Introduction
In vitro fertilization (IVF) failure, and
recurrent IVF failure, are known problems for couples (1–3).
Endometrial receptiveness has an important role in IVF embryo
transfer (ET) treatments, and a lack of consistency between embryo
development and endometrial ripening causes failure (3,4). The
luminal epithelium is responsible for non-receptivity in the
expression, organization or activation of adhesion systems
(4). Implantation is affected by
numerous variables involved in recurrent implantation failure
(RIF). It has been suggested that this process may be hampered if
either of these variables is defective. RIF is diagnosed when
high-quality embryos repeatedly fail to implant following
transference in several IVF treatment cycles (5). Chromosomal abnormalities, sperm DNA
damage and inadequate culture conditions are all of importance in
the etiology of RIF (6). It was
observed that ovarian hyperstimulation itself is a factor for
reduced endometrial receptivity (7). The Vero co-culture system is
considered to be useful for IVF in terms of prolonging in
vitro culture and enabling the transfer of embryos, as well as
eliminating early-blocked eggs and freezing embryos at the
blastocyst stage (8). Successful
implantation requires the appropriately timed arrival of a viable
blastocyst into a receptive endometrium. The endometrium is
remodeled throughout the menstrual cycle, and exhibits a short
period of receptivity, known as the 'implantation window' (IW)
(9). Poor embryo quality has been
identified as a major cause of implantation failure (10).
Cell adhesion molecules (CAMs) have been determined
to serve specific roles in various phases in reproductive
physiology. The functions of CAMs have been reported in biological
and pathological states, and require cursory examination (11).
Trophinin is an intrinsic membrane protein, and its
marked expression has been detected in the trophectoderm surface of
monkey blastocysts. Expression of trophinin has also been observed
in human endometrial surface epithelium on day 16/17 at the early
secretory phase, the time consistent with that expected for the IW
(12). Trophinin is synthesized in
implantation-associated cells of humans and primates, and is
expressed in a restricted area of the human endometrial luminal
epithelium during the early secretory phase. Restricted, but marked
expression of trophinin in the IW indicates the specific role of
trophinin throughout implantation in humans (12–14).
In spite of this, trophinin is not limited to cells that are
associated with implantation. Trophinin has been detected in the
luminal and glandular epithelium of the endometrium, whether or not
it includes the implanted blastocyst (15). Trophinin mediates homophilic and
apical cell adhesion between trophoblastic cells and endometrial
epithelial cells, which is potentially the initial attachment step
in human embryo implantation (16). Trophinin is a dual signaling
molecule. In embryonic cells, it promotes proliferation and
invasion, whereas in maternal cells it promotes cell death in order
to accept the invading embryo (17).
Dipeptidyl peptidase IV (CD26) is a membrane-binding
extracellular glycoprotein expressed on extravillous trophoblasts
(EVTs) at the decidua, and its enzymic activation leads to EVT
invasion in women throughout the IW. It is known as an indicator
molecule for the endometrium implantation phase expressed on the
cell surface, and it can be reduced to various biological active
peptidases in extracellular domains (18,19).
Overexpression of CD26 has been stated to cause a high blastocyst
adhesion rate and high outgrowth domain in the trophectoderm
(19). Therefore, the present
study assessed the expression levels of trophinin and CD26 by
immunofluorescence in embryos from the zygote to the blastocyst
stage in co-culture medium of endometrial cells obtained from women
patients with RIF.
Materials and methods
Patients
Statements of approval were received from the
participants of the present study and the study was approved by the
Kocaeli University Human Research Ethical Committee (approval no.
KOU HREC:4/20; 2009 Feb 10/30). A total of 13 patients with RIF,
aged between 26 and 36 years, were included in the present study.
Common factors, including uterine polyps, leiomyomata and
endometriosis, besides hormonal pathology, were excluded prior to
the study by the gynecologist. Basal (menstrual day 3) levels of
follicle-stimulating hormone (FSH), luteinizing hormone (LH),
estradiol, thyroid-stimulating hormone, free triiodothyronine, free
thyroxine and prolactin precursor were measured prior to the
patients undergoing pituitary desensitization, followed by
gonadotrophin ovarian stimulation and IVF treatment. Endometrial
biopsies were obtained from the patients on day 21 of their
menstrual cycle, termed the luteal phase. Controlled ovarian
hyperstimulation (COH) was performed in the patients to obtain
plenty of oocytes. Long protocol was selected for decreasing cycle
and sufficient ovarium response (poor response to ovulation
induction results in increasing of cycle cancellation and
implantation failure). In the same cycle, the patients were
administered Lucrin depot (leuprolid asetat, 3M, 11.25 mg; AbbVie,
Chicago, IL, USA) on day 21 and human chorionic gonadotrophin (hCG;
Sigma Chemical Co., St. Louis MO, USA) on day 2 of menstrual
bleeding. The dose was decreased to 5 mg hCG per injection when at
least three follicles were >17 mm in diameter.
Endometrial co-culture
Autologous endometrial co-culture was performed, as
described previously (20).
Briefly, a patient's fertilized eggs were placed on top of a layer
of cells from her own uterine lining, creating a more natural
environment for embryo development. Endometrial biopsies containing
Hanks' balanced salt solution (HBSS) with 5%
penicillin-streptomycin-amphotericin (dilution: 5,000 µg/100
ml; Biological Industries, Ltd., Kibbutz Beit Haemek, Israel) were
obtained. In 15 mm centrifuge tubes, endometrial cells were
isolated by digesting with 0.5% collagenase type II (Sigma-Aldrich,
St. Louis, MO, USA) in Ca2+/Mg2+-free HBSS at
37°C for 5 min. These cells were seeded onto poly-L-lysine coated
four-well chamber slides (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Endometrial cells were allowed to proliferate in the
presence of recombinant human basic fibroblast growth factor (10
ng/ml; Biological Industries, Ltd.) in serum-free medium. The
implantation was performed by putting lamella into two pieces of
the four-well slide in order that each pit contained 500,000 cells.
The patients' names and their dates of birth were written on the
Petri dish as identifiers. The following day, after washing the
cells with fresh medium, the solution was replaced and its top was
covered with oil. Prepared as 5 pieces, one of the 4-well petries
were suspended as in non-lubricated manner in the incubator for
control endometrial co-culure. The cell culture procedure was
performed in a laminar flow hood under sterile conditions.
Conventional culture protocol
All manipulations of oocytes and embryos were
performed as previously described (1). The zygotes were cultured in a
protein-free medium and cultured for 96 h (Sage IVF Inc., Trumbull,
CT, USA) in a laminar flow hood under sterile conditions. The
identical process was applied, and blastocysts were visualized on
an inverted microscope (DMI 4000; Leica Microsystems GmbH, Wetzlar,
Germany).
Oocyte pick up (OPU) and intracytoplasmic
sperm injection
Two days after the hCG injection, the OPU process
was performed in a sterile tube, accompanied by an OPU injection
fixed in the ultrasonography probe, and collected oocytes were
taken to the embryology laboratory. Oocytes in the sterile tube
were taken by means of disposable sterile glass pipettes from
folliculin liquid in a sterile container under the
stereo-microscope (SZX7; Olympus Corporation, Tokyo, Japan) in the
embryology laboratory. The cumulus cells around the eggs, obtained
as a result of the OPU procedure, were cleared up, and subsequently
the mature cells available for use were determined. For the
microinjection procedure, the oocyte was located, and the sperm
exhibiting a normal appearance in terms of form, and if available,
liveliness, was selected under an inverted microscope. The sperm
was inactivated by pressing in the middle of its tail by means of a
microinjection pipette. The sperm was injected into the oocyte,
fixed with a holding pipette. This procedure was performed for all
oocytes in turn, with subsequent incubation at 37°C of the eggs in
an incubator (MCO-18 M; Sanyo, Tokyo, Japan) and performance of a
fertilization check 18–20 h after the procedure.
Culture environments and days
After taking a reading under an inverted microscope,
the majority of the embryos were moved into a conventional culture
environment (n=80), the others into an endometrial co-culture
environment (n=25) on day 1 following OPU. Images of the embryo
were captured using an inverted microscope (Olympus, Tokyo,
Japan).
Day 1 after OPU
The RPMI-1640 medium (Sigma-Aldrich) in four-well
dishes was refreshed three to five times, and loose dead cells,
unlysed cells and erythrocytes were removed from the culture. The
medium was prepared with human serum albumin (HSA; 1:10 in 0.75 ml;
Life Global Medium) and was added into the wells, covered with 0.5
ml oil and incubated. The endometrial cells were fixed with cold
methanol on four-well dishes following ET to another
co-culture.
Days 2–5 after OPU
The development of embryos was checked and recorded
daily. The endometrial co-cultures were washed with new medium in
the morning. The same process was applied between days 2–5.
Day 5 after OPU
The high-quality embryos were transferred, and
endometrial cells were fixed with cold methanol on four-well dishes
after ET.
The control endometrial co-cultures were fixed on
four-well dishes without embryos and immunofluorescence staining
was performed, as described below.
ET
Good-quality embryos were transferred with distended
urinary bladder, using ultrasonography to determine the most
appropriate place where the embryos would implant into the uterus.
During ET, a speculum was placed in the vagina, and the cervix was
cleansed with a sterile saline solution, and cervical mucus was
cleaned with a sterile stick. The embryos were transferred into the
uterus with the aid of a thin and soft catheter. Since the edges of
the catheter can be monitored with ultrasonography, the area in the
uterus where the embryos were transferred to was clearly
determined. Loaded to the catheter in a laminar flow hood by an
embryologist, the ET was performed gently by a surgeon, and the
catheter was removed slowly and controlled under the microscope by
the embryologist to determine whether all embryos were transferred
or not. If present, any developing embryos not used in the transfer
were frozen, according to their quality, or destroyed.
Immunocytochemistry
Endometrial cells were stained using an indirect
immunofluorescent technique, similarly to a previously described
protocol (19). Briefly, during
the fixation process, the coverslip on the endometrial co-culture
Petri dishes was removed. Endometrial cells were rinsed briefly in
phosphate-buffered saline (PBS) and fixed in cold methanol for 10
min. The coverslips were then allowed to dry completely. Following
permeabilization with 0.025% Triton X-100 (Merck, Darmstadt,
Germany), endometrial cells were incubated with 1.5% normal goat
blocking serum (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) in PBS for 30 min at 37°C to suppress non-specific binding of
immunoglobulin (Ig)Gs. Following washing three times with PBS (5
min each), the endometrial cells were incubated overnight at 4°C
with primary antibodies against rabbit anti-human CD26 (H-270; cat.
no. SC-9153; Santa Cruz Biotechnology, Inc.) and mouse anti-human
trophinin (cat. no. SC-80002; Santa Cruz Biotechnology, Inc.) at
dilutions of 1:100 in PBS supplemented with 1% (w/v) bovine serum
albumin (Santa Cruz Biotechnology, Inc.). After three PBS washes,
the cells were incubated with a 1:100 dilution of either goat
anti-mouse IgM conjugated to fluorescein isothiocyanate (FITC; cat.
no. SC-2082; Santa Cruz Biotechnology, Inc.) or a 1:100 dilution of
goat anti-rabbit IgG conjugated to Texas red (cat. no. SC-2780;
Santa Cruz Biotechnology, Inc.) secondary antibodies for 25 min in
the dark. Following washing three times with PBS, the cells were
mounted with mounting medium containing
4′,6-diamidino-2-phenylindole (DAPI; 1 mg/ml; Santa Cruz
Biotechnology, Inc.) to counter-stain the nucleus. A negative
control of immunofluorescence staining was incubated with PBS as a
primary antibody and then secondary antibody to determine any
non-specific binding. Immunofluorescent staining was observed as
green with FITC for trophinin, and red with Texas red for CD26,
using an inverted wide-field fluorescence microscope. Images were
captured using a Leica camera (DMI 4000B; Microsystems GmbH).
Enumeration of cells in the
micrograph
In each endometrial co-culture Petri dish (n=4) of
each patient, quantification of positive immunofluorescence
staining was performed in 0.20 mm2 fields with a x40
objective, using Image J version 1.44a software with Java™ by
National Institutes of Health (Bethesda, MD, USA). Endometrial
cells were examined by two independent observers in a blinded
manner, using the Image J software for analysis and digitization
(21). Looping cell enumeration
was used on images obtained for groups by the Fero lab (Fred
Hutchinson Cancer Research Center, Seattle, WA, USA), and Image J
for analysis and digitization.
Statistical analysis
The data were analyzed using SPSS software version
13.0 (SPSS, Inc., Chicago, IL, USA) for Windows. The Spearman test
for correlation analysis among ongoing variables, the Mann
Whitney-U test to identify the difference between each group, the
Wilcoxon signed-rank test for the comparison of two associated
samples, and the Friedman test to compare all groups were
performed. P<0.05 was considered to indicate a statistically
significant difference. The data are presented as the mean ±
standard deviation.
Results
Patients and embryos
The age, basal hormonal values and endometrial
co-cultures were evaluated in 11 patients. No difference in terms
of the number of pregnancies or embryo development were observed
between each culture environment. The embryos of 11 women had
developed normally, with the exception of 2 women from the 13 women
involved in the present study, the embryos were arrested in 2
patients. No difference was observed on the first to the fourth
days between the two culture groups in terms of the grade (cell
number, irregular blastomeres, fragmentation, multinucleation of
embryo). In the unsuccessful group with implantation failure on the
third day, the morphology of morula in the endometrial co-culture
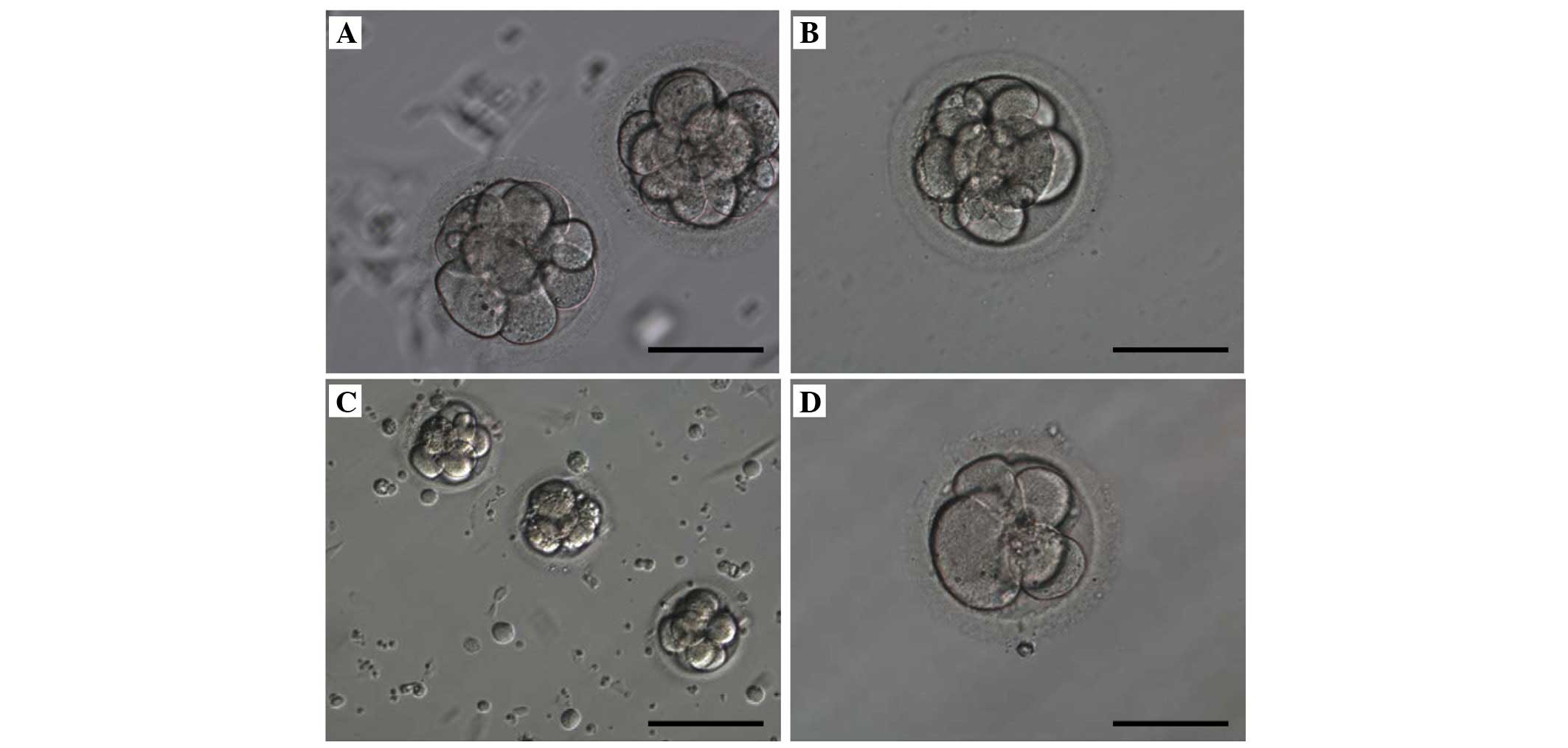
(Fig. 1A) and conventional
co-culture (Fig. 1B) was
identical. The number of cells was important in embryo development
for pregnancy. These eight-cell embryos were moderately fragmented
and had irregular cells on the third day. In the successful
pregnancy group, on same day, the microscopic appearance of the
morula in endometrial co-culture (Fig.
1C) and conventional co-culture (Fig. 1D) exhibited no morphological
difference.
The patients were grouped according to development
of the embryo, implantation and pregnancy status as either
unsuccessful, unsuccessful with implantation failure or successful
pregnancy groups.
Unsuccessful groups
Patients in the unsuccessful group exhibited
developmentally arrested embryos and non-implanted embryos. These
embryos failed to develop, and pregnancy was unsuccessful in these
patients (n=2).
Unsuccessful group with implantation
failure
Patients in this group exhibited well-developed
embryos; however, implantation failure was observed (n=6). Although
the developed embryos were transferred, due to implantation
failure, pregnancy was unsuccessful in this group.
Successful pregnancy group
Patients exhibited well-developed embryos and
appeared pregnant, with successful implantation (n=5). The
developed embryos were transferred and pregnancy occurred in this
group.
While the average age of women involved in the
present study was 28±3.54 in the successful group, the average age
in the unsuccessful group was 32.67±2.81. A difference between the
average ages of the groups was clear; however, no difference was
observed between the basal hormonal values in each group (P=0.035;
Table I). The ratio of successful
pregnancy was 0.38 (n=5/13).
 | Table IAge and basal hormonal values in the
unsuccessful and the successful pregnancy groups. |
Table I
Age and basal hormonal values in the
unsuccessful and the successful pregnancy groups.
| Parameter | Group
| P-value |
|---|
| Unsuccessful
(n=6) | Successful (n=5) |
|---|
| Age | 32.67±2.81 | 28.00±3.54 | 0.035a |
| FSH | 6.33±1.15 | 7.26±2.54 | 0.583 |
| LH | 4.52±1.16 | 4.71± 0.34 | 0.783 |
| E2 | 46.78±16.44 | 36.62±5.60 | 0.465 |
| TSH | 2.53±1.33 | 1.42±0.75 | 0.054 |
| FT3 | 3.38±0.21 | 3.61±0.25 | 0.198 |
| FT4 | 1.33±0.29 | 1.37±0.15 | 0.279 |
| PRL | 23.18±5.19 | 18.88±13.55 | 0.273 |
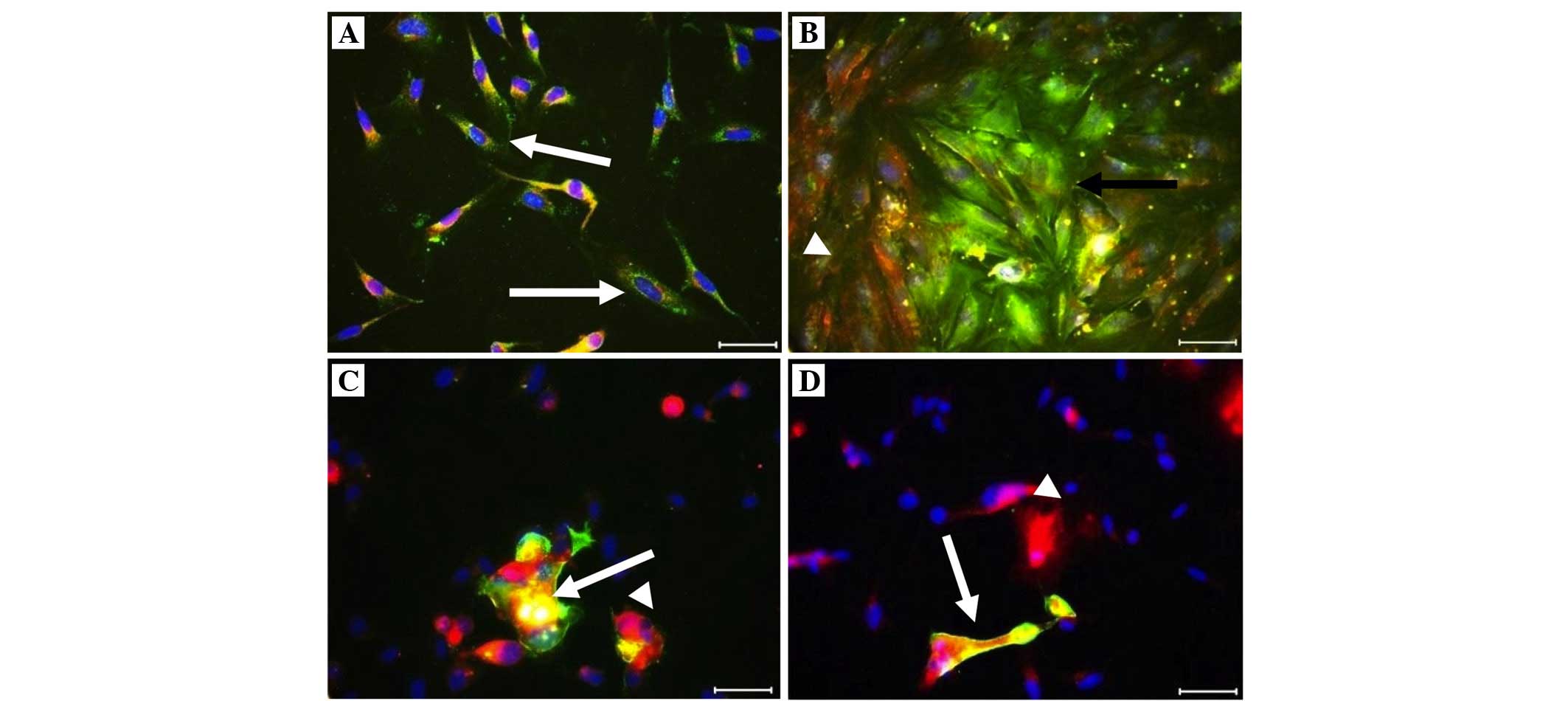
Immunofluorescence staining was performed for the
co-culture: Trophinin (+) cells were yellow-green (FITC), CD26 (+)
cells were red (Texas red) and the nuclei were blue with DAPI, on
endometrial co-culture cells (Fig.
2). No data regarding trophinin and CD26 (+) cells were
obtained for the co-culture of the unsuccessful group as a result
of arrested embryos. The number of trophinin and CD26 (+) cells
were observed on the first to the fourth days of embryo development
in both culture groups. A significant difference was observed in
the number of trophinin (+) cells on the first day between each
group (P=0.046). The number of CD26 (+) cells was higher, with the
exception of the third to the fourth days, and trophinin (+) cells
were lower in the successful group. No difference was observed
between the number of trophinin and CD26 (+) cells on the second to
the fourth days. Additionally, the number of control CD26 (+) cells
were higher in the successful group (Table II; Figs. 2 and 3). A negative correlation was determined
between control CD26 and trophinin parameters (r=−0.836; P=0.005;
Table II). A positive correlation
between ages and the number of CD26 (+) cells was observed on the
third day (r=0.678; P=0.045). A positive correlation was also
observed between the first and second days in the number of CD26
(+) cells (r=0.817; P=0.007) and between the first day number of
CD26 (+) cells and the fourth day number of trophinin (+) cells
(r=0.763; P=0.017; P<0.05). Therefore, the highest (+) cell
numbers in the number of CD26 and trophinin (+) cells were on the
fourth day. An inequality between the number of control trophinin
(+) cells on the first (P=0.036) and second days (P=0.021;
P<0.05) was associated with the number of trophinin (+) cells.
Briefly, the number of trophinin (+) cells on the first and second
days was lower compared with the control trophinin (+) cells
(Table II; Figs. 2 and 3).
 | Table IIQuantification of the number of
trophinin and CD26 (+) cells in the absence or presence of embryos
on the first to the fourth days in the endometrial co-culture of
the unsuccessful and the successful pregnancy groups. |
Table II
Quantification of the number of
trophinin and CD26 (+) cells in the absence or presence of embryos
on the first to the fourth days in the endometrial co-culture of
the unsuccessful and the successful pregnancy groups.
| Immunoreactivity of
ECs | Group
| P-value |
|---|
| Unsuccessful
(n=6) | Successful
(n=5) |
|---|
| Control | | | |
| Trophinin
ECsb | 25.40±15.82 | 17.25±19.76 | 0.140 |
| CD26 ECsb | 22.00±9.77 | 30.75±12.92 | 0.268 |
| Day 1 | | | |
| Trophinin
ECsc | 11.00±4.30a | 5.00±1.63a | 0.046a |
| CD26 ECsc | 17.60±5.32 | 32.00±25.86 | 0.624 |
| Day 2 | | | |
| Trophinin
ECsc | 9.20±7.43 | 5.00±2.16 | 0.537 |
| CD26 ECsc | 20.80±9.15 | 25.25±12.34 | 0.462 |
| Day 3 | | | |
| Trophinin
ECsc | 16.40±11.78 | 3.50±2.38 | 0.138 |
| CD26 ECsc | 28.00±11.77 | 18.50±17.62 | 0.221 |
| Day 4 | | | |
| Trophinin
ECsc | 10.00±9.46 | 9.25±11.33 | 0.803 |
| CD26 ECsc | 38.00±34.91 | 27.00±19.20 | 0.806 |
Discussion
Observing no difference between the basal hormone
levels in each group in the present study may be due to the
patients with RIF. Serum FSH and LH were known prognostic
indicators on the second day in the treatment with IVF, and FSH was
particularly useful in predicting the ovarian response to
superovulation (22). The basal
FSH concentration is also known as a better predictor of the
cancellation rate and of the number of oocytes collected in IVF
treatment compared with age; however, age was a stronger predictor
of the pregnancy rate (23).
Autologous endometrial co-culture, more commonly
known as co-culture, is a state-of-the-art technique co-developed
by Abington Reproductive Medicine's Dr. Larry Barmat (20). This procedure has a more natural
environment for embryo development and maximizes the chance for a
successful IVF pregnancy. It is known that co-culture may be an
effective treatment for patients who have failed previous IVF
cycles, or who have poor embryo quality (20). The quality of embryos in autologous
endometrial co-culture has been determined to be better than that
of embryos in non-co-culture (24). No difference in terms of embryo
quality was observed between the two culture environments, due to
the development of mediums. The difference between each group was
significant in terms of the number of trophinin (+) and CD26 (+)
cells, as control in the absence of an embryo on the first to the
fourth days. The adhesion mechanism has been previously shown to be
involved in human blastocyst implantation by endometrial CD26 (+)
and embryonal fibronectin (19).
IVF embryos developed in vitro in culture media, allowing
them to be maintained alive for a longer period of time, has led to
a rise in pregnancy rates (8). In
the present study, at the blastocyst stage, the ET was possible due
to continuation of pregnancy. A statistically significant
difference was observed between the average ages of the groups. Age
has been reported as a clear predictor of the pregnancy rate
(23).
The successful group exhibited a higher number of
CD26 (+) cells, with an exception on the third day. A significant
difference was observed with regard to the first to the fourth
days, the early growth phase, and the expression of trophinin (+)
and CD26 (+) (P=0.046). The successful group exhibited a lower
number of trophinin (+) and CD26 (+) cells in the controls, unlike
the unsuccessful group. Since the trophoectoderm of the human
blastocyst secretes hCG prior to and following implantation, these
results suggested that hCG from the human embryo induces trophinin
expression by maternal cells (25). Trophinin-mediated signal
transduction has been described in trophectoderm cells and
endometrial epithelial cells (17).
The highest cell numbers of CD26 (+) and trophinin
(+) were on the fourth day. The expression of trophinin has been
investigated in human blastomeres and blastocysts by
immunofluorescence and laser scanning confocal microscopy. This
expression was intensified in the course of embryonic development
(26). Trophinin expression of
endometrial cells was stronger in the control groups. However, no
consistent increase or decrease of trophinin/CD26 (+) cells was
obtained in the course of the pre-implantation embryos, since
endometrial biopsies and oocytes were obtained from the patients
with RIF.
In a clinical program with in vitro models,
the embryos can be co-cultured with endometrial cells until the
blastocyst stage, and subsequently transferred back into the mother
(27). These models have provided
information about embryonic regulation of endometrial epithelial
molecules, including anti-adhesion molecules (28), cytoskeletal proteins (29) and chemokines (30), during human implantation.
Structural and hormonal changes occur in blastocyst invasion, and
these changes have been demonstrated using time-lapse photography,
immunostaining and hCG levels for human-hatched blastocyst
co-culture with human endometrial stromal cell monolayers (31).
Immunostaining of trophinin and CD26 suggested that
endometrial co-culture cells may influence implantation with CAMs.
It may be suitable, both in terms of enabling improvements of
conventional culture medium with immunohistochemical studies
performed in endometrial co-culture, and in providing connections
in the early period among cells of the gravid endometrium and
embryo in unexplained infertility. Natural growth factors, proteins
and nutrients may support embryo development in the co-culture
environment. Therefore, co-culture may be a considerable
alternative for patients with RIF. It is important that the
development of endometrial co-culture techniques is performed,
instead of the conventional culture methods for patients with
RIF.
Acknowledgments
The authors would like to thank Associate Professor
Dr. Dogan Yuksel and lecturer Mrs Ayca Ozguven from Kocaeli
University Department of Foreign Languages for editing of our
manuscript. The present study was financially supported by the
Scientific Research Foundation of Kocaeli University Scientific
Research Foundation (no. 2007/70).
Abbreviations:
|
CD26
|
dipeptidyl peptidase IV
|
|
IVF
|
in vitro fertilization
|
|
ART
|
assisted reproductive techniques
|
|
ET
|
embryo transfer
|
|
RIF
|
recurrent implantation failure
|
|
IW
|
implantation window
|
|
EVT
|
extravillous trophoblast
|
|
FSH
|
follicle-stimulating hormone
|
|
LT
|
luteinizing hormone
|
|
hCG
|
human chorionic gonadotrophin
|
|
COH
|
controlled ovarian
hyperstimulation
|
|
HBSS
|
Hanks' balanced salt solution
|
|
OPU
|
oocyte pick up
|
|
PBS
|
phosphate buffer saline
|
|
FITC
|
fluorescein isothiocyanate
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
RGB
|
red/green/blue in Image J
threshold
|
|
CAM
|
cell adhesion molecules
|
References
|
1
|
Gardner DK and Lane M: Culture systems for
the human embryo. Gardner DK, Weissman A, Howles CM and Shoham Z:
Textbook of Assisted Reproductive Technologies Laboratory and
Clinical Perspectives. 3rd ed. UK, Informa Healthcare Taylor &
Francis; USA: pp. 219–240. 2009
|
|
2
|
Penzias AS: Recurrent IVF failure: Other
factors. Fertil Steril. 97:1033–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Revel A: Defective endometrial
receptivity. Fertil Steril. 97:1028–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aplin JD: Embryo implantation: The
molecular mechanism remains elusive. Reprod Biomed Online. 14(Spec
No 1): 49–55. 2007.PubMed/NCBI
|
|
5
|
Laufer N and Simon A: Recurrent
implantation failure: Current update and clinical approach to an
ongoing challenge. Fertil Steril. 97:1019–1020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das M and Holzer HE: Recurrent
implantation failure: Gamete and embryo factors. Fertil Steril.
97:1021–1027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Tong X, Jiang L, Li T, Zhou F and
Zhang S: A comparison of implantation, miscarriage and pregnancy
rates of single and double day 3 embryo transfer between fresh and
frozen thawed transfer cycles: A retrospective study. Chin Med J
(Engl). 127:911–915. 2014.
|
|
8
|
Menezo YJ, Guerin JF and Czyba JC:
Improvement of human early embryo development in vitro by coculture
on monolayers of Vero cells. Biol Reprod. 42:301–306. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moore KL, Persaud TVN and Torchia MG:
First and second week of human development, Chapter: 2, 3. The
Developing Human. Clinically Oriented Embryology. 9th ed.
International ed.Elsevier Saunders; ISBN: 978-0-8089-2444-9pp.
32–51. 2013
|
|
10
|
Urman B, Yakin K and Balaban B: Recurrent
implantation failure in assisted reproduction: How to counsel and
manage. A. General considerations and treatment options that may
benefit the couple. Reprod Biomed Online. 11:371–381. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Atabekoğlu CS, Engin Y, Üstün Y and Aytaç
R: Reproductive physiology and adhesion molecules. J Ankara Univ
Faculty Med. 55:85–92. 2002.In Turkish.
|
|
12
|
Fukuda MN, Sato T, Nakayama J, Klier G,
Mikami M, Aoki D and Nozawa S: Trophinin and tastin, a novel cell
adhesion molecule complex with potential involvement in embryo
implantation. Genes Dev. 9:1199–1210. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki N, Zara J, Sato T, Ong E, Bakhiet
N, Oshima RG, Watson KL and Fukuda MN: A cytoplasmic protein,
bystin, interacts with trophinin, tastin, and cytokeratin and may
be involved in trophinin-mediated cell adhesion between trophoblast
and endometrial epithelial cells. Proc Natl Acad Sci USA.
95:5027–5032. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki N, Nadano D, Paria BC, Kupriyanov
S, Sugihara K and Fukuda MN: Trophinin expression in the mouse
uterus coincides with implantation and is hormonally regulated but
not induced by implanting blastocysts. Endocrinology.
141:4247–4254. 2000.PubMed/NCBI
|
|
15
|
Nadano D, Sugihara K, Paria BC, Saburi S,
Copeland NG, Gilbert DJ, Jenkis NA, Nakayama J and Fukuda MN:
Significant differences between mouse and human trophinins are
revealed by their expression patterns and targeted disruption of
mouse trophinin. Gene Biol Reprod. 66:313–321. 2002. View Article : Google Scholar
|
|
16
|
Aoyama J, Nakayama Y, Sugiyama D, Saburi
S, Nadano D, Fukuda MN and Yamaguchi N: Apical cell adhesion
molecule, trophinin, localizes to the nuclear envelope. FEBS Lett.
579:6326–6332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukuda MN and Sugihara K: Cell adhesion
molecules in human embryo implantation. Sheng Li Xue Bao.
64:247–258. 2012.PubMed/NCBI
|
|
18
|
Sato Y, Fujiwara H, Higuchi T, Yoshioka S,
Tatsumi K, Maeda M and Fujii S: Involvement of dipeptidyl peptidase
IV in extravillous trophoblast invasion and differentiation. J Clin
Endocrinol Metab. 87:4287–4296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimomura Y, Ando H, Furugori K, Kajiyama
H, Suzuki M, Iwase A, Mizutani S and Kikkawa F: Possible
involvement of crosstalk cell-adhesion mechanism by endometrial
CD26/dipeptidyl peptidase IV and embryonal fibronectin in human
blastocyst implantation. Mol Hum Reprod. 12:491–495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barmat LI, Liu H, Spandorfer SD, Kowalik
A, Mele C, Xu K, Veeck L, Damario M and Rozenwaks Z: Autologous
endometrial co-culture in patients with repeated failures of
implantation after in vitro fertilization-embryo transfer. J Assist
Reprod Genet. 16:121–127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collins TJ: ImageJ for microscopy.
Biotechniques. 43(1 Suppl): 25–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan YF, Ho PC, So WW and Yeung WS: Basal
serum pituitary hormone levels and outcome of in vitro
fertilization utilizing a flare nasal gonadotropin releasing
hormone agonist and fixed low-dose follicle-stimulating
hormone/human menopausal gonadotropin regimen. J Assist Reprod
Genet. 10:251–254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharif K, Elgendy M, Lashen H and Afnan M:
Age and basal follicle stimulating hormone as predictors of in
vitro fertilisation outcome. Br J Obstet Gynaecol. 105:107–112.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spandorfer SD, Soslow R, Clark R,
Fasouliotis S, Davis OK and Rosenwaks Z: Histologic characteristics
of the endometrium predicts success when utilizing autologous
endometrial coculture in patients with IVF failure. J Assist Reprod
Genet. 23:185–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakayama J, Aoki D, Suga T, Akama TO,
Ishizone S, Yamaguchi H, Imakawa K, Nadano D, Fazleabas AT,
Katsuyama T, et al: Implantation-dependent expression of trophinin
by maternal fallopian tube epithelia during tubal pregnancies:
Possible role of human chorionic gonadotrophin on ectopic
pregnancy. Am J Pathol. 163:2211–2219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HY, Xing FQ and Chen SL: Expression
of trophinin in human oocytes and preimplantation embryos. Nan Fang
Yi Ke Da Xue Xue Bao. 28:122–124. 2008.In Chinese. PubMed/NCBI
|
|
27
|
Mercader A, Garcia-Velasco JA, Escudero E,
Remohí J, Pellicer A and Simón C: Clinical experience and perinatal
outcome of blastocyst transfer after coculture of human embryos
with human endometrial epithelial cells: A 5-year follow-up study.
Fertil Steril. 80:1162–1168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meseguer M, Aplin JD, Caballero-Campo P,
O'Connor JE, Martín JC, Remohí J, Pellicer A and Simón C: Human
endo-metrial mucin MUC1 is up-regulated by progesterone and
down-regulated in vitro by the human blastocyst. Biol Reprod.
64:590–601. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin JC, Jasper MJ, Valbuena D, Meseguer
M, Remohí J, Pellicer A and Simón C: Increased adhesiveness in
cultured endometrial-derived cells is related to the absence of
moesin expression. Biol Reprod. 63:1370–1376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dominguez F, Galan A, Martin JJ, Remohi J,
Pellicer A and Simón C: Hormonal and embryonic regulation of
chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human
endometrium and the human blastocyst. Mol Hum Reprod. 9:189–198.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carver J, Martin K, Spyropoulou I, Barlow
D, Sargent I and Mardon H: An in-vitro model for stromal invasion
during implantation of the human blastocyst. Hum Reprod.
18:283–290. 2003. View Article : Google Scholar : PubMed/NCBI
|