Introduction
Doxorubicin (DOX), an anthracycline antibiotic, is a
potent anticancer drug commonly used in the treatment of numerous
types of hemotological malignancy and solid tumors (1). However, the clinical application of
DOX in cancer chemotherapy is largely limited by its serious side
effects on non-target organs (2,3).
Despite the well-known side effects of DOX treatment associated
with the heart, increasing evidence demonstrates that DOX also
affects other organs, including the liver, kidney and brain
(4,5). Studies have also suggested that ~40%
of patients suffered from liver injury following treatment with
doxorubicin (6). However, little
is known regarding the effects and mechanisms of DOX toxicity on
the liver and kidney system.
The exact mechanisms involved in DOX-induced
cellular damage are complex and remain to be fully elucidated. A
previous study demonstrated that oxidative stress was critical in
the development of DOX-induced systemic injury (7). In the process of oxidative stress
injury, excessive reactive oxygen species (ROS) are produced by DOX
treatment, which is associated with cell death and is responsible
for organ injury. It was reported that DOX predominantly caused
liver injury via the generation of free radicals and the activation
of nuclear factor-κB (4).
Therefore, effective strategies against DOX-induced oxidative
stress injury would be expected to preserve or enhance the
therapeutic effects of DOX in anticancer therapy. Thus far, to
reduce the toxic effects of DOX, several pharmacologic agents, such
as antioxidants, hematopoietic cytokines and iron-chelating agents
have been investigated (8,9). Although the majority of these agents
have shown beneficial effects in terms of inducing oxidative
stress, pharmacological and clinical attempts to reduce the
hepatorenal toxicity of DOX have had little success thus far.
Therefore, identification of novel effective strategies against
DOX-induced complications is required.
Berberine (Ber), an isoquinoline alkaloid,
originally extracted from the traditional Chinese herb Coptis
chinensis, is used for the treatment of bacterial infectious
diseases, and has a long history for treating diarrhea in
traditional Chinese medicine (10). Recently, an increasing number of
studies have revealed that Ber displayed a wide range of
pharmacological activities, such as reducing the risk of cancer,
cardiovascular diseases and brain diseases due to its radical
scavenging and antioxidant activities (11,12).
In addition, animal and clinical studies have suggested that Ber is
beneficial in preventing reactive oxygen species (ROS) formation
(13–15). In addition, it has been reported
that Ber improved cardiac function in patients with severe
congestive heart failure (16).
Zhao et al (17) also
reported that Ber may have a potential protective effect against
DOX-induced cardiotoxicity. However, to the best of our knowledge,
no study has been conducted concerning the protective effects of
Ber on DOX-induced hepatorenal toxicity. Therefore, the present
experiment was designed to investigate the possible protective
effects of Ber on acute hepatorenal toxicity induced by DOX in rat
model, and to further elucidated the mechanisms underlying these
effects.
Materials and methods
Drugs and reagents
DOX was obtained from Sigma-Aldrich (St. Louis, MO,
USA). Ber was obtained from Acros Organics (Geel, Belgium).
Superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT)
and glutathione peroxidase (GPx) assay kits were all obtained from
Jiancheng Bioengineering Institute (Nanjing, China). Alanine
aminotransferase (ALT), aspartate aminotransferase (AST), blood
urea nitrogen (BUN) and total cholesterol (TCHO) diagnostic kits
were all obtained from Sysmex Corporation (Kobe, Japan). The
paraffin, hematoxylin and eoisin were obtained from Beijing
Solarbio Science & Technology Co., Ltd. (Beijing, China).
Animals and experimental protocol
Male Sprague Dawley rats (experimental animal
quality certificate no. 808046), weighing 250–300 g, were obtained
from the experimental animal center of Hebei Medical University
[license no. SCXK (Hebei) 2013-003]. The animals were housed under
standard laboratory conditions with a 12 h light dark cycle at a
24±3°C, and food and water provided ad libitum. Fifty rats
were randomly divided into five groups: i) Control group; ii) DOX
(20 mg kg) group; iii) DOX + Ber (5 mg kg) group; iv) DOX + Ber (10
mg kg); and v) DOX + Ber (20 mg kg) group. Ber was administered
intragastrically once a day for 10 consecutive days in the DOX +
Ber groups. Rats in groups the control and DOX groups received an
equal volume of saline. DOX was injected intraperitoneally as a
single dose (20 mg kg in saline) on the 8th day of Ber
administration in the DOX and DOX + BER groups while an equal
volume of saline in the control group. Mortality, general condition
and body weight of the animals were observed during throughout the
experiment. Then, on 10th day, 30 min after the final dose of Ber
was administered, all rats were sacrificed with 80 mg kg
pentobarbital sodium (Merck Millipore, Dramstadt, Germany) via an
intraperitoneal injection. The abdomen of each rat was opened, and
blood samples were collected from the abdominal aorta. Next, the
organs of liver and kidney were rapidly removed and washed with
ice-cold saline, part of which was kept in liquid nitrogen and part
of which was fixed in 4% paraformaldehyde. Subsequently, all
analyses were performed according to the experimental protocol. The
experiments were conducted in accordance with the Guide for Care
and Use of Laboratory Animals published by the US National
Institutes of Health, and were approved by the ethical committee
for Animal Experiments.
Assessment of survival and general
toxicity
The general condition, body weight and mortality of
the experimental rats were recorded daily during throughout the
experimental period. At the end of the experiment, all rats were
anesthetized with 45 mg kg pentobarbital sodium via an
intraperitoneal injection, and the abdomen of each rat was opened,
then the fluid accumulation in the abdominal cavity was collected
with a syringe and scored according to a graded scale of 0 to +++
(0: non; +: mild; ++: moderate and +++: severe) (18). Next, the organs of liver and kidney
were removed and weighed, and the organ indexes were calculated
according to the following formula: Organ index = organ weight body
weight.
Determination of serum biochemical
indicators
Blood samples collected from the abdominal aorta
were stored on ice prior to centrifugation at 2,100 × g for 10 min
within 1 h of collection. Then, the supernatant was collected for
the determination of biochemical indicators. The levels of ALT,
AST, TCHO and BUN in the serum were determined as sensitive
indicators of liver damage, according to the manufacturer's
protocol of diagnostic kits with a CHEMIX-180 automatic
biochemistry analyzer (Sysmex Corporation).
Histopathological analysis
At the end of the study period, the liver and kidney
were excised from all study animals, and sections were fixed in 4%
formaldehyde directly after excision. The tissues were then
dehydrated in an ascending series of ethanol (70, 80, 96 and 100%).
Following paraffin embedding, transverse sections with a thickness
of 5 µm were stained with hematoxylin-eosin, and
histological examination was conducted using a light microscope
(Olympus BX-50 Olympus Corporation, Tokyo, Japan).
Determination of lipid peroxides
The preparation of serum was conducted as described.
The liver or kidney tissues were homogenized in ice-cold saline to
form a 10% homogenate. The homogenate was then centrifuged at 2,100
× g for 15 min at 4°C, then the supernatants were collected and the
total protein content was detected with a BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Next,
the levels of SOD, CAT, GPx and MDA in the serum and tissue
homogenate were determined using a commercially available assay
kits according to the manufacturer's protocol.
Statistical analysis
All values are presented as the mean ± standard
deviation. Statistical analyses were performed using one-way
analysis of variance, a χ2 was used for the count data
and Dunnett's test (SPSS for Windows v11.0, SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of Ber on survival and general
toxicity in DOX-induced acute injury in rats
The general condition, body weight and mortality of
the experimental rats were recorded daily throughout the
experimental period. Two rats died in the DOX-treated group.
However, no fatalities were observed in any of the other groups.
Moreover, reduced appetite, decreased activity and progressive
physical exhaustion were observed in the rats from the DOX-treated
group (data not shown). These animals also presented with scruffy
and a light yellow-tinged fur compared to the control and
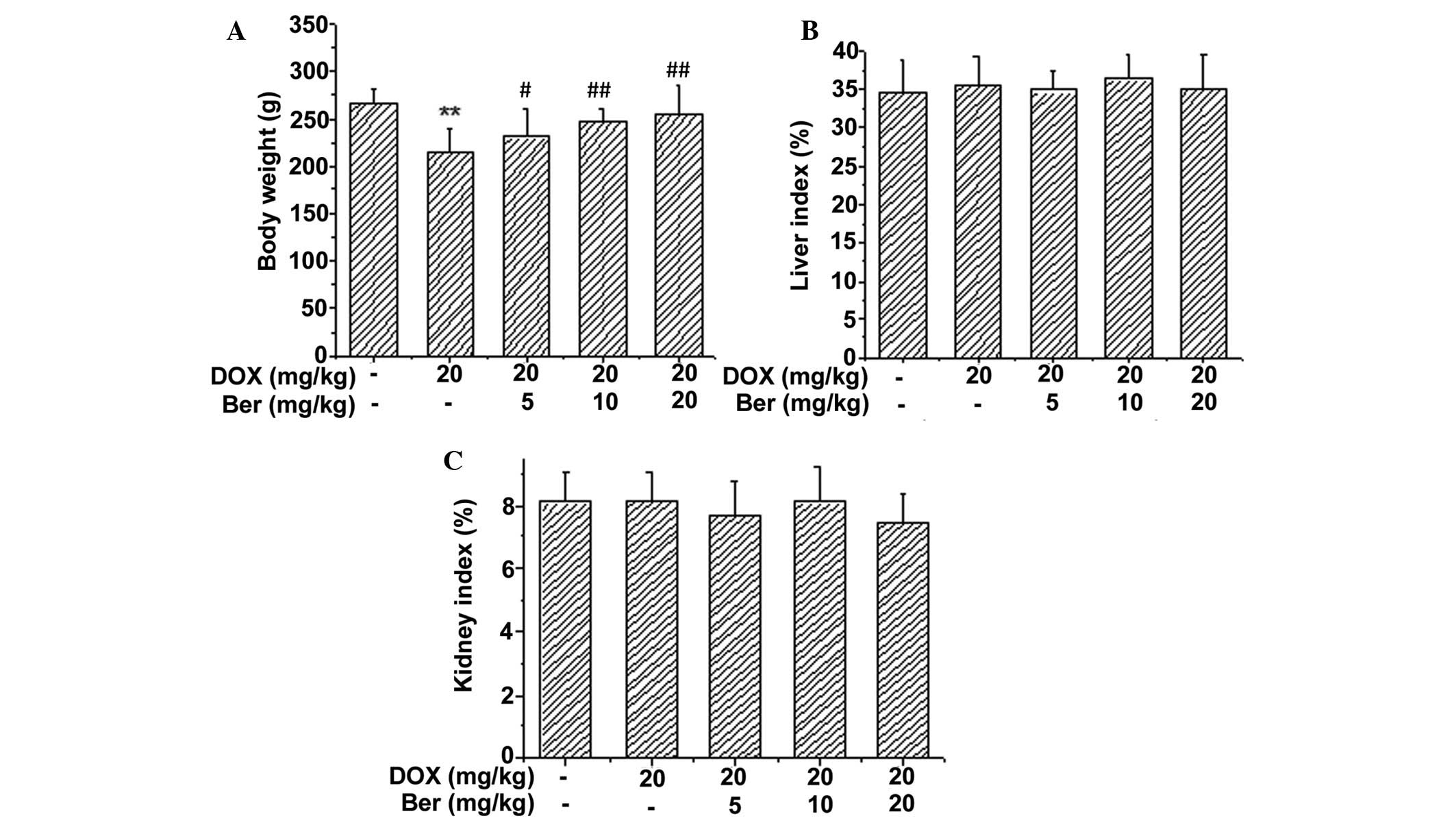
Ber+DOX-treated group. The body weight and organ indexes 30 min
after the last Ber dose was administered (day 10) are shown in
Fig. 1. It was demonstrated that
DOX treatment resulted in reduced body weights compared with the
control group (P<0.01). The body weight was significantly
increased in the Ber-treated groups (5, 10 and 20 mg kg) compared
with the DOX group (P<0.05, P<0.01 and P<0.01,
respectively) (Fig. 1A). However,
Fig. 1B and C suggested that there
was no significant difference in the liver and kidney indexes among
the five groups.
Notably, the rats in the DOX-alone group were also
observed to develop ascites, as determined by a grossly distended
abdomen and later confirmed during necropsy. At necropsy, the most
prominent gross pathological change in the rats treated with DOX
was the presence of excessive pericardial, pleural and peritoneal
fluid. The effusion intensity score was severe in 100% of the rats
in the DOX-treated group compared with 0% of the control group
(P<0.01). However, treatment with Ber significantly decreased
the amount of pericardial, pleural and peritoneal fluids. Compared
with the DOX-treated group, the effusion intensity score was
improved in the three Ber-treated groups (5, 10, 20 mg kg)
(P<0.01; Table I).
 | Table IInfluence of Ber on DOX-induced
abdominal, pleural and pericardial effusion intensity score in
surviving rats. |
Table I
Influence of Ber on DOX-induced
abdominal, pleural and pericardial effusion intensity score in
surviving rats.
| Group | n | Effusion intensity
score
|
|---|
0
| +
| ++
| +++
|
|---|
| N | % | N | % | N | % | N | % |
|---|
| Con | 10 | 10 | 100 | 0 | 0 | 0 |
0 | 0 |
0 |
| DOX | 8 | 0 |
0 | 0 | 0 | 0 |
0 | 8 | 100a |
| Ber 5 mg/kg | 10 | 0 |
0 | 4 | 40 | 3 | 30 | 3 |
30a,b |
| Ber 10 mg/kg | 10 | 1 | 10 | 4 | 40 | 2 | 20 | 3 |
30a,b |
| Ber 20 mg/kg | 10 | 1 | 10 | 5 | 50 | 2 | 33.3 | 2 |
20a,b |
Effects of Ber on the serum levels of
ALT, AST, TCHO and BUN
The serum levels of ALT, AST, TCHO and BUN have been
widely used clinically as parameters for the diagnosis of
hepatorenal diseases. As shown in Fig.
2, DOX alone induced significant increases in serum ALT, AST,
TCHO and BUN levels compared with the control group (P<0.01),
and these increases were effectively attenuated by treatment with
5, 10 and 20 mg kg Ber (P<0.05 or P<0.01). However, although
these levels declined following treatment with Ber, they did not
return to the baseline levels. These results suggest that Ber
effectively protects the liver and kidney against DOX-induced
hepatorenal toxicity.
 | Figure 2Effects of Ber on the activity of ALT,
AST, TCHO and the content of BUN in the serum of rats with
DOX-induced acute injury. Values are presented as the mean ±
standard deviation. *P<0.05, **P<0.01
vs. the control group, and #P<0.05,
##P<0.01 vs. the DOX-treated group. Ber, berberine;
ALT, alanine transaminase; AST, aspartate aminotransferase; TCHO,
total cholesterol; DOX, doxorubicin. |
Histopathological examination
Hepatorenal toxicity induced by DOX in rats was
further assessed using hematoxylin and eosin stained sections.
Representative examples of the histological appearance in the
control, DOX-treated and Ber+DOX-treated groups are shown in
Figs. 3 and 4. Fig. 3
shows the histopathological changes of the rat liver. The liver
sections from control group showed regular cell distribution and
normal integrated structures of liver lobules (Fig. 3A). Following administration of DOX
the liver exhibited notable histopathological change. DOX induced
tissue injuries, including degeneration of the hepatocytes, focal
necrosis and hemorrhage. Moreover, visible congestion and expansion
also appeared in the central veins. (Fig. 3B). By contrast, the severe hepatic
injury induced by DOX was alleviated by Ber treatment (Fig. 3C and D).
Representative examples of the histological
appearance of rat kidney are shown in Fig. 4. The microscopic examination for
the kidney in the control group revealed normal histology
parameters (Fig. 4A). Whereas,
kidney tissues from the DOX-treated group showed widespread
structural abnormalities, with congestion in glomerular tissues and
irregular hemorrhages in the renal interstitial (Fig. 4B). By contrast, the severe
histopathological changes in the kidney induced by DOX was limited
by Ber treatment (Fig. 4C and
D).
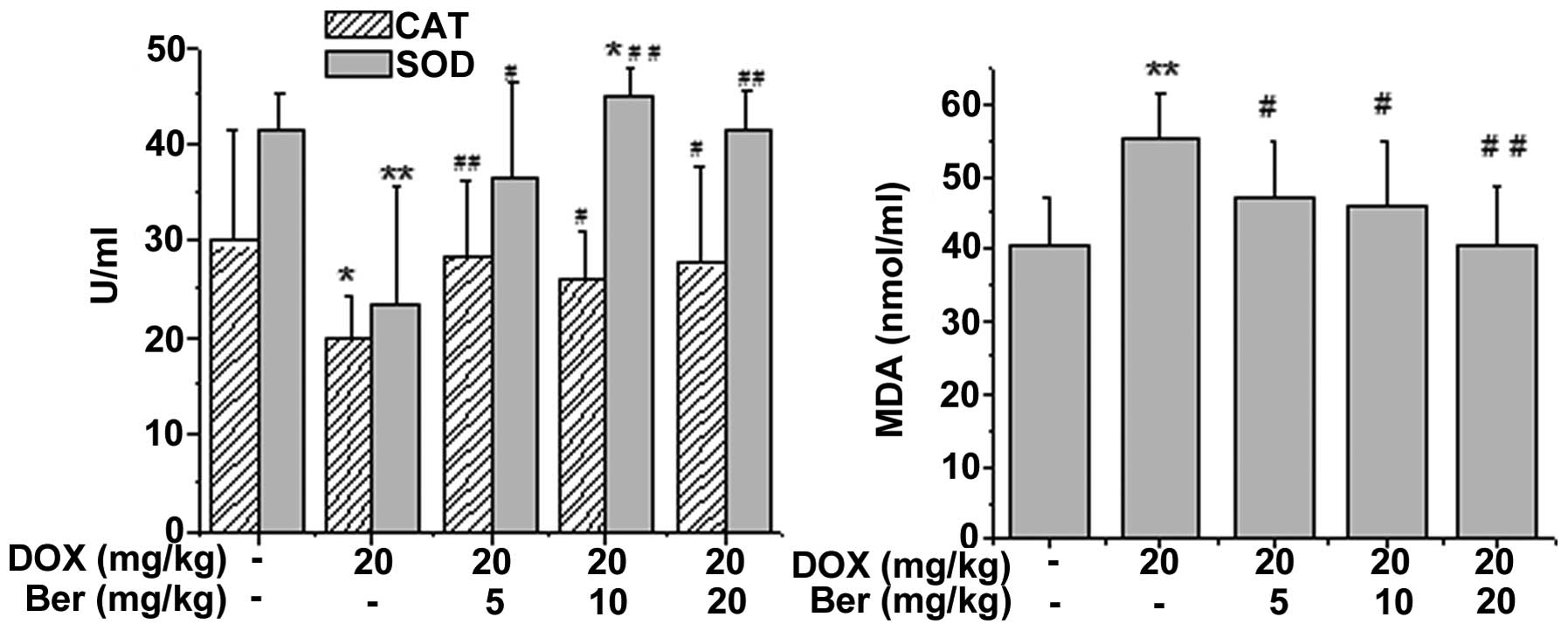
Ber alleviates DOX-induced oxidative
damage
To determine whether Ber alleviated DOX-induced
oxidative damage in rats, the SOD, CAT and GPx activity, as well as
the content of MDA in rat serum or tissue homogenate, were
assessed. As shown in Fig. 5, DOX
treatment led to a significant increase in the content of MDA and a
manifest depletion in the activity of CAT and SOD in the serum
compared with that of the control group (P<0.05 or P<0.01).
Whereas, Ber (5, 10 and 20 mg kg) treatment notably reversed the
DOX-induced changes in the MDA, CAT and SOD levels in the rat serum
(P<0.05 or P<0.01).
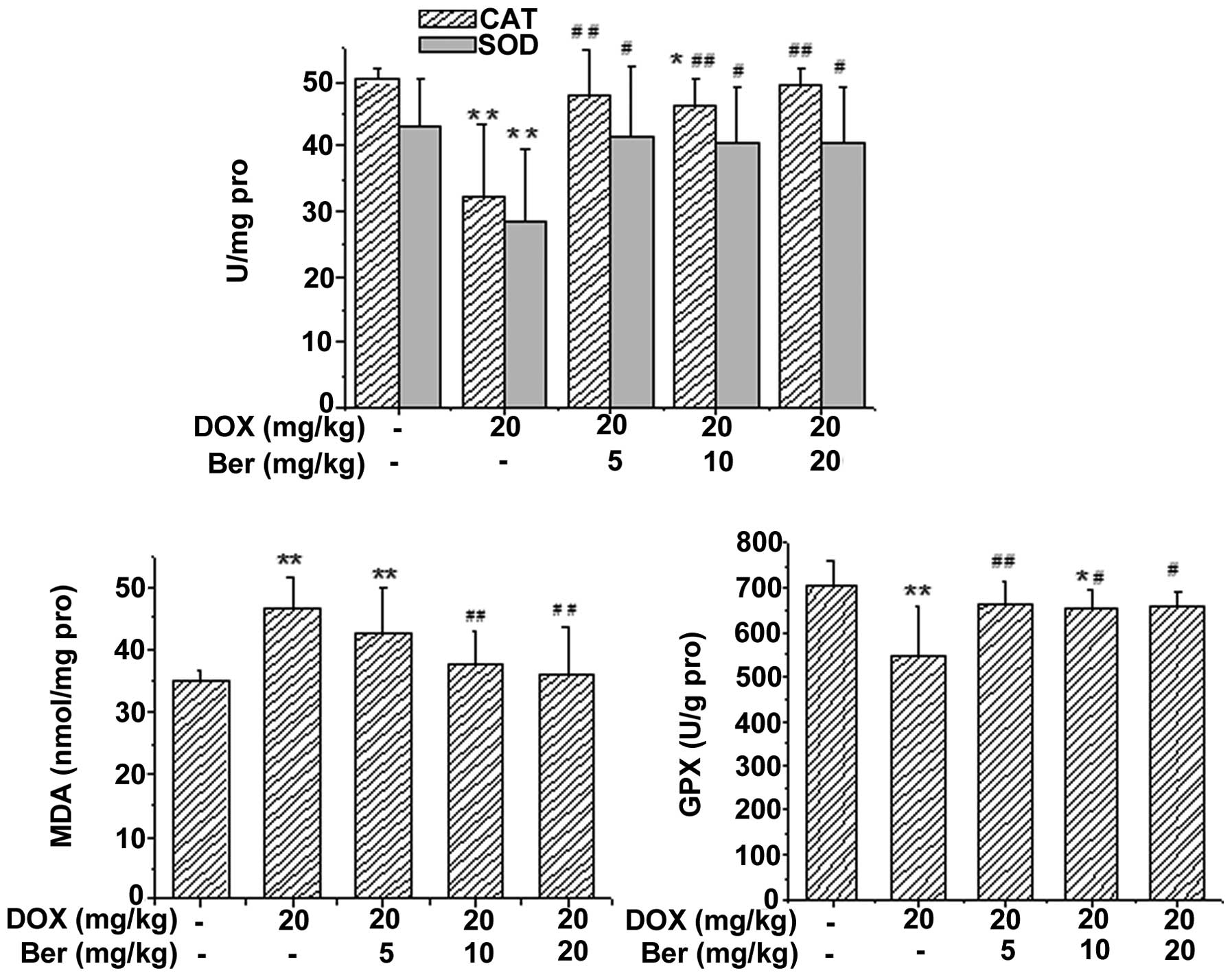
In addition, the activity of SOD, CAT and GPx, and
the content of MDA in rat tissue homogenate were also assessed. As
shown in Figs. 6 and 7, DOX treatment led to a significant
increase in the content of MDA and a manifest depletion in the
activity of GPx, CAT and SOD in the liver and kidney tissue
homogenate compared with that of the control group (P<0.05 or
P<0.01). Whereas, Ber (10 and 20 mg kg) treatment reversed the
DOX-induced changes in the MDA, SOD and GPx levels in rat liver
tissue homogenate (P<0.05 or P<0.01), and Ber (5 and 10 mg
kg) also reversed the DOX-induced reduction in the activity of CAT
(P<0.05 or P<0.01, Fig. 6).
Additionally, in rat kidney tissue homogenate, Ber (5, 10, 20 mg
kg) significantly increased the activity of CAT, SOD and GPx
compared with that of the DOX-treated group (P<0.05 or
P<0.01, Fig. 7). The elevated
content of MDA induced by DOX was also decreased by Ber (10 and 20
mg kg) treatment (P<0.01, Fig.
7).
 | Figure 6Effects of Ber on the activity of CAT,
SOD, GPx and the content of MDA in the hepatic tissue of
DOX-treated rats. Values are expressed as the mean ± standard
deviation. *P<0.05, **P<0.01 vs. the
control group, #P<0.05, ##P<0.01 vs.
the DOX-treated group. CAT, catalase; SOD, superoxide dismutase;
MDA, malondialdehyde; GPx, glutathione peroxidase; DOX,
doxorubicin; Ber, berberine. |
 | Figure 7Effects of Ber on the activity of CAT,
SOD and GPx, and the content of MDA in the renal tissue of
DOX-treated rats. Values are expressed as the mean ± standard
deviation. *P<0.05, **P<0.01 vs. the
control group, #P<0.05, ##P<0.01 vs.
the DOX-treated group. CAT, catalase; SOD, superoxide dismutase;
MDA, malondialdehyde; GPx, glutathione peroxidase; DOX,
doxorubicin; Ber, berberine. |
Discussion
With the wide application of chemotherapeutic drugs
in tumor therapy, adverse reactions that limit the dosage and
duration of treatment, and affect the quality of life have gained
attention. Therefore, it is important to develop effective parts of
Chinese herbal medicine against the adverse reactions of
chemotherapeutic drugs. The present study demonstrated that Ber, an
isoquinoline alkaloid, originally extracted from the traditional
Chinese herb Coptis chinensis, suppressed the hepatorenal
toxicity induced by DOX administration. Pretreatment with Ber
ameliorated the DOX-induced liver and kidney injury by lowering the
serum levels of ALT, AST, TCHO and BUN, and the histological
damage, such as hemorrhage and focal necrosis of the liver and
kidney tissues induced by DOX were also attenuated by Ber. This
suggested that Ber may exhibit a protective role in DOX-induced
hepatorenal toxicity, and may be a potential agent for attenuation
and prevention of the serious complications of DOX in clinical
practice.
DOX has been reported to be a potent and effective
anticancer agent (19). However,
the therapeutic application of DOX has been greatly limited by its
dose-dependent toxicity, particularly severe cardiac and hepatic
toxicity (20,21). DOX poisoning is usually divided
into acute toxicity, subacute toxicity and chronic toxicity
categories. Acute poisoning commonly occurs following single use or
after a period of treatment, with the most common symptoms
including hypotension, arrhythmia and cardiac dysfunction may occur
occasionally, and often accompanied by hepatic and renal damage
(22,23). In the current study of DOX
toxicity, the single high-dose model is widely used, which provides
valuable biological insights into DOX-induced organ injury. In the
single high-dose model, the dosage is equivalent to a high-dose
single injection in cancer patients, similar to the experimental
design implemented in the present study.
Concurrent with clinical trials on DOX-induced organ
injury, the present study showed that the significant increase in
the activity of AST, ALT, TCHO, BUN, and the histopathological
changes in the liver and kidney were observed in the DOX treatment
group. These biochemical and pathological alterations in the rat
model resemble the acute liver and kidney failure observed in
humans (24). ALT and AST are the
enzymes required in the mutual transformation of sugar and protein
in the body. ALT predominantly exists in liver cells, and AST is
mainly located in myocardial cells; however, the serum level of AST
is also increased when the liver is damaged; thus, the increase in
the serum level of ALT and AST suggests liver damage. Higher serum
aminotransferase activity may be the result of leakage from damaged
liver cell membranes following DOX treatment (25). BUN is the major end metabolic
product of human protein, and is one of the main indexes for the
evaluation of renal function. Therefore, the increase in these
biochemical indexes suggest that DOX causes acute damage of the
liver and kidney. Moreover, DOX-induced hepatorenal toxicity was
further evaluated by analyzing the histopathology.
Since DOX has significant antitumor activity, novel
methods to reduce or prevent its detrimental side effects are
expected to increase its effectiveness in anticancer therapy. The
traditional Chinese medicine Rhizoma Coptidis has also been
demonstrated to exhibit notable antitumor effects (26). In Rhizoma Coptidis and its active
ingredients, Ber is the most valuable active component, and
exhibits antidiabetic, antitumor, antibacterial and
anti-inflammatory effects (12,27).
Therefore, it was hypothesized that Ber may have synergistic
antitumor effects with DOX, and relieve the systemic toxicity
induced by DOX. Tong et al (26) revealed that Ber treatment
potentiated the sensitivity of cancer cells to DOX. It was also
reported that Ber suppressed tumor growth through the induction of
apoptosis and cell cycle arrest in cancer cells (28). The present study was designed to
investigate the potential protective effects of Ber against
DOX-induced hepatorenal toxicity in rats. In the current study, DOX
administration resulted in increased mortality rates compared with
control rats. Live rats showed excessive pericardial, pleural and
peritoneal effusion when treated with DOX. However, pretreatment
with Ber showed protective effects against DOX-induced mortality
and effusion intensity score. Moreover, Ber also protected the
liver and kidney function by lowering serum ALT, AST, TCHO and BUN
levels. Furthermore, the protective effect of Ber against DOX was
also evaluated histopathologically. The histological damages such
as congestion, necrosis and severe destruction of hepatic
architecture induced by DOX were also markedly attenuated by Ber
pretreatment. These findings indicated that Ber exhibited a
potential protective agent against DOX injury.
However, the mechanism underlying the effects of Ber
is unclear. Further research is required to identify with precision
the protective effect of Ber, and the exact mechanism of Ber on
DOX-induced liver and kidney injury. Some hypotheses as to the
mechanism underlying the effect of Ber have been reported, such as
free radical scavenging ability, antiapoptotic and
anti-carcinogenic actions (29,30).
Ber is an effective antioxidant and free radical scavenger which
can prevent ROS formation and produce protective effects on cardiac
function (31). Additionally,
animal and clinical investigations showed that Ber was beneficial
in combating against ROS formation (32,33).
Whereas, it is still unknown whether the protective effect of Ber
on hepatic and renal function was due to its antioxidative
properties. Thus, it is reasonable to investigate the antioxidant
status of Ber in DOX-induced acute hepatorenal toxicity in rats. In
the present study, the antioxidant activity of Ber was revealed
through the oxidative markers as well as antioxidant enzymes. The
oxidative marker MDA is a product of lipid peroxidation which is
released during oxidative stress. SOD, CAT and GPx act as the
antioxidant defense system of the body (34). Current data showed that the MDA
levels in serum and hepatorenal tissues were significantly
elevated. Moreover, the activity of antioxidant enzymes CAT, SOD
and GPx was significantly reduced following DOX administration.
Whereas, Ber significantly decreased the DOX-induced elevation of
MDA, and increased the activity of CAT, SOD and GPx as compared
with the DOX group. The findings of the present study suggested
that elevated lipid peroxidation, accompanied by deteriorating
antioxidant status, was evident in the DOX group. Pretreatment of
rats with Ber significantly alleviated the oxidative stress induced
by DOX. Thus, Ber protected the DOX-induced hepatic and renal
injury due to its antioxidative properties.
In conclusion, the present study reported the
protective effect of Ber against DOX-induced acute hepatorenal
toxicity in a rat model. Pretreatment with Ber significantly
inhibited DOX-induced liver and kidney dysfunction and
histopathological changes. The mechanism underlying the effects of
Ber is likely to be through attenuating the oxidative stress
injury. This suggests that Ber may have a role in the attenuation
and prevention of the serious complications of DOX. Thus the
combination of Ber with DOX is a novel strategy that has the
potential for protecting against DOX-induced hepatorenal toxicity
in clinical practice.
Acknowledgments
This study was supported by the Natural Science
Foundation of China (grant nos. 81273600 and 81302773) and the
natural science Foundation of Hebei Province (grant nos.
C2011206145 and H2013206147).
References
|
1
|
Cheng C, Xue W, Diao H, Xia S, Zuo L, He
A, Gao F, Huang Z, Chen J and Zhang J: Antitumor activity and
toxicological properties of doxorubicin conjugated to [alpha],
[beta]-poly [(2-hydroxyethyl)-L-aspartamide] administered
intraperitoneally in mice. Anticancer Drugs. 21:362–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tangpong J, Miriyala S, Noel T,
Sinthupibulyakit C, Jungsuwadee P and St Clair DK:
Doxorubicin-induced central nervous system toxicity and protection
by xanthone derivative of Garcinia mangostana. Neuroscience.
175:292–299. 2011. View Article : Google Scholar :
|
|
3
|
Cardoso S, Santos RX, Carvalho C, Correia
S, Pereira GC, Pereira SS, Oliveira PJ, Santos MS, Proença T and
Moreira PI: Doxorubicin increases the susceptibility of brain
mitochondria to Ca(2+)-induced permeability transition and
oxidative damage. Free Radic Biol Med. 45:1395–1402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalender Y, Yel M and Kalender S:
Doxorubicin hepatotoxicity and hepatic free radical metabolism in
rats. The effects of vitamin E and catechin. Toxicology. 209:39–45.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bárdi E, Bobok I, Voláh A, Kappelmayer J
and Kiss C: Anthracycline antibiotics induce acute renal tubular
toxicity in children with cancer. Pathol Oncol Res. 13:249–253.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang XL, Fan CH and Zhu HS: Photo-induced
cytotoxicity of malonic acid [C(60)]fullerene derivatives and its
mechanism. Toxicol In Vitro. 16:41–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Badkoobeh P, Parivar K, Kalantar SM,
Hosseini SD and Salabat A: Effect of nano-zinc oxide on
doxorubicin-induced oxidative stress and sperm disorders in adult
male Wistar rats. Iran J Reprod Med. 11:355–364. 2013.
|
|
8
|
Li L, Takemura G, Li Y, Miyata S, Esaki M,
Okada H, Kanamori H, Khai NC, Maruyama R, Ogino A, et al:
Preventive effect of erythropoietin on cardiac dysfunction in
doxorubicin-induced cardiomyopathy. Circulation. 113:535–543. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeh YC, Lai HC, Ting CT, Lee WL, Wang LC,
Wang KY, Lai HC and Liu TJ: Protection by doxycycline against
doxorubicin-induced oxidative stress and apoptosis in mouse testes.
Biochem Pharmacol. 74:969–980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang LQ, Wang FL, Zhu LN, Lv F, Liu S and
Zhang ST: Berberine ameliorates renal injury by regulating G
proteins-AC- cAMP signaling in diabetic rats with nephropathy. Mol
Biol Rep. 40:3913–3923. 2013. View Article : Google Scholar
|
|
11
|
Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye
H, Lau CW, Vanhoutte PM and Xu A: Berberine prevents
hyperglycemia-induced endothelial injury and enhances
vasodilatation via adenosine monophosphate-activated protein kinase
and endothelial nitric oxide synthase. Cardiovasc Res. 82:484–492.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Derosa G, Maffioli P and Cicero AF:
Berberine on metabolic and cardiovascular risk factors: An analysis
from preclinical evidences to clinical trials. Expert Opin Biol
Ther. 12:1113–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho BJ, Im EK, Kwon JH, Lee KH, Shin HJ,
Oh J, Kang SM, Chung JH and Jang Y: Berberine inhibits the
production of lysophosphatidylcholine-induced reactive oxygen
species and the ERK1 2 pathway in vascular smooth muscle cells. Mol
Cells. 20:429–434. 2005.
|
|
14
|
Li J, Pan Y, Kan M, Xiao X, Wang Y, Guan
F, Zhang X and Chen L: Hepatoprotective effects of berberine on
liver fibrosis via activation of AMP-activated protein kinase. Life
Sci. 98:24–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu YY, Tseng YT and Lo YC: Berberine, a
natural antidiabetes drug, attenuates glucose neurotoxicity and
promotes Nrf2-related neurite outgrowth. Toxicol Appl Pharmacol.
272:787–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marin-Neto JA, Maciel BC, Secches AL and
Gallo Junior L: Cardiovascular effects of berberine in patients
with severe congestive heart failure. Clin Cardiol. 11:253–260.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao X, Zhang J, Tong N, Liao X, Wang E,
Li Z, Luo Y and Zuo H: Berberine attenuates doxorubicin-induced
cardiotoxicity in mice. J Int Med Res. 39:1720–1727. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelishomi RB, Ejtemaeemehr S, Tavangar SM,
Rahimian R, Mobarakeh JI and Dehpour AR: Morphine is protective
against doxorubicin-induced cardiotoxicity in rat. Toxicology.
243:96–104. 2008. View Article : Google Scholar
|
|
19
|
Jing L, Wu Y, Wu J, Zhao J, Zuo D and Peng
S: Peroxiredoxins are involved in metallothionein protection from
doxorubicin cardiotoxicity. Eur J Pharmacol. 659:224–232. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou XW, Jiang Y, Wang LF, Xu HY, Lin HM,
He XY, He JJ and Zhang S: Protective role of granulocyte
colony-stimulating factor against adriamycin induced cardiac, renal
and hepatic toxicities. Toxicol Lett. 187:40–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
You JS, Pan TL and Lee YS: Protective
effects of Danshen (Salvia miltiorrhiza) on adriamycin-induced
cardiac and hepatic toxicity in rats. Phytother Res. 21:1146–1152.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Outomuro D, Grana DR, Azzato F and Milei
J: Adriamycin-induced myocardial toxicity: New solutions for an ole
problems? Int J Cardiol. 117:6–15. 2007. View Article : Google Scholar
|
|
23
|
Piscitelli SC, Rodvold KA, Rushing DA and
Tewksbury DA: Pharmacokinetics and pharmacodynamics of doxorubicin
in patients with small-cell lung cancer. Clin Pharmacol Ther.
53:555–561. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: A
retrospective analysis of three trials. Cancer. 97:2869–2879. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
King SL, Mohiuddin JJ and Dekaney CM:
Paneth cells expand from newly created and preexisting cells during
repair after doxorubicin-induced damage. Am J Physiol Gastrointest
Liver Physiol. 305:G151–G162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tong N, Zhang J, Chen Y, Li Z, Luo Y, Zuo
H and Zhao X: Berberine sensitizes mutliple human cancer cells to
the anticancer effects of doxorubicin in vitro. Oncol Lett.
3:1263–1267. 2012.PubMed/NCBI
|
|
27
|
Abd El-Wahab AE, Ghareeb DA, Sarhan EE,
Abu-Serie MM and El Demellawy MA: In vitro biological assessment of
Berberis vulgaris and its active constituent, berberine:
Antioxidants, anti-acetylcholinesterase, anti-diabetic and
anticancer effects. BMC Complement Altern Med. 13:2182013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan K, Zhang C, Feng J, Hou L, Yan L, Zhou
Z, Liu Z, Liu C, Fan Y, Zheng B and Xu Z: Induction of G1 cell
cycle arrest and apoptosis by berberine in bladder cancer cells.
Eur J Pharmacol. 661:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Youn MJ, So HS, Cho HJ, Kim HJ, Kim Y, Lee
JH, Sohn JS, Kim YK, Chung SY and Park R: Berberine, a natural
product, combined with cisplatin enhanced apoptosis through a
mitochondria caspase-mediated pathway in HeLa cells. Biol Pharm
Bull. 31:789–795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsu YY, Chen CS, Wu SN, Jong YJ and Lo YC:
Berberine activates Nrf2 nuclear translocation and protects against
oxidative damage via a phosphatidylinositol 3-kinase Akt-dependent
mechanism in NSC34 motor neuron-like cells. Eur J Pharm Sci.
46:415–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv XX, Yu XH, Wang HD, Yan YX, Wang YP, Lu
DX, Qi RB, Hu CF and Li HM: Berberine inhibits
norepinephrine-induced apoptosis in neonatal rat cardiomyocytes via
inhibiting ROS-TNF-α-caspase signaling pathway. Chin J Integr Med.
19:424–431. 2013. View Article : Google Scholar
|
|
32
|
Cheng F, Wang Y, Li J, Su C, Wu F, Xia WH,
Yang Z, Yu BB, Qiu YX and Tao J: Berberine improves endothelial
function by reducing endothelial microparticles-mediated oxidative
stress in humans. Int J Cardiol. 167:936–942. 2013. View Article : Google Scholar
|
|
33
|
Shen L and Ji HF: The mechanisms of
ROS-photogeneration by berberine, a natural isoquinoline alkaloid.
J Photochem Photobiol B. 99:154–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X, Zhang J, Tong N, Chen Y and Luo Y:
Protective effects of berberine on doxorubicin-induced
hepatotoxicity in mice. Biol Pharm Bull. 35:796–800. 2012.
View Article : Google Scholar : PubMed/NCBI
|