Introduction
Autoimmune hepatitis (AIH) is a chronic inflammatory
liver disorder with an etiology that remains unclear. The
pathogenesis may be a result of alterations in immune tolerance, a
genetic predisposition and environmental conditions, which in
collaboration induce a T-cell-mediated attack on liver antigens,
leading to necro-inflammation and liver damage (1). Monocytes/macrophages are a class of
specialized antigen-presenting cells that serve an important role
in the recruitment and activation of innate immune cells.
Monocytes/macrophages are also able to deliver co-stimulatory
signals to activate naive T cells, thus triggering the initiation
of the adaptive immune responses. Therefore, they act as the bridge
between the innate and adaptive immune systems. Previous studies
indicate that dysfunction of monocytes/macrophages is important in
the pathogenesis of numerous autoimmune diseases (2,3),
however the role of monocytes/macrophages in AIH remains unclear.
Kupffer cells (KCs) are present throughout the liver, representing
80~90% of all tissue macrophages in the body (4). Liver damage has been previously
reported to result from the dysfunction of KCs (5).
Monocytes/macrophages serve three main functions
including phagocytosis, antigen presentation and inflammatory
cytokine production (6).
Phagocytosis of pathogens or antigens is a central process in the
host defense mechanism against infections and the immune responses
(7). Antigen presentation is
critical for activation of the adaptive immune response, and the
process is closely associated with HLA-DR and CD80 expression
levels (8,9). The members of the ras homolog gene
family (Rho) guanosine triphosphatase (GTPase) family are known to
regulate signaling pathways leading to remodeling of the actin
cytoskeleton, transcriptional regulation and the cell cycle. It has
been suggested that the Rho GTPase family serves a critical role in
cell adhesion, antigen presentation, migration, chemotaxis and
phagocytosis (10). VAV1 and
p21-activated kinase 1 (PAK1) have been previously described as
effectors of the Rho GTPases (11,12).
The aim of the current study was to measure the
abundance of VAV1 and PAK1 in the livers of patients with AIH and
further evaluate their expression in KCs. The expression levels of
HLA-DR and CD80 in the peripheral blood monocytes (PBMs) of
patients with AIH were also measured to assess
antigen-presentation. In addition, the phagocytic functions of PBMs
were evaluated by co-culture with fluorescent-labeled bacteria.
Subjects and methods
Patients
Subsequent to obtaining informed consent, 21
patients at the Department of Digestive Diseases, Tianjin Medical
University General Hospital (Tianjin, China) with histologically
confirmed AIH of different disease stages, were enrolled in the
present study between January 2011 and February 2013. A total of 7
patients with non-alcoholic fatty liver disease (NAFLD), who were
age- and gender-matched, were selected as the controls. The
diagnosis of AIH was performed according to a simplified criteria
for the diagnosis of AIH (13),
and the diagnosis of NAFLD was performed according to the NAFLD
guidelines by the American Gastroenterological Association,
American Association for the Study of Liver Diseases and American
Collage of Gastroenterology (14).
The AIH patients included 2 men and 19 women with a mean age of
51.2±21.4 years. The serum levels of alanine aminotransferase (ALT)
were assessed using a commercially available kit (cat. no. 70911;
normal level, ≤40 U/l; Biobase, Shandong, China), and based on
these results, and ultrasound or computerized tomography of the
abdomen, the patients were classified into the AIH normal liver
function group (ALT ≤40 U/l, no cirrhosis), AIH abnormal liver
function group (ALT >40 U/l, no cirrhosis) or the AIH cirrhosis
group (cirrhosis). The 7 patients with NAFLD analyzed as the
controls comprised 1 men and 6 women with a mean age of 53.1±19.9
years. None of the patients had previously received any
treatment.
The institutional review board and ethical committee
of Tianjin Medical University General Hospital approved the study
protocol. All patients provided written informed consent for their
participation in the current study.
Western blotting of VAV1 and PAK1
Liver tissue samples were obtained by
ultrasound-guided percutaneous liver biopsy and stored at −80°C.
Liver tissue samples were homogenized on ice in lysis buffer
(Well-Biology, Changsha, China) containing 50 mM Tris (pH 8.0), 150
mM NaCl, 1% Triton X-100, 100 µg/ml phenylmethanesulfonyl fluoride
(Roche Diagnositcs, Basel, Switzerland). Lysates were clarified by
centrifugation at 12,000 × g and 4°C for 15 min, and the protein
concentration was determined using the Bicinchoninic Acid Assay kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Supernatants
were separated by 10% sodium dodecyl sulfate-polyacrylimide gel
electrophoresis, and transferred onto a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% skimmed milk in phosphate-buffered saline (PBS;
Well-Biology) for 1 h, followed by incubation with the following
primary antibodies overnight at 4°C: Mouse anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH) monoclonal antibody (1:1,000;
sc-365062; Santa Cruz Biotechnology, Inc., Danvers, TX, USA); mouse
anti-VAV1 polyclonal antibody (1:1,000; ab58106; Abcam, Cambridge,
MA, USA); and rabbit anti-PAK1 monoclonal antibody (1:1,000;
ab40852; Abcam). Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-mouse (1:1,000;
ZB-5305; ZSGB-BIO, Beijing, China) and goat anti-rabbit (1:1,000;
ZB-5301; ZSGB-BIO) IgG for 1 h at room temperature. The blots were
detected using an enhanced chemiluminescence system (Syngene,
Frederick, MD, USA).
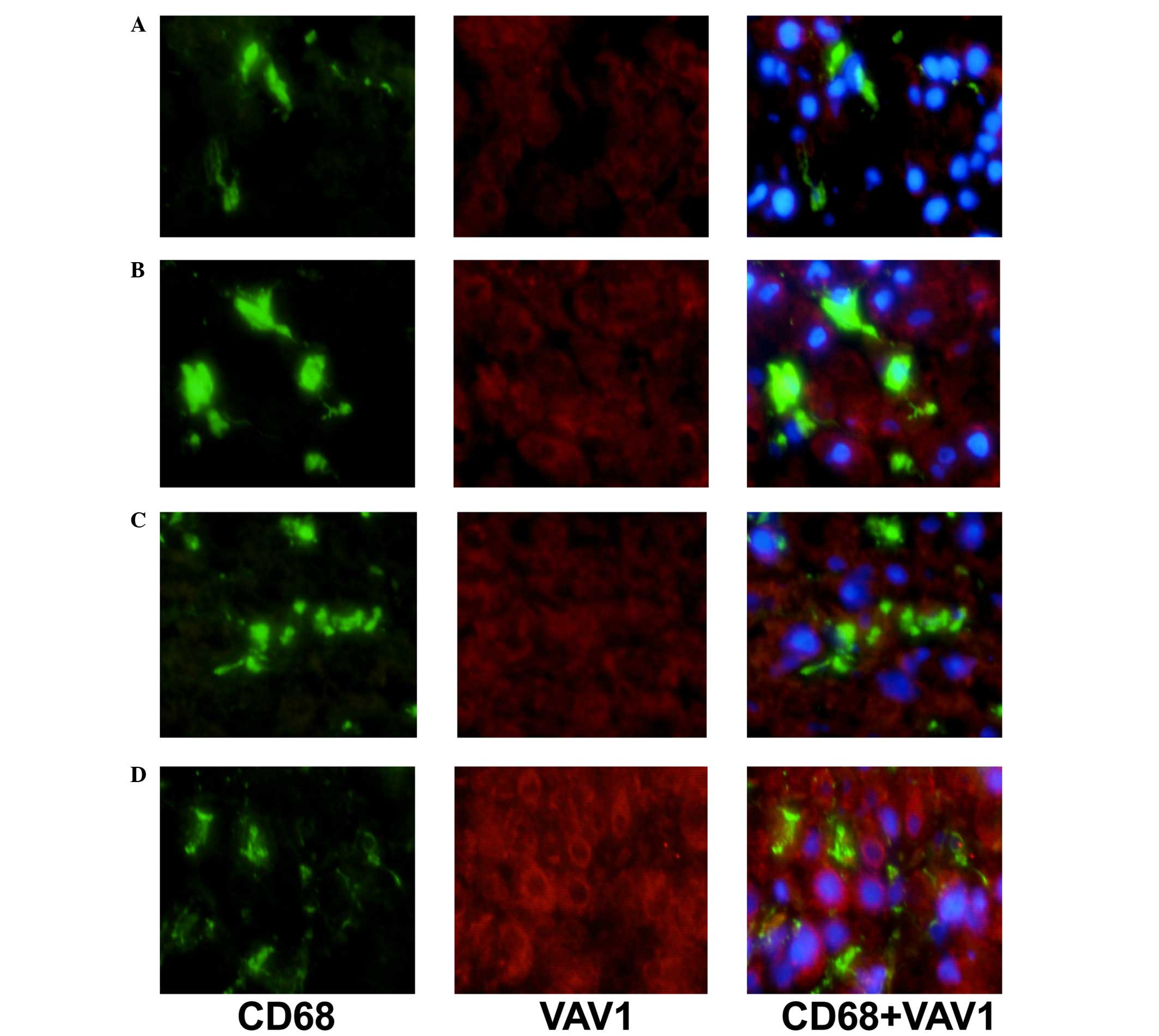
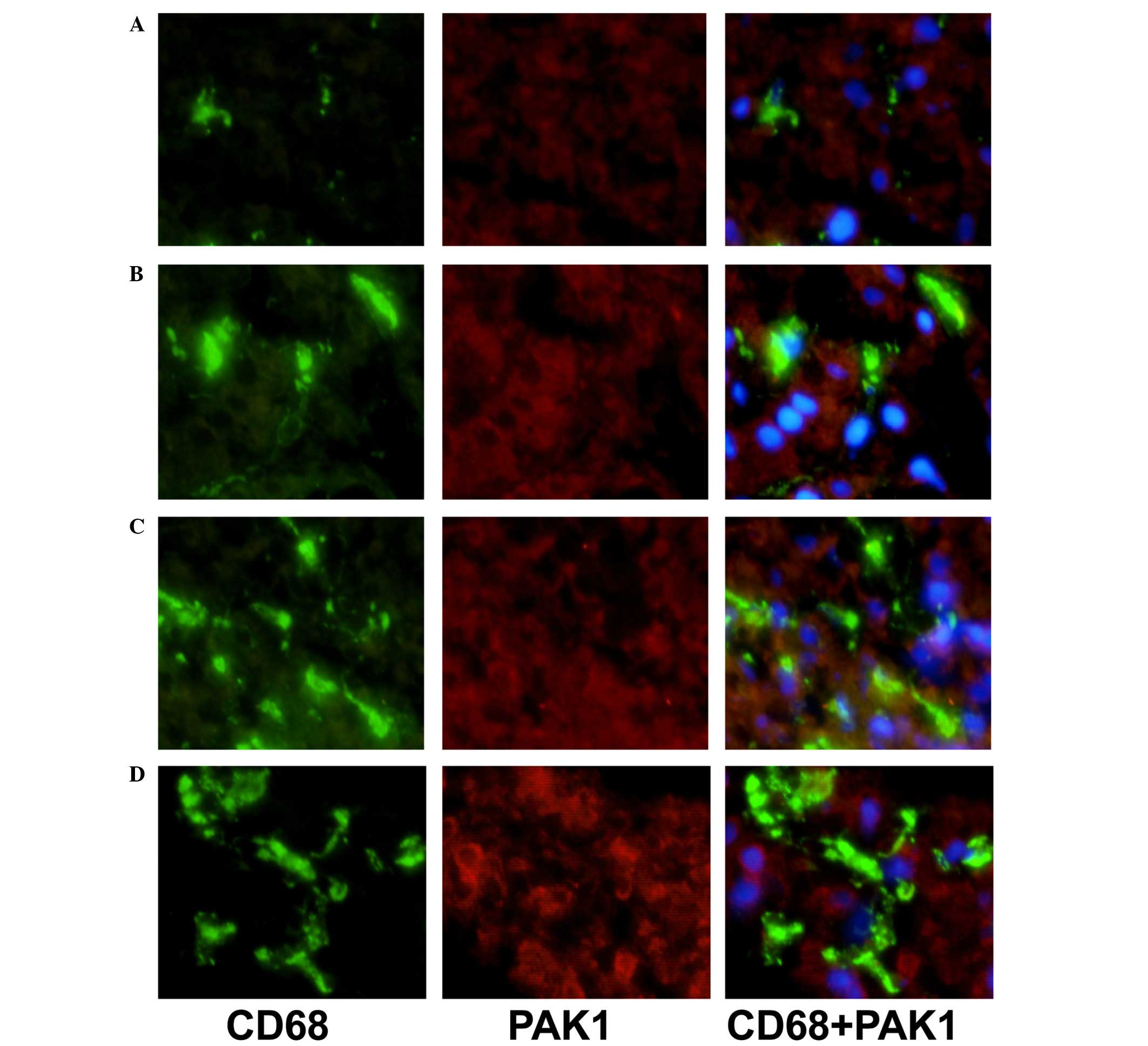
Double immunostaining of CD68/VAV1 or
PAK1
To characterize the expression of VAV1 and PAK1 on
KCs, double-immunostaining for CD68 and either VAV1 or PAK1 was
performed in the livers of patients with NAFLD and AIH. Sections
were incubated with the monoclonal mouse anti-CD68 antibody (1:50;
ab955; Abcam) and either polyclonal mouse anti-VAV1 (1:50) or
monoclonal rabbit anti-PAK1 (1:50). Subsequent to treatment with
fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG
(1:100; sc-2010; Santa Cruz Biotechnology, Inc.) and
PerCP-Cy5.5-conjugated goat anti-rabbit IgG (1:100; sc-45101; Santa
Cruz Biotechnology, Inc.), fluorescence was observed under a
fluorescent microscope (BX51; Olympus Corporation, Tokyo,
Japan).
Flow cytometry analysis
Blood samples (10 ml) were drawn using a needle and
syringe from the peripheral vein of all patients, after which PBMs
were isolated from the heparinized (Well-Biology) blood samples by
Ficoll-Hypaque density gradient centrifugation at 150 × g and 4°C
for 20 min. Cells were then incubated at 4°C for 45 min in the dark
with monoclonal PE-conjugated mouse anti-CD14 (1:10; sc-52457;
Santa Cruz Biotechnology, Inc.) monoclonal FITC-conjugated mouse
anti-HLA-DR (1:10; sc-33718; Santa Cruz Biotechnology, Inc.) and
monoclonal PerCP-conjugated mouse anti-CD80 antibodies (1:10,
sc-73382; Santa Cruz Biotechnology, Inc.). Samples were assayed
using a FACSCalibur system (Beckman Coulter, Inc., Brea, CA, USA)
and analysis was performed using CellQuest software, version 3.0
(BD Biosciences, Franklin Lakes, NJ, USA).
Phagocytic activity assay
According to the method described by Gille et
al (15), monocytes were
isolated from PBMs using CD14 MicroBeads™ (Miltenyi Biotec GmbH,
Bergisch Gladbach, Germany) and were passed through a MACS column
(Miltenyi Biotec GmbH) to positively select for CD14+
cells by immunomagnetic selection, according to the manufacturer's
instructions. This procedure yielded a minimum of a 90% pure
population of monocytes, as assessed by fluorescence-activated cell
sorter analysis. The adherent monocytes were treated for 60 min in
the dark with FITC-conjugated E. coli (a gift from Dr Jie
Yin, Chinese Academy of Sciences), and were then washed with PBS
and centrifuged at 3,000 × g and 4°C for 5 min to remove free
bacteria. Samples were assayed using the FACSCalibur system.
Phagocytic activity was expressed as a percentage of the
FITC-conjugated cells.
Statistics
Data are presented as the mean ± standard error.
Significant differences between the means were evaluated by
Student's t-test or analysis of variance. Spearman's rank
correlation coefficient was used to examine the correlation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Increased expression of VAV1 and
PAK1
The expression levels of VAV1 and PAK1 in the liver
of patients with AIH and NAFLD controls were measured using western
blotting. As presented in Table I,
the expression levels of VAV1 and PAK1 were significantly increased
in patients with AIH, as compared with the NAFLD group (P<0.05).
In addition, a correlation between increased expression of VAV1 and
PAK1 and an advanced disease stage was observed (data not
shown).
 | Table IWestern blotting of VAV1 and PAK1
expression in liver tissues of AIH and controls. |
Table I
Western blotting of VAV1 and PAK1
expression in liver tissues of AIH and controls.
| Group | N | VAV1/GAPDH | PAK1/GAPDH |
|---|
| NAFLD | 7 | 1.00±0.07 | 1.00±0.08 |
| AIH normal liver
function | 7 | 1.79±0.78a | 1.64±0.52a |
| AIH abnormal liver
function | 7 | 2.37±0.81a | 2.41±0.53a |
| AIH cirrhosis | 7 | 4.64±1.37a | 4.71±1.26a |
The expression levels of VAV1 and PAK1 in KCs of AIH
patients in comparison with NAFLD controls were additionally
analyzed by immunofluorescence double staining (Figs. 1 and 2). Expression levels of VAV1 and PAK1 in
KCs were markedly increased in patients with AIH, as compared with
the NAFLD group. In addition, a correlation between increased
levels of VAV1 and PAK1 expression and an advanced disease stage
was observed (data not shown).
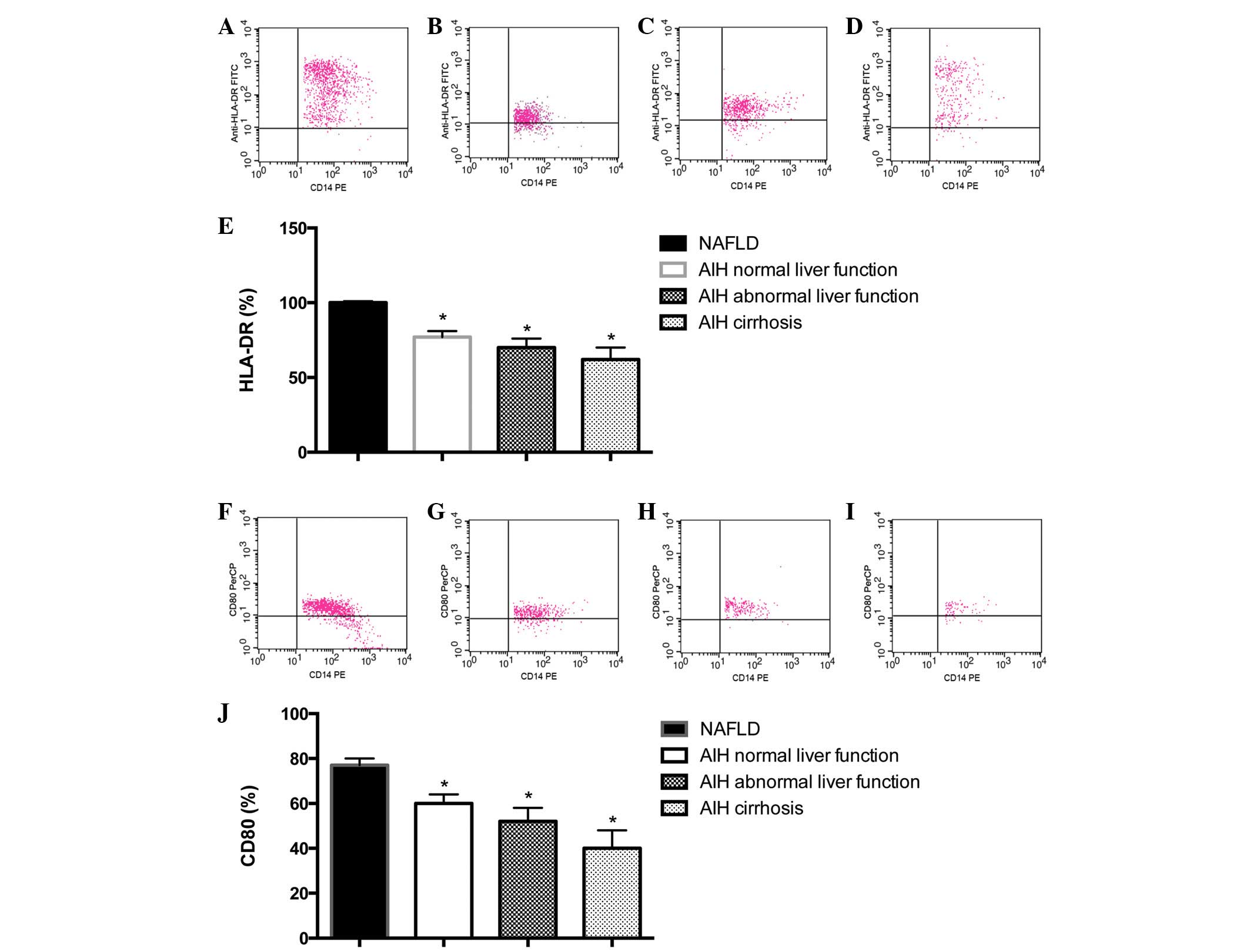
Reduced expression of HLA-DR and
CD80
As presented in Fig.
3A–E, the expression level of HLA-DR in CD14+ cells
was 99.06±0.61% in the NAFLD group, 82.37±4.62% in the AIH normal
liver function group, 73.54±8.53% in the AIH abnormal liver
function group and 60.63±11.47% in the AIH cirrhosis group. Cell
surface expression of HLA-DR was significantly reduced on PBMs from
patients with AIH, as compared with those from NAFLD control
subjects (P<0.05). In addition, a correlation between reduced
expression of HLA-DR and an advanced disease stage was observed
(data not shown).
As presented in Fig.
3F–J, the expression level of CD80 on CD14+ cells
was 81.46±5.08% in the NAFLD group, 69.15±11.37% in the AIH normal
liver function group, 54.72±9.72% in the AIH abnormal liver
function group and 43.24±15.52% in the AIH cirrhosis group. Cell
surface expression of CD80 was significantly reduced on PBMs from
patients with AIH, as compared with the NAFLD control subjects
(P<0.05; Fig. 3J). In addition,
a correlation between reduced expression of CD80 and an advanced
disease stage was observed (Fig.
3J).
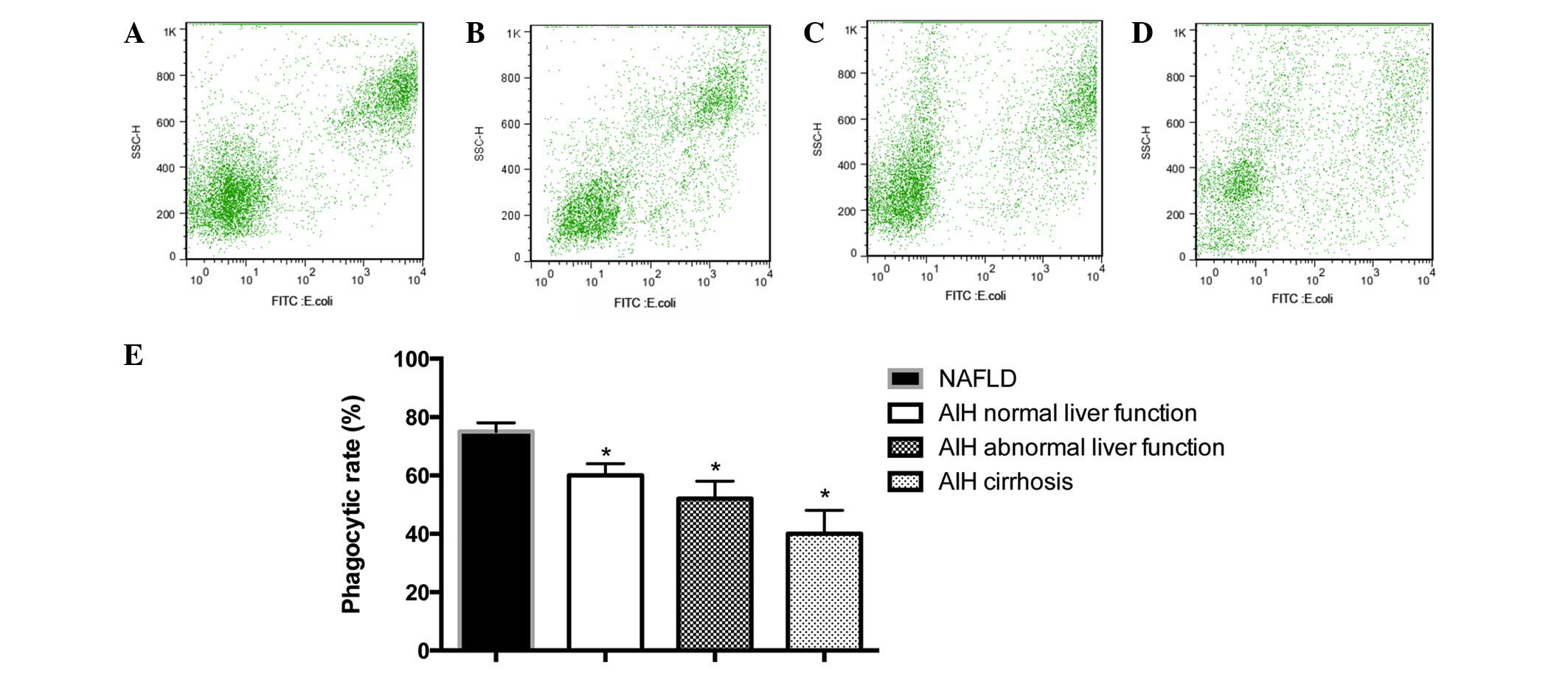
Reduced phagocytic activity of PBMs
As presented in Fig.
4, the percentage of FITC-conjugated PBMs was 75.73±6.32% in
the NAFLD group, 63.28±8.24% in the AIH normal liver function
group, 52.69±10.36% in the AIH abnormal liver function group and
36.21±14.29% in the AIH cirrhosis group. Phagocytic activity was
observed to be significantly reduced in monocytes from patients
with AIH, as compared with the NAFLD control subjects (P<0.05;
Fig. 4). In addition, a
correlation between reduction in phagocytic activity and an
advanced disease stage was observed (Fig. 4).
Discussion
AIH is an inflammatory liver disease that is
characterized by the presence of auto-antibodies,
hypergammaglobulinemia, a histological evidence of interface
hepatitis and an optimal response to steroids in the majority of
patients (16). A previous study
on the pathogenesis of AIH focused on the adaptive immune system,
since lymphocytic abnormalities have previously been hypothesized
to be the primary cause of autoimmunity (1). In the past decade, however, this
focus has shifted with advances in the field of innate immunity.
Monocytes/macrophages are a key component of the innate immune
system with numerous immunological functions, including
phagocytosis, antigen presentation and cytokine production.
Phagocytosis is the process of the clearance of dead or dying
cells, cellular debris, microbes and other foreign materials. The
phagocytic capacity of monocytes/macrophages is essential for the
host's defense against pathogens and homeostatic clearance of dead
or dying cells. Monocytes/macrophages serve a pivotal role in the
initiation of immunologically-mediated liver injury (2,17).
Phagocytic activity of peritoneal macrophages has been observed to
be significantly impaired in mice with concanavalin A-induced
hepatitis (18). Aberrant
activation of macrophages may trigger inflammation that contributes
to the initiation and progression of liver diseases. However, the
function of monocytes/macrophages in patients with AIH remains to
be fully elucidated.
KCs represent approximately 10% of the resting total
liver cell population, however constitute the largest component
(80~90%) of all tissue macrophages in the body. Central to innate
immunity, KCs are responsible for clearance of exogenous
particulate and immunoreactive material, and antigen presentation,
which aid in the maintenance of immune homeostasis (19). In the current study, KCs from
patients with AIH were investigated. Obtaining liver biopsies from
healthy individuals is unlikely to be approved by an institutional
review board, thus in the current study, control KCs were obtained
from patients with NAFLD. Due to the fact that the number of KCs
present in a liver biopsy is too few to allow for conducting
functional assays, and that monocytes serve as direct precursors to
tissue macrophages, PBMCs were isolated to evaluate phagocytosis
and antigen presenting functions. The phagocytic activity of PBMCs
in patients with AIH was investigated with the FITC-conjugated
E. coli phagocytosis assay. It was identified that
phagocytosis was significantly impaired in PBMCs from patients with
AIH compared with the NAFLD controls. This clearance defect may
result in the accumulation of apoptotic cells, which can serve as
antigens. Booth et al (8)
demonstrated that the antigen-presentation capablities are closely
associated with HLA-DR and CD80 expression levels. HLA-DR can
provide an initial signal leading to the development of an
effective immune response via formation of the specific
peptide-major histocompatibility complex (MHC) antigen complex.
CD80 is a co-stimulatory molecule present on activated monocytes
and B cells and provides a co-stimulatory signal necessary for the
activation and survival of T lymphocytes. In the current study, the
expression levels of HLA-DR and CD80 on PBMCs were reduced in
patients with AIH compared with the NAFLD individuals, suggesting
an ineffective antigen-presenting function may contribute to an
impaired antigen-specific immune response in these patients. These
results support the theory that monocyte function may be defective,
and thus may therefore be indicative of an impaired immune response
status in patients with AIH.
Previous studies have demonstrated that Rho GTPases
are the essential regulators of cell behavior, linking
extracellular stimuli to intracellular signal transduction events
(20,21). The activity is controlled by
guanine nucleotide exchange factors (GEF) that regulate the
exchange from guanosine diphosphate (inactive Rho GTPase) to
guanosine triphosphate (active Rho GTPase). Rho GTPases, including
Cdc42, Rac, and Rho, then interact with downstream effectors to
regulate cytoplasmic signaling pathways that control the vital
cellular processes, including cytoskeletal dynamics, cell cycle
progression, gene transcription and cell transformation (22). Antigen presentation is associated
with phagocytosis, which occurs via remodeling of the actin
cytoskeleton and shares numerous core cytoskeletal components
involved in adhesion and migration (23). VAV1 is a GEF for Rho GTPases and is
able to regulate the activation of Rac, Rho and Cdc42 (24). It has been reported that VAV1 may
serve a crucial role in actin rearrangement at the phagocytic cup
and MHC II expression in dendritic cells and macrophages (25,26).
PAK1, a serine/threonine kinase, has been identified as a major
downstream effector of the Rho GTPases Rac1 and Cdc42 (27). PAK1 serves an essential role in
regulating cellular processes such as cytoskeletal remodeling, cell
motility, cell proliferation and cell survival (28). The expression levels of VAV1 and
PAK1 on KCs were observed to be significantly increased in patients
with AIH. The PBMC functions of phagocytosis and
antigen-presentation were reduced, and correlated with the disease
progression. This may have been due to aberrant activation of Rho
GTPase signaling associated with the breakdown of actin patches and
the delayed closure of the phagocytic cup (29), which induces dysfunction of
monocytes/macrophages.
Taken together, these data suggest a potential
mechanism for Rho GTPases in the regulation of the function of
monocytes/macrophages. Liver damage may result from the inability
of KCs to eliminate immunoreactive materials, which are then able
to initiate the immune process. These observations aid in the
elucidation of the pathogenesis of AIH, and may provide a novel
therapeutic target. Due to the small number of patients enrolled in
the current study and the limitations of the biopsy tissues,
further investigation into the precise signaling pathway of Rho
GTPases, and how these are involved in the regulation of KC
function in an AIH model are required.
Abbreviations:
|
AIH
|
autoimmune hepatitis
|
|
KCs
|
Kupffer cells
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
PAK1
|
p21-activated kinase 1
|
|
PBMs
|
peripheral blood monocytes
|
References
|
1
|
Ma Y, Bogdanos DP, Hussain MJ, Underhill
J, Bansal S, Longhi MS, Cheeseman P, Mieli-Vergani G and Vergani D:
Polyclonal T-cell responses to cytochrome P450IID6 are associated
with disease activity in autoimmune hepatitis type 2.
Gastroenterology. 130:868–882. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seki E and Schnabl B: Role of innate
immunity and the microbiota in liver fibrosis: Crosstalk between
the liver and gut. J Physiol. 590:447–458. 2012. View Article : Google Scholar :
|
|
3
|
Lleo A, Bowlus CL, Yang GX, Invernizzi P,
Podda M, Van de Water J, Ansari AA, Coppel RL, Worman HJ, Gores GJ
and Gershwin ME: Biliary apotopes and anti-mitochondrial antibodies
activate innate immune responses in primary biliary cirrhosis.
Hepatology. 52:987–998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bouwens L, Baekeland M, De Zanger R and
Wisse E: Quantitation, tissue distribution and proliferation
kinetics of Kupffer cells in normal rat liver. Hepatology.
6:718–722. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baffy G: Kupffer cells in non-alcoholic
fatty liver disease: The emerging view. J Hepatol. 51:212–223.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burdo TH, Lackner A and Williams KC:
Monocyte/macrophages and their role in HIV neuropathogenesis.
Immunol Rev. 254:102–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pul R, Morbiducci F, Škuljec J, Skripuletz
T, Singh V, Diederichs U, Garde N, Voss EV, Trebst C and Stangel M:
Glatiramer acetate increases phagocytic activity of human monocytes
in vitro and in multiple sclerosis patients. PLoS One.
7:e518672012. View Article : Google Scholar
|
|
8
|
Booth S, Florida-James GD, McFarlin BK,
Spielmann G, O'Connor DP and Simpson RJ: The impact of acute
strenuous exercise on TLR2, TLR4 and HLA.DR expression on human
blood monocytes induced by autologous serum. Eur J Appl Physiol.
110:1259–1268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang D, Yuan F, Wang L and Wei W:
Paeoniflorin inhibits function and down-regulates HLA-DR and CD80
expression of human peripheral blood monocytes stimulated by
rhIL-1β. Int Immunopharmacol. 14:172–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jennings RT and Knaus UG: Rho family and
Rap GTPase activation assays. Methods Mol Biol. 1124:79–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katzav S: Vav1:A hematopoietic signal
transduction molecule involved in human malignancies. Int J Biochem
Cell Biol. 41:1245–1248. 2009. View Article : Google Scholar
|
|
12
|
Jhaveri KA, Debnath P, Chernoff J, Sanders
J and Schwartz MA: The role of p21-activated kinase in the
initiation of atherosclerosis. BMC Cardiovasc Disord.
12(55)2012.PubMed/NCBI
|
|
13
|
Hennes EM, Zeniya M, Czaja AJ, Parés A,
Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer
H, et al: Simplified criteria for the diagnosis of autoimmune
hepatitis. Hepatology. 48:169–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ; American
Gastroenterological Association; American Association for the Study
of Liver Diseases; American College of Gastroenterologyh: The
diagnosis and management of non-alcoholic fatty liver disease:
Practice guideline by the American gastroenterological association,
American association for the study of liver diseases and American
college of gastroenterology. Gastroenterology. 142:1592–1609. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gille C, Leiber A, Mundle I, Spring B,
Abele H, Spellerberg B, Hartmann H, Poets ChF and Orlikowsky TW:
Phagocytosis and postphagocytic reaction of cord blood and adult
blood monocyte after infection with green fluorescent
protein-labeled Escherichia coli and group B Streptococci.
Cytometry B Clin Cytom. 76:271–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liberal R, Longhi MS, Mieli-Vergani G and
Vergani D: Pathogenesis of autoimmune hepatitis. Best Pract Res
Clin Gastroenterol. 25:653–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heymann F, Hammerich L, Storch D, Bartneck
M, Huss S, Rüsseler V, Gassler N, Lira SA, Luedde T, Trautwein C
and Tacke F: Hepatic macrophage migration and differentiation
critical for liver fibrosis is mediated by the chemokine receptor
C-C motif chemokine receptor 8 in mice. Hepatology. 55:898–909.
2012. View Article : Google Scholar
|
|
18
|
Yu Z, Otsuka H, Yamaguchi K, Kuroishi T,
Sasano T, Sugawara S, Nakamura M and Endo Y: Roles of platelets and
macrophages in the protective effects of lipopolysaccharide against
concanavalin A-induced murine hepatitis. Biochim Biophys Acta.
1812.1069–1079. 2011.
|
|
19
|
Naito M, Hasegawa G and Takahashi K:
Development, differentiation and maturation of Kupffer cells.
Microsc Res Tech. 39:350–364. 1997. View Article : Google Scholar
|
|
20
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swetman CA, Leverrier Y, Garg R, Gan CH,
Ridley AJ, Katz DR and Chain BM: Extension, retraction and
contraction in the formation of a dendritic cell dendrite: Distinct
roles for Rho GTPases. Eur J Immunol. 32:2074–2083. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ridley AJ: Rho proteins, PI 3-kinases and
monocyte/macrophage motility. FEBS Lett. 498:168–171. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tse SM, Furuya W, Gold E, Schreiber AD,
Sandvig K, Inman RD and Grinstein S: Differential role of actin,
clathrin and dynamin in Fc gamma receptor-mediated endocytosis and
phagocytosis. J Biol Chem. 278:3331–3338. 2003. View Article : Google Scholar
|
|
24
|
Chrencik JE, Brooun A, Zhang H, Mathews
II, Hura GL, Foster SA, Perry JJ, Streiff M, Ramage P, Widmer H, et
al: Structural basis of guanine nucleotide exchange mediated by the
T-cell essential Vav1. J Mol Biol. 380:828–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wells CM, Bhavsar PJ, Evans IR, Vigorito
E, Turner M, Tybulewicz V and Ridley AJ: Vav1 and Vav2 play
different roles in macrophage migration and cytoskeletal
organization. Exp Cell Res. 310:303–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de la Fuente H, Mittelbrunn M,
Sánchez-Martín L, Vicente-Manzanares M, Lamana A, Pardi R, Cabañas
C and Sánchez-Madrid F: Synaptic clusters of MHC class II molecules
induced on DCs by adhesion molecule-mediated initial T-cell
scanning. Mol Biol Cell. 16:3314–3322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arias-Romero LE and Chernoff J: A tale of
two Paks. Biol Cell. 100:97–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Delorme V, Machacek M, DerMardirossian C,
Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G and
Bokoch GM: Cofilin activity downstream of Pak1 regulates cell
protrusion efficiency by organizing lamellipodium and lamella actin
networks. Dev Cell. 13:646–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsu HY and Twu YC: Tumor necrosis
factor-alpha-mediated protein kinases in regulation of scavenger
receptor and foam cell. J Biol Chem. 275:41035–41048. 2000.
View Article : Google Scholar : PubMed/NCBI
|