IL, USA; cat. no. 60008-01). The secondary antibody was
horseradish peroxidase-conjugated goat anti-mouse antibody
(1:6,000; EarthOx Life Sciences, Millbrae, CA, USA; cat. no.
E030110-01). Protein was detected using chemiluminescence reagents
(Beyotime Institute of Biotechnology).
Sleep assays
Individual female virgin flies (3–7 days post
eclosion; n=32 in each group) were transferred into monitor tubes
(5×65 mm) containing 5% sucrose and 2% agar media at one end,
enabling the continuous recording of behavior over a number of
days. Locomotor activity was recorded by the Drosophila
Activity Monitoring System (DAMS; TriKinetics, Inc., Waltham, MA,
USA). Sleep was defined as periods of 5 min without recorded
activity. Data were collected for 3 days. Experiments were
performed in an incubator at a temperature of 25±1°C and a relative
humidity of 60±5%. Lights were turned on at zeitgeber time (ZT)0
(circadian time 06:00) and off at ZT12 (circadian time 18:00).
Electrophysiological recordings from KCs
in isolated whole brains
All brains were obtained from flies 1–3 days in age.
The entire brain including the optic lobes was removed from the
head. The dissected brains were then mounted in an RC-26 perfusion
chamber (Warner Instruments, LLC, Hamden, CT, USA) containing the
recording solution bubbled with 95% O2 and 5%
CO2 (2 ml/min) throughout the experiments with the
ventral surface of the brain facing up (26). The standard external solution
contained (in mM): 101 NaCl, 1 CaCl2, 4
MgCl2, 3 KCl, 5 glucose, 1.25 NaH2PO4, and
20.7 NaHCO3, at pH 7.2, osmolarity 250 Osm. Recording
pipettes were fabricated from capillary glass using a four stage
micropipette puller, and had tip resistances of 15–20 MΩ, when
filled with the intracellular solution of the following composition
(in mM): 102 K-gluconate, 17 KCl, 0.94 ethylene glycol tetraacetic
acid, 8.5 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1.7
MgCl2, 17 NaCl, at pH 7.2, osmolarity 235 Osm. Pipettes
were targeted to GFP-labeled KCs in the MBs. Current-clamp (n=6 in
each group) and voltage-clamp (n=6 in each group) recordings were
performed using patch-clamp electrodes. Giga-ohm seals were
achieved prior to recording in an on-cell configuration, followed
by whole-cell configuration while in voltage-clamp mode. Recordings
were made at room temperature, and only a single KC neuron was
examined in each brain. Excitatory postsynaptic potential (EPSP)
frequency and amplitude were recorded using whole-cell
current-clamp. For measurements of cholinergic miniature excitatory
postsynaptic current (mEPSC), neurons were held at −75 mV followed
by whole-cell configuration in voltage clamp, tetrodotoxin (1
µM) was added to the external solution to block
voltage-gated sodium currents and γ-aminobutyric acid-ergic
synaptic currents were blocked by picrotoxin (10 µM). mEPSC
amplitudes <20 pA were detected. All electrophysiological
recordings were conducted using a BX51WI upright microscope
(Olympus Corporation, Tokyo, Japan). Data were acquired by
MultiClamp 700B amplifier and an Axon Digidata1440A (Molecular
Devices, LLC, Sunnyvale, CA, USA), and were filtered at 5 kHz using
a built-in filter and digitized at 5 kHz. Data were analyzed
offline using Clampfit 10.2 (Molecular Devices, LLC).
Biocytin staining and two-photon laser
scanning fluorescence microscopy
To examine the morphology of the recorded single
neurons, post hoc staining with biocytin was utilized. In these
cells (n=7 in each group), 0.4% biocytin was added to the internal
pipette solution. Following electrophysiological recordings, the
brain was fixed in phosphate buffered 4% formaldehyde for 3 h on
ice. The brains were then washed 5 times with phosphate-buffered
saline-Tween 20 on the orbital shaker for 10 min at room
temperature and then blocked in blocking buffer (0.1 M Tris-HCl,
0.1% Triton X-100, 10% goat serum) for 3 h on ice. Brains were
incubated in Cy3 (1:100 dilution) for 24 h at 4°C.
A Leica TCS SP5 microscope (Leica Microsystems GmbH,
Wetzlar, Germany) with a 40× objective was used to acquire optical
slices through the MBs. The position of the soma was determined by
both the position of electrode tip and the intense biocytin
staining. An argon laser provided the excitation line at 546 nm
with the gate for the multiplier opened between 500 and 600 nm.
Slices of the brain (~120) were generated at a thickness of 1
µm per step (from the top of the soma to bottom of the
dendrite in the z-axis through the tissue), at a 1024×2048 Hz
scanning speed. Two photon images were saved as lif, the preferred
file format for image processing using Imarisx64 software, version
7.2.1 (Bitplane AG, Zurich, Switzerland), which were used to create
3D optical volumes of the neuronal dendrites, from which the
synaptic boutons can be detected.
Data analysis
All statistical analysis was conducted using SPSS
19.0 software (IBM SPSS, Armonk, NY, USA). The Shapiro-Wilks test
was used to determine the normality of data. The normally
distributed data were analyzed by 2-tailed, unpaired one-way
analysis of variance followed by the Newman-Keuls multiple
comparison post hoc test. Data are presented as the mean ± standard
error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
MB expression of hLRRK2 did not result in
gross morphological alterations
hLRRK2 was selectively expressed in the MBs using
the OK107 promoter to target GAL4 expression in all lobes of the
MBs. In these transgenic flies, hLRRK2 was expressed in all MB
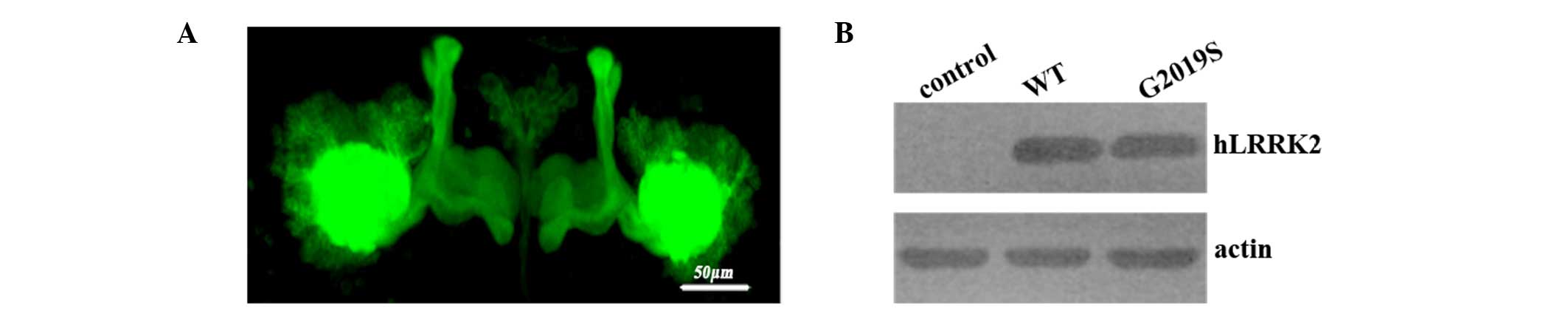
lobes as indicated by the GFP fluorescence (Fig. 1A). Structurally, MB lobes remained
intact in flies expressing either hLRRK2-WT or hLRRK2-G2019S. The
size and thickness of the MB lobes were similar between control and
hLRRK2 flies (Fig. 1A). Confocal
microscopy did not reveal any cell loss and axonal degeneration of
KCs in all the examined LRRK2 files compared with the control.
Western blotting demonstrated that the protein expression was
stable and at similar levels between the hLRRK2-WT and
hLRRK2-G2019S flies (Fig. 1B).
Taken together, these results indicate that expression of hLRRK2 in
the MBs does not result in any significant morphological
alterations.
MB expression of hLRRK2 disrupted normal
sleep patterns
To explore whether MB expression of hLRRK2 results
in sleep problems, sleep patterns were investigated in hLRRK2
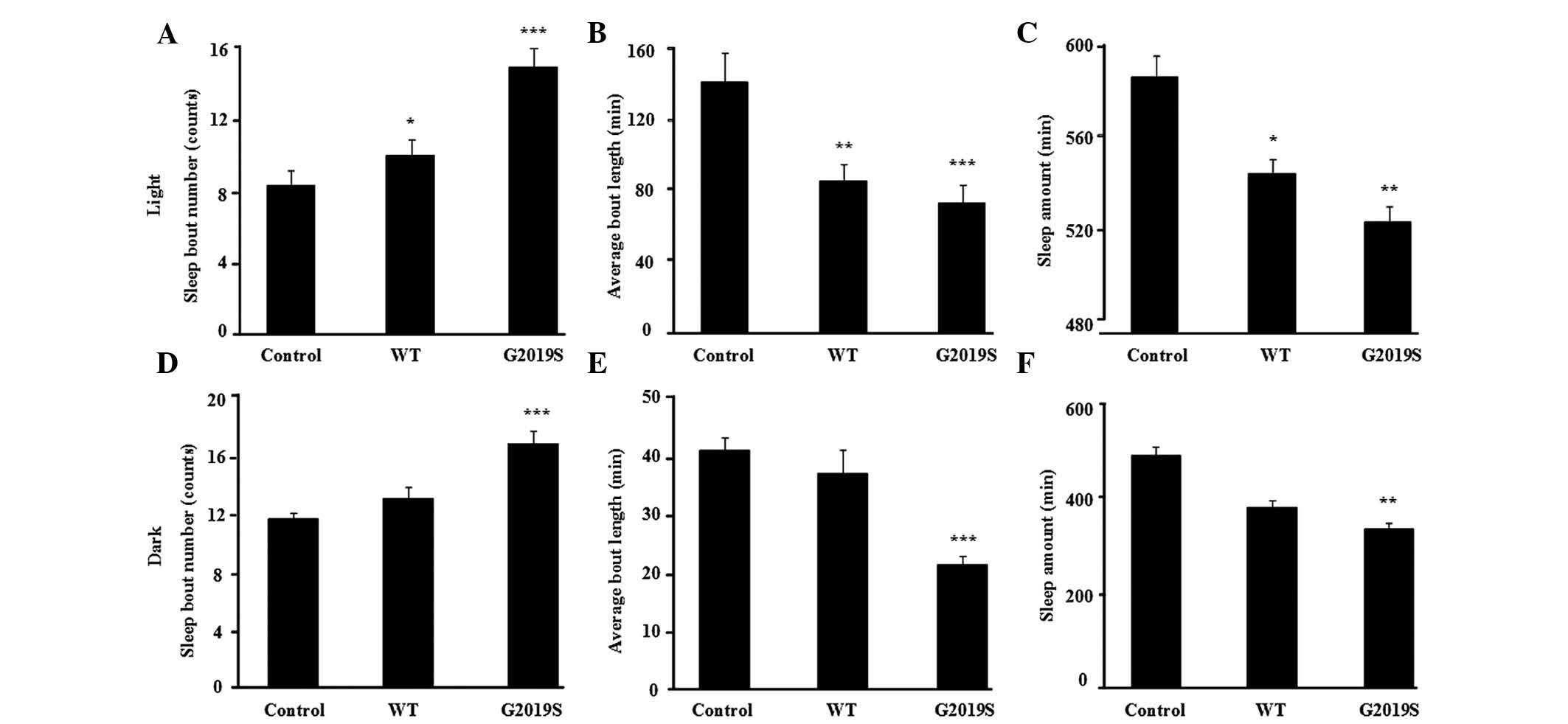
flies. Compared with the control flies, the bout number of
hLRRK2-WT flies were increased (P=0.035; Fig. 2A) and the mean length of each sleep
episode in the light phase were significantly reduced (Fig. 2B, P= 0.002), however, not in the
dark phase (Fig. 2D and E). By
contrast, the expression of hLRRK2-G2019S markedly increased bout
number (counts; Fig. 2A,
P<0.001; Fig. 2D, P<0.001)
and reduced bout length (min) in both the light and dark phases
(Fig. 2B, P<0.001; Fig. 2E, P<0.001). Furthermore, the
total sleep (min) of the hLRRK2-G2019S mutant flies was
significantly reduced (Fig. 2C, P=
0.007; Fig. 2F, P= 0.004),
however, not in the hLRRK2-WT flies compared with the control group
(Fig. 2C and F). The sleep
behavior data is presented in Table
I. Collectively, the data suggest that MB expression of hLRRK2
disrupted normal sleep patterns in flies, predominantly by
increasing arousal during sleep and reducing total sleep time.
 | Table ISleep behavior of flies expressing
hLRRK2-WT and hLRRK2-G2019S (with and without melatonin treatment),
driven by the OK107-GAL4-mediated expression of upstream activating
sequence transgenes and w1118. |
Table I
Sleep behavior of flies expressing
hLRRK2-WT and hLRRK2-G2019S (with and without melatonin treatment),
driven by the OK107-GAL4-mediated expression of upstream activating
sequence transgenes and w1118.
| Group | Light phase
| Sleep bout
(number) | Dark phase
| Sleep (min) |
|---|
| Sleep bout
(number) | Mean bout length
(min) | Sleep (min) | Mean bout length
(min) |
|---|
| Control | 8.281±0.743 |
141.997±19.541b |
579.484±9.063b |
11.500±1.067b |
41.449±1.170c |
470.687±11.698b |
| WT | 9.938±0.853d |
85.648±9.493e |
542.078±9.282d | 13.859±1.778 | 39.627±4.256 | 387.625±12.625 |
| G2019S |
14.875±1.162f |
74.109±10.287f |
523.797±11.293e |
17.906±0.867f |
22.975±1.463f |
348.125±10.718e |
| G2019S (M) | 9.094±0.714c |
121.475±16.085b |
552.313±9.083a |
15.359±0.892a | 26.100±2.219 | 310.016±13.212 |
MB expression of hLRRK2 resulted in
presynaptic dysfunction
The synaptic functions of KCs, such as membrane
excitability, are thought to be critical for sleep regulation. To
investigate whether hLRRK2 modulates the synaptic function of KCs,
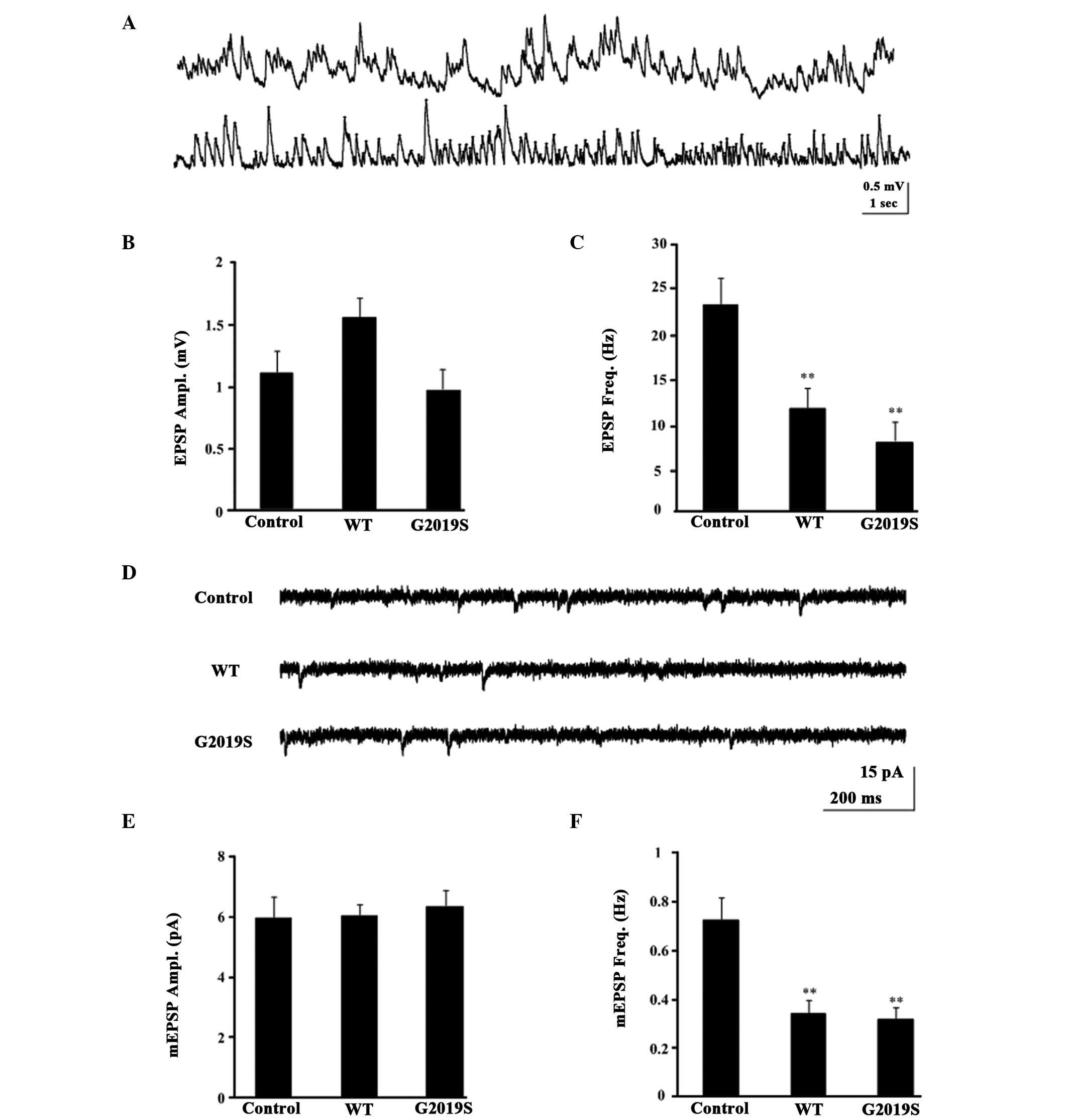
whole-cell current-clamp recording (Fig. 3A) and voltage-clamp recordings in
MB KCs was performed (Fig. 3D).
During the recordings, the frequency and amplitude of the EPSP and
mEPSC were detected, with the amplitude and frequency of the mEPSC
indicating the postsynaptic function and presynaptic function,
respectively. When the holding potential was −75 mV in
voltage-clamp mode, and the mean resting potential was −60±1.25 mV
in current-clamp mode, none of the examined KCs exhibited
spontaneous firing activities, however, some exhibited subthreshold
spontaneous activity in normal control flies (Fig. 3A and D). The EPSP amplitude in WT
(1.624±0.1759 mV; P=0.572) and G2019S (0.954±0.169 mV, P=0.994)
were not significantly different from the control flies
(1.091±0.219 mV; Fig. 3B). By
contrast, the EPSP frequency in WT (12.300±1.174 Hz, P=0.008) and
G2019S (6.850±1.053 Hz, P=0.004) were significantly lower compared
with the control flies (25.525±3.957 Hz; Fig. 3C). Similarly, the mEPSCs frequency
of WT (0.388±0.0557 Hz; P<0.05) and G2019S (0.3101±0.462 Hz;
P<0.01; Fig. 3F) were
significantly lower than the control w1118 (0.778±0.078;
Fig. 3F), however, the mEPSCs
amplitude of WT and G2019S indicated no significant difference
compared with the control w1118 (Fig. 3E). However, the amplitude but not
the frequency of either mEPSCs or EPSPs were similar between the
hLRRK2 flies and control flies (Fig.
3B and E), indicating that postsynaptic but not presynaptic
function remained intact in hLRRK2 flies. Taken together, these
results indicate that hLRRK2 expression results in presynaptic
dysfunction, as evidenced by the reduction in the cellular
excitability of KCs.
MB expression of hLRRK2-G2019S increases
the density of synaptic boutons
To investigate whether hLRRK2-G2019S affects
synaptic plasticity, the synaptic bouton density (synaptic
terminals) was investigated. Biocytin staining was used to label
the synaptic boutons of the KCs in the MBs (Fig. 4Aa) and Fig. 4Ab presents a single KC neuron. To
identify the density of neurons accurately and efficiency, an
imaging method of the KCs was tested, based on two-photon laser
scanning fluorescence microscopy, which enabled the visualization
of the density of KCs synaptic boutons. The density of boutons was
significantly higher in hLRRK2-G2019S mutant flies (0.2010±0.0238
µm; P=0.025) while the density of boutons remained unaltered
in hLRRK2-WT flies (0.1145±0.0465 µm; P=0.89) compared with
the w1118 control flies (0.1246±0.0145 µm;
Fig. 4B). Thus, these results
demonstrate that hLRRK2-G2019S induces excessive synaptic
plasticity (Fig. 4C).
Melatonin attenuates sleep disturbances
and rescues presynaptic dysfunction in hLRRK2 flies
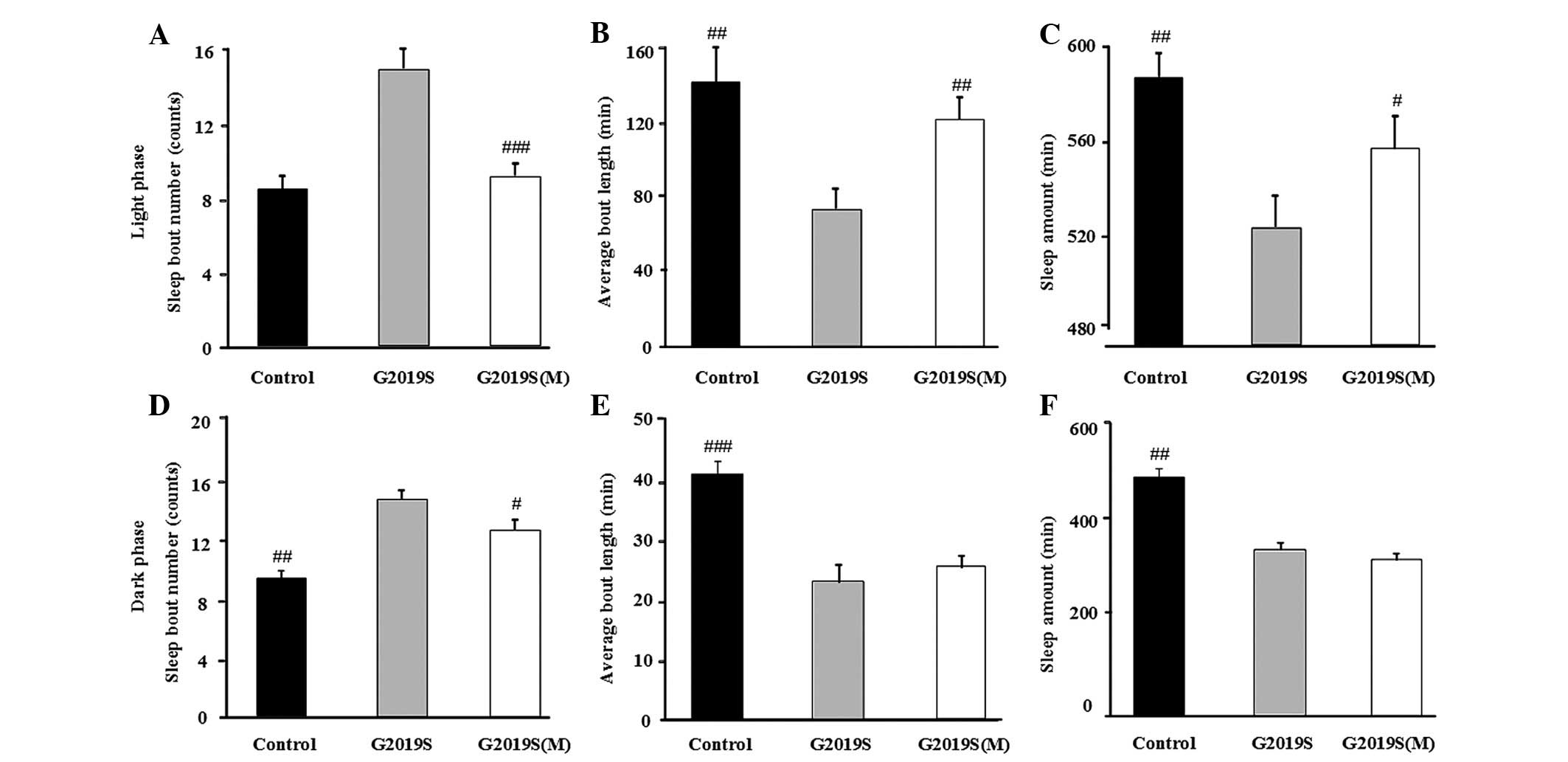
Whether melatonin attenuates hLRRK2-associated sleep
problems and the relationship between sleep disorder and synaptic
dysfunction was investigated. It was observed that melatonin
significantly attenuated the hLRRK2-induced increase in light and
dark phase sleep bouts (Fig. 5A and
D), and melatonin attenuated the hLRRK2-induced reduction in
the mean bout length and the amount of sleep in the light phase
compared with the G2019S flies without melatonin (Fig. 5B and C). However, the mean bout
length and the amount of sleep in the light phase were not altered
(Fig. 5E and F). All sleep
behavior data is presented in Table
I. Furthermore, the EPSP and mEPSC frequencies in the hLRRK2
flies were restored to the levels of the control group following
melatonin treatment compared with the flies without melatonin
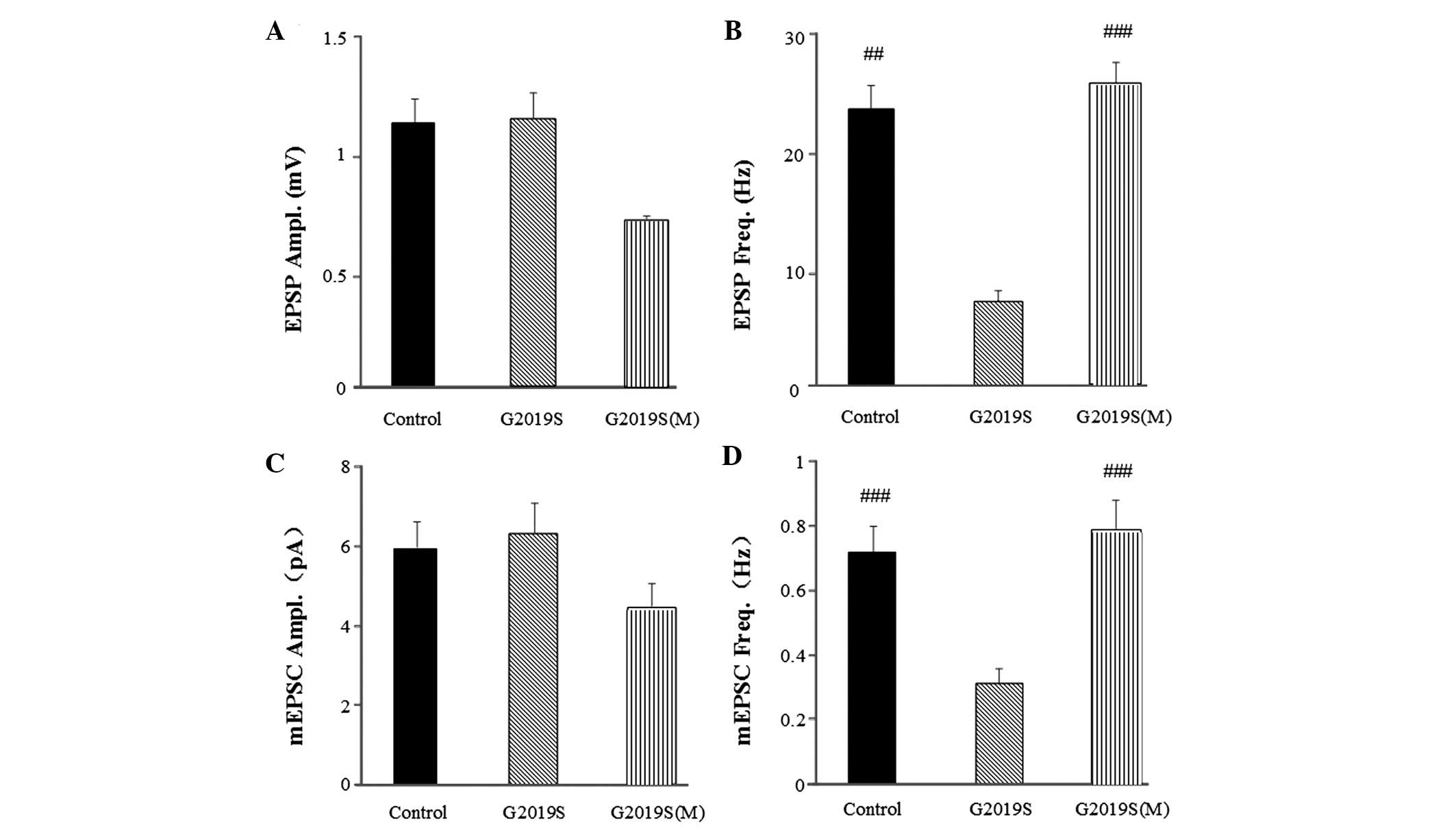
treatment (Fig. 6). The EPSP
frequency of G2019S flies treated with melatonin was restored to
26.022±6.534 Hz (P<0.001; Fig.
6B), which was significantly different from the EPSP frequency
of the G2019S flies without melatonin (6.850±1.953 Hz). The mEPSC
frequency in the G2019S flies treated with melatonin was restored
to 0.785±0.104 Hz (P<0.001; Fig.
6D), which was significantly different from the mEPSC frequency
of the G2019S flies without melatonin (0.310±0.046 Hz). Amplitude
differences in mEPSCs suggest changes to postsynaptic function and
frequency differences suggest changes to presynaptic function. The
results from the present study indicated neither the EPSP (Fig. 6A) or mEPSC (Fig. 6C) amplitudes were altered in the
hLRRK2 flies, with or without melatonin treatment. However, the
frequency of EPSP (Fig. 6B) and
mEPSC (Fig. 6D was altered with
melatonin, thus, suggesting that melatonin improves sleep problems
and presynaptic dysfunction in hLRRK2 flies.
Discussion
Mutations in LRRK2 are the most common genetic cause
for familial and sporadic PD (27). Thus, studying the role of hLRRK2 in
PD is important to further understand the pathophysiology of PD.
The present study reports that the expression of hLRRK2 in the MBs
induced sleep disorders in flies. hLRRK2-induced sleep problems
were associated with reduced frequency of EPSPs and increased
synaptic bouton density, indicating the important role of
hLRRK2-induced synaptic dysfunction in sleep disorders.
Furthermore, melatonin significantly attenuated hLRRK2-induced
sleep problems and rescued hLRRK2-induced synaptic dysfunction,
suggesting a potential clinical application of melatonin in
patients with PD carrying LRRK2 mutations. Sleep problems are a
major contributor to impairment in PD, with 80–90% of patients with
PD experiencing disturbances of sleep patterns (28,29).
Although the manifestations of sleep problems vary, sleep
fragmentation is one of the most common sleep complaints of
patients with PD (30–32). Consistently, MB expression of
hLRRK2 in flies resulted in sleep fragmentation as indicated by the
increase in arousal during sleep. Thus, the transgenic flies
expressing hLRRK2 in the MBs are able to recapitulate the sleep
disturbances observed in clinical PD. The underlying causes of
sleep problems in PD are complex. Previous studies have
demonstrated that a large proportion of sleep problems are
associated with the involvement of non-dopaminergic brain regions
with multiple neurotransmitter deficiencies such as cholinergic
system degeneration (33–36). The MBs serve a central role in
sleep regulation in flies (22).
The MBs are a paired brain structure, and are composed of small
neurons known as KCs. The synaptic functions of the KCs are
critical for sleep regulation (21). In the present study, expression of
hLRRK2 in the MBs did not result in any gross morphological damage
however, reduced cholinergic synaptic mEPSC and EPSP frequency was
observed, suggesting a negative modulation of hLRRK2 on presynaptic
properties. Notably, in addition to sleep problems, the synaptic
bouton density was increased in hLRRK2-G2019S flies. Synaptic
boutons are the presynaptic terminals that contain
neurotransmitters stored in synaptic vesicles. The increase in the
number of synaptic boutons means greater levels of
neurotransmitters present, which in turn increases the probability
of the neurotransmitter release. Thus, the increase in synaptic
bouton density seems contradictory to the negative modulation of
hLRRK2 on synapses. This may be explained by the inhibitory effect
of hLRRK2 on synaptic efficacy. Synaptic efficacy is the capacity
of a presynaptic input to influence postsynaptic output.
Previously, hLRRK2 has been reported to disrupt synaptic vesicle
trafficking and distribution within the bouton (37). By doing so, hLRRK2 may prevent the
release of neurotransmitters from the vesicles in the synaptic
boutons and reduce synaptic efficacy. Furthermore, the increase in
the number of synaptic boutons may lead to synaptic stress due to
the increase in synaptic boutons occupying greater space and
demanding increased cellular supply to synapses (38). As a result, low synaptic efficacy
and synaptic stress may further disrupt synaptic homeostasis,
leading to an exaggeration of hLRRK2-induced sleep problems.
The primary function of sleep is to restore brain
energy metabolism, with sleep problems increasing energy
consumption and exaggerating the metabolic disturbances in PD. In
this regard, treatment of sleep disturbances with appropriate drugs
may help to not only to solve sleep problems, but also to prevent
the progression of PD. In the present study, melatonin
significantly attenuated hLRRK2-induced sleep problems and rescued
the reduced cholinergic synaptic mEPSC and EPSP frequencies,
however, had no effect on the increase in synaptic bouton density.
These results suggest that the beneficial effects of melatonin may
be associated with the promotion of synaptic transmission. The
direct action of melatonin on synaptic transmission remains
inconclusive due to inconsistent results from different studies
(39). Alternatively, the
promotion of synaptic transmission may be explained by the
antioxidant role of melatonin. Reactive oxygen species (ROS) have
been reported to be involved in vesicular neurotransmitter release
(40). At presynaptic sites,
certain essential proteins responsible for the exocytosis of
neurotransmitters such as synaptosomal-associated protein, 25kDa
are vulnerable to ROS attack due to their chemical structures
(41). The oxidation of these key
proteins impairs the neurotransmitter release machinery. Indeed,
ROS scavengers have been reported to prevent ROS attack and enhance
synaptic transmission (41). Thus,
melatonin may reduce ROS and attenuate hLRRK2-induced synaptic
dysfunction. As a result, melatonin may break the cycle involving
sleep disturbances and metabolic stress, and prevent the
progression of LRRK2-associated PD. However, the precise mechanisms
underlying the beneficial effects of melatonin require further
study. Nevertheless, the present study highlights the potential of
melatonin as a candidate neuroprotective agent with sleep-promoting
properties in future clinical trials for patients with PD carrying
hLRRK2 mutations.
Acknowledgments
The current study was supported by grants from the
National Natural Science Foundation of China (grant no. 81371255),
the Doctoral Program of Higher Education of China (grant no.
20110171110058) and the Guangdong Province Science and Technology
Department Project (grant nos. 2011B050400031 and
2012B031800107).
References
|
1
|
Braak H, Del Tredici K, Rüb U, de Vos RA,
Jansen Steur EN and Braak E: Staging of brain pathology related to
sporadic Parkinson's disease. Neurobiol Aging. 24:197–211. 2003.
View Article : Google Scholar
|
|
2
|
Dauer W and Przedborski S: Parkinson's
disease: Mechanisms and models. Neuron. 39:889–909. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkinson J: An essay on the shaking
palsy. 1817. J Neuropsychiatry Clin Neurosci. 14:223–236;
discussion 222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Comella CL: Sleep disorders in Parkinson's
disease: An overview. Mov Disord. 22(Suppl 17): S367–S373. 2007.
View Article : Google Scholar
|
|
5
|
Van Hilten JJ, Weggeman M, Van der Velde
EA, Kerkhof GA, Van Dijk JG and Roos RA: Sleep, excessive daytime
sleepiness and fatigue in Parkinson's disease. J Neural Transm Park
Dis Dement Sect. 5:235–244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mehta SH, Morgan JC and Sethi KD: Sleep
disorders associated with Parkinson's disease: Role of dopamine,
epidemiology and clinical scales of assessment. CNS Spectr. 13(3
Suppl 4): 6S–11S. 2008.
|
|
7
|
Nausieda PA, Weiner WJ, Kaplan LR, Weber S
and Klawans HL: Sleep disruption in the course of chronic levodopa
therapy: An early feature of the levodopa psychosis. Clin
Neuropharmacol. 5:183–194. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cookson MR: The role of leucine-rich
repeat kinase 2 (LRRK2) in Parkinson's disease. Nat Rev Neurosci.
11:791–797. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greggio E and Cookson MR: Leucine-rich
repeat kinase 2 mutations and Parkinson's disease: Three questions.
ASN Neuro. 1:e000022009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paisán-Ruı́z C, Jain S, Evans EW, Gilks
WP, Simón J, van der Brug M, López de Munain A, Aparicio S, Gil AM,
Khan N, et al: Cloning of the gene containing mutations that cause
PARK8-linked Parkinson's disease. Neuron. 44:595–600. 2004.
View Article : Google Scholar
|
|
11
|
Zimprich A, Müller-Myhsok B, Farrer M,
Leitner P, Sharma M, Hulihan M, Lockhart P, Strongosky A, Kachergus
J, Calne DB, et al: The PARK8 locus in autosomal dominant
parkinsonism: Confirmation of linkage and further delineation of
the disease-containing interval. Am J Hum Genet. 74:11–19. 2004.
View Article : Google Scholar
|
|
12
|
Berg D, Schweitzer KJ, Leitner P, Zimprich
A, Lichtner P, Belcredi P, Brüssel T, Schulte C, Maass S, Nägele T,
et al: Type and frequency of mutations in the LRRK2 gene in
familial and sporadic Parkinson's disease*. Brain. 128:3000–3011.
2005.PubMed/NCBI
|
|
13
|
Dawson TM, Ko HS and Dawson VL: Genetic
animal models of Parkinson's disease. Neuron. 66:646–661. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harbison ST and Sehgal A: Quantitative
genetic analysis of sleep in Drosophila melanogaster. Genetics.
178:2341–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huber R, Hill SL, Holladay C, Biesiadecki
M, Tononi G and Cirelli C: Sleep homeostasis in Drosophila
melanogaster. Sleep. 27:628–639. 2004.PubMed/NCBI
|
|
16
|
Koh K, Evans JM, Hendricks JC and Sehgal
A: A Drosophila model for age-associated changes in sleep: Wake
cycles. Proc Natl Acad Sci USA. 103:13843–13847. 2006. View Article : Google Scholar
|
|
17
|
Mackay TF and Anholt RR: Of flies and man:
Drosophila as a model for human complex traits. Annu Rev Genomics
Hum Genet. 7:339–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li T, Yang D, Sushchky S, Liu Z and Smith
WW: Models for LRRK2-linked parkinsonism. Parkinsons Dis.
2011:9424122011.PubMed/NCBI
|
|
19
|
Liu Z, Wang X, Yu Y, Li X, Wang T, Jiang
H, Ren Q, Jiao Y, Sawa A, Moran T, et al: A Drosophila model for
LRRK2-linked parkinsonism. Proc Natl Acad Sci USA. 105:2693–2698.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SB, Kim W, Lee S and Chung J: Loss of
LRRK2/PARK8 induces degeneration of dopaminergic neurons in
Drosophila. Biochem Biophys Res Commun. 358:534–539. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joiner WJ, Crocker A, White BH and Sehgal
A: Sleep in Drosophila is regulated by adult mushroom bodies.
Nature. 441:757–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pitman JL, McGill JJ, Keegan KP and Allada
R: A dynamic role for the mushroom bodies in promoting sleep in
Drosophila. Nature. 441:753–756. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fertl E, Auff E, Doppelbauer A and
Waldhauser F: Circadian secretion pattern of melatonin in
Parkinson's disease. J Neural Transm Park Dis Dement Sect. 3:41–47.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olakowska E, Marcol W, Kotulska K and
Lewin-Kowalik J: The role of melatonin in the neurodegenerative
diseases. Bratisl Lek Listy. 106:171–174. 2005.PubMed/NCBI
|
|
25
|
Polimeni G, Esposito E, Bevelacqua V,
Guarneri C and Cuzzocrea S: Role of melatonin supplementation in
neurodegenerative disorders. Front Biosci (Landmark Ed).
19:429–446. 2014. View
Article : Google Scholar
|
|
26
|
Gu H and O'Dowd DK: Whole cell recordings
from brain of adult Drosophila. J Vis Exp. 6:2482007.
|
|
27
|
Thaler A, Ash E, Gan-Or Z, Orr-Urtreger A
and Giladi N: The LRRK2 G2019S mutation as the cause of Parkinson's
disease in Ashkenazi Jews. J Neural Transm. 116:1473–1482. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar S, Bhatia M and Behari M: Sleep
disorders in Parkinson's disease. Mov Disord. 17:775–781. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stacy M: Sleep disorders in Parkinson's
disease: epidemiology and management. Drugs Aging. 19:733–739.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedman A: Sleep pattern in Parkinson's
disease. Acta Med Pol. 21:193–199. 1980.PubMed/NCBI
|
|
31
|
Friedman JH and Chou KL: Sleep and fatigue
in Parkinson's disease. Parkinsonism Relat Disord. 10(Suppl 1):
S27–S35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu SY, Sun L, Liu Z, Huang XY, Zuo LJ, Cao
CJ, Zhang W and Wang XM: Sleep disorders in Parkinson's disease:
Clinical features, iron metabolism and related mechanism. PLoS One.
8:e829242013. View Article : Google Scholar
|
|
33
|
Cirelli C: The genetic and molecular
regulation of sleep: From fruit flies to humans. Nat Rev Neurosci.
10:549–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Comella CL, Tanner CM and Ristanovic RK:
Polysomnographic sleep measures in Parkinson's disease patients
with treatment-induced hallucinations. Ann Neurol. 34:710–714.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ozekmekçi S, Apaydin H and Kiliç E:
Clinical features of 35 patients with Parkinson's disease
displaying REM behavior disorder. Clin Neurol Neurosurg.
107:306–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Poryazova R, Oberholzer M, Baumann CR and
Bassetti CL: REM sleep behavior disorder in Parkinson's disease: A
questionnaire-based survey. J Clin Sleep Med. 9:55–59A.
2013.PubMed/NCBI
|
|
37
|
Piccoli G, Condliffe SB, Bauer M, Giesert
F, Boldt K, De Astis S, Meixner A, Sarioglu H, Vogt-Weisenhorn DM,
Wurst W, et al: LRRK2 controls synaptic vesicle storage and
mobilization within the recycling pool. J Neurosci. 31:2225–2237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tononi G and Cirelli C: Sleep and synaptic
homeostasis: A hypothesis. Brain Res Bull. 62:143–150. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosales-Corral SA, Acuña-Castroviejo D,
Coto-Montes A, Boga JA, Manchester LC, Fuentes-Broto L, Korkmaz A,
Ma S, Tan DX and Reiter RJ: Alzheimer's disease: Pathological
mechanisms and the beneficial role of melatonin. J Pineal Res.
52:167–202. 2012. View Article : Google Scholar
|
|
40
|
Tarasenko A, Krupko O and Himmelreich N:
Reactive oxygen species induced by presynaptic glutamate receptor
activation is involved in [(3)H]GABA release from rat brain
cortical nerve terminals. Neurochem Int. 61:1044–1051. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Giniatullin AR, Darios F, Shakirzyanova A,
Davletov B and Giniatullin R: SNAP25 is a pre-synaptic target for
the depressant action of reactive oxygen species on transmitter
release. J Neurochem. 98:1789–1797. 2006. View Article : Google Scholar : PubMed/NCBI
|