Introduction
Calcitonin gene-related peptide (CGRP), a 37-residue
peptide produced in specific neurons by alternative splicing of the
calcitonin gene, is an important neuropeptide involved in bone
growth and metabolism (1).
Previous studies have provided evidence suggesting that CGRP
innervation is associated with bone formation (2,3).
CGRP can stimulate the proliferation and differentiation of
osteoblasts, and improve bone fracture healing and bone metabolism
(4). The overexpression of CGRP in
the osteoblasts of transgenic mice has been shown to increase bone
density (5). Valentijn et
al indicated that CGRP administration inhibited bone
resorption, but not bone formation, in ovariectomized rats
(6). In a previous study by
Schinke et al, it was shown that α-CGRP only regulated the
functional activity of osteoblasts in vivo (7). Another study indicated that CGRP may
modulate the balance between osteoblast and osteoclast activity,
which is involved in fine-tuning all the bone remodeling phases
necessary for the subsequent anabolic effect (8).
The interaction of CGRP with specific
G-protein-coupled receptors is known to activate multiple signaling
transduction pathways. Numerous mechanisms of action of CGRP in
osteoblast-associated cells have been suggested for bone growth and
metabolism (4,9,10).
However, the detailed regulatory mechanism underlying the effect of
CGRP in bone metabolism remains to be fully elucidated. The
expression of osteocalcin (OC) is parallel with osteogenic
differentiation and is utilized as a characteristic marker of
osteogenic differentiation. Treatment with CGRP increases the mRNA
expression of OC and has been suggested to induce osteoblast
differentiation (11). Activating
transcription factor-4 (ATF4), also known as cyclic adenosine
monophosphate (cAMP)-response element-binding protein 2 (CREB2), is
a leucine zipper-containing transcription factor, which regulates
OC transcription and osteoblast terminal differentiation. However,
the detailed effects of CGRP treatment on the expression of ATF4
has not been investigated previously.
The receptor activator of nuclear factor κB ligand
(RANKL) and osteoprotegerin (OPG) are important transcription
factors in the regulation of bone formation and resorption
(12–14). The balance between RANKL and OPG is
a critical determinant for osteoclast differentiation. Neuropeptide
CGRP has been reported to be important in suppressing bone
resorptive activities through the RANKL/OPG pathway (15). However, CGRP administration has
also demonstrated a significant depressive effect on the expression
of RANKL, without an effect on the expression of OPG in primary
human osteoblasts (16).
Furthermore, a study by Villa et al found that CGRP
inhibited OPG production in human osteoblast-like cells, with no
detectable expression of RANKL (8). The present study was performed to
further clarify the potential mechanism of CGRP on bone metabolism
in primary osteoblasts, predominantly focussing on the osteoblast-
and osteoclast-associated mechanisms.
Materials and methods
Cell isolation and identification
Primary osteoblasts were digested from newborn
Chinese rabbit (purchased from Shanghai SLAC Laboratory Animal Co.,
Ltd., Shanghai, China) calvaria using a method described previously
(17). Briefly, the calvaria were
dissected from 8 newly born Chinese rabbits at <24 h of age
(weight, 60–120 g; male and female), and subjected to sequential
digestion with 0.25% trypsin (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 0.15% collagenase II (Sigma-Aldrich,
St. Louis, MO, USA) at 37°C for 15 and 60 min, respectively.
Osteoblasts were collected following centrifugation at 2,000 × g at
4°C for 5 min and a second collagenase digestion step, and were
resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 15% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.). The cells were then
counted using a hemocytometer (Baxter, Deerfield, IL, USA). The
suspension was inoculated into a 100 ml culture flask at a density
of 5×105 cells/ml, cultured at 37°C in a humidified
incubator with 5% CO2, and passaged every 2–3 days. The
enriched osteoblasts were purified using a differential adhesion
method, where cells were cultured for 10 min at 37°C in 5%
CO2 to allow adherence of fibroblasts to the surface of
the flask. The cells suspended in the medium were then moved to
fresh medium and cultured to obtain purified osteoblasts. Then,
alkaline phosphatase (ALP) activity was examined using a modified
Gomori calcium-cobalt method to identify the osteoblasts, where
cells were fixed with 4% paraformaldehyde (Beijing Chemical Reagent
Company, Beijing, China), rinsed with distilled water 3 times,
placed in an incubation solution at 37°C for 4 h and washed with
running water for 10 min. The cells were then incubated with cobalt
nitrate solution (20 g/l; Sigma-Aldrich) for 5 min, washed with
running water, incubated with sulfurated amine solution (10 g/l;
Chongqing Huabo Co., Ltd., Chongqing, China) for 2 min, and washed
with running water. The cells were counter-stained with eosin
(Sigma-Aldrich) and dried (18).
The present study was ethically approved by the Animal Care
Committee of the Third Military Medical University (Daping,
China).
hCGRP treatment
Osteoblasts (1×105 cells/ml) at passage
five were incubated in serum-free DMEM culture medium for 24 h at
37°C to induce synchronization at the G0 phase, in order to compare
the effect of hCHRP treatment on bone metabolism in primary
osteoblasts. The synchronized cells were then distributed into six
groups and cultured for 24 h at 37°C under the following
conditions: Group 1, DMEM only as a negative control; Group 2, DMEM
with 10−9 mol/l hCGRP and 10−6 mol/l hCGRP
(8–37) (both purchased from Sigma-Aldrich); Group 3, DMEM with
10−9 mol/l hCGRP; Group 4, DMEM with 10−8
mol/l hCGRP; Group 5, DMEM with 10−7 mol/l hCGRP; Group
6, DMEM with 10−10 mol/l hCGRP.
Intracellular Ca2+
measurement
The osteoblasts were seeded at a density of
1×105/ml in each well of a 6-plate, cultured until
70–80% confluence, and incubated for 24 h at 37°C in serum-free
medium. The cells were then incubated in a working solution
containing Fluo-3/AM (5 µmol/l; Sigma-Aldrich) and Pluronic
F-127 (18%; Sigma-Aldrich) at 37°C, 5% CO2 for 30 min.
The cells were then washed with Ca2+-free DMEM 2–3
times, re-suspended in Ca2+-free DMEM, and incubated for
another 15 min. The Fluo-3 fluorescence responses to intracellular
Ca2+ concentrations were detected immediately after the
addition of hCGRP or hCGRP (8-37), according to the group design,
under a laser scanning confocal microscope (LSCM; Leica TCS NT
type; Leica Microystems GmbH, Wetzlar, Germany).
cAMP radioimmunoassay
The synchronized osteoblasts were treated with the
phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (0.5
µmol/l; Sigma-Aldrich)) for 15 min, and were then incubated
with different concentrations of hCGRP and hCGRP (8-37) for 10 min
at 37°C, or without treatment, according to the particular
treatment group. The levels of cAMP were assayed using a commercial
cAMP assay kit (Nuclear Medicine Laboratory of Shanghai University
of Traditional Chinese Medicine, Shanghai, China), according to the
manufacturer's protocol.
Electrophoretic mobility shift assay
(EMSA)
Following treatment of the osteoblasts in each group
for 24 h, nuclear extracts were isolated for EMSA analysis.
Briefly, cells were harvested and resuspended in 1 ml cold buffer A
[10 mmol/l KCl, 1.5 mmo1/l MgCl2, 1 mmol/l
dithiothreitol (DTT), 0.2 mmol/l ethylenediaminetetraacetic acid
(EDTA), l mmol/l phenylmethylsulfonyl fluoride (PMSF), 5%
glycerinum, 3 mg/l aprotinin, 3 mg/l phenanthroline, 1% NP40 and 10
mmol/l HEPES] and homogenized. The cells were then placed on ice
for 15 min and centrifuged at 16,000 × g for 15 min. The cell
pellets were gently resuspended in 500 µl buffer B (420
mmo1/l NaCl, 1.5 mmo1/l MgCl2, 0.5 mmol/l DTT, 0.2
mmol/l EDTA, 0.5 mmol/l PMSF, 25% glycerinum, 5 mg/l aprotinin, 5
mg/l phenanthroline, 3 mg/l pepstatin A and 20 mmol/l HEPES),
vortex blended for 15 sec and placed on ice for 10 min. The
procedures were repeated for 4 times, then the nuclear lysates were
centrifuged at 16,000 × g for 15 min, aliquoted, and stored at
−80°C. The protein concentrations were measured using the Bradford
method (19). The oligonucleotide
sequences used as the ATF4 probe (5-AGG ACG AAT GTA GTC TCT C-3)
was synthesized by Shanghai Shenggong Bioengineering Co., Ltd.
(Shanghai, China) and was end-labeled using γ-32p-ATP
(Beijing Furui Biotech Co., Ltd., Beijing, China). For the specific
competitive experiment, a molar excess of unlabeled ATF4
oligonucleotide was added to the binding reaction, together with
the labeled ATF4 probe. For the super shift assay, polyclonal
rabbit anti-ATF4 antibody (1:200 dilution; sc-22800; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was incubated with the
nuclear extracts prior to the addition of the other components for
20 min at 37°C. The Light Shift Chemiluminescent Electrophoretic
Mobility Shift Assay kit (Pierce Biotechnology, Inc., Rockford, IL,
USA) was used to perform EMSA, as described previously (20). The samples (1.7
µg/µl) were separated by electrophoresis on a 6%
polyacrylamide gel (Promega Corporation, Madison, WI, USA) and
analyzed using the Tanon GIS-2010 image analysis system (Shanghai
Tanon Science & Technology Co., Ltd., Shanghai, China).
Northern blot analysis
Total RNA was extracted from the osteoblasts using a
modified guanidinium isothiocyanate method (21). Briefly, cells were resuspended in 1
ml guanidinium thiocyanate buffer (4 mol/l; Fluka Chemical
Corporation, Hauppauge, NY, USA) at 4°C for 5 min and
ultrasonicated for 5 sec 3 times. Chloroform (100 µl;
Tianjin Hengxing Chemical Reagent Co., Ltd., Tianjin, China) was
added to each tube, vibrated, and maintained at 4°C for 10 min.
Following centrifugation at 12,000 × g for 15 min at 4°C,
supernatants were collected, to which equal volumes of isopropanol
(Beijing Baishun Chemical Technology Co., Ltd., Beijing, China),
precooled at −20°C, were added and blended. The mixture was then
centrifuged at 12,000 × g for 10 min at 4°C. The pellets were
washed with 80% ethanol (0.5 ml) for 5 sec and centrifuged at 7,500
× g for 5 min at 4°C. The final pellets were dried in a DNA-mini
vacuum dryer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
dissolved in 30 µl diethylpyrocarbonate ultrapure water. For
quantitative analysis, the purity and concentration of the mRNA was
measured using a Ultraviolet-visible Beckman DU 640
spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA). The RNA
(5.4 µg/µl) was separated on a urea-polyacrylamide
gel electrophoresis (PAGE) gel and transferred onto a
nitrocellulose membrane (Bio-Rad Laboratories, Inc.). Hybridization
was performed according to a standard protocol. Following
hybridization, the membranes were washed 3 times with hybridization
stringency washing buffer (Pierce Biotechnology, Inc.), and the
probe was detected with horseradish peroxidase (HRP) using a
North2South Chemiluminescent Hybridization kit (Pierce
Biotechnology, Inc.). The probes used are summarized in Table I.
 | Table IProbes used for northern blot
analysis. |
Table I
Probes used for northern blot
analysis.
| Gene | Sequence
(5′–3′) | Length (bp) |
|---|
| RANKL-1 |
TTCAGCCCTTTGCCCATCTCACGA | 24 |
| RANKL-2 |
AAGTCGGGAAACGGGTAGAGTGCT | 24 |
| OC-1 |
TCTCTCTAGCCCAGCACCCTCCCC | 24 |
| OC-2 |
AGAGAGATCGGGTCGTGGGAGGGG | 24 |
| OPG-1 |
TCCTCTCTACACTCTCTGCGTTTACTTTGGTGC | 33 |
| OPG-2 |
AGGAGAGATGTGAGAGACGCAAATGAAACCACG | 33 |
| β-actin-1 |
CCACCAGACAGCACTGTGTTGGCA | 24 |
| β-aactin-2 |
GGTGGTCTGTCGTGACACAACCGT | 24 |
Western blot analysis
The protein expression levels of ATF4, OC, RANKL and
OPG was evaluated using Western blot analysis. Briefly, the cells
were harvested and lysed using a Radioimmunoprecipitation kit
(Shanghai Shenneng Bocai Biotechnology Co., Ltd., Shanghai, China),
and protein concentration was determined using Coomassie Brilliant
Blue staining (Sigma-Aldrich) and a Beckman DU 640 ultraviolet
spectrophotometer. The protein samples (15 µl) were
subjected to SDS-PAGE (11%; Sigma-Aldrich) and transferred onto a
polyvinylidene fluoride membrane (DuPont, Boston, MA, USA). The
membrane was blocked with 5% nonfat dry milk for 2 h, and then
incubated at 4°C overnight with primary antibodies against ATF4,
OC, RANKL and OPG (1:200; Santa Cruz Biotechnology, Inc.), followed
by incubation with the HRP-conjugated secondary antibodies
(1:2,500; Santa Cruz Biotechnology, Inc.). The blots were
visualized using an ECL Western Blotting Substrate kit (Pierce
Biotechnology, Inc.). The mRNA expression levels were quantified
using a Beckman DU 640 ultraviolet spectrophotometer. The protein
expression of β-actin was used as an internal control.
Statistical analysis
Data are expressed as the mean ± standard deviation,
and were subjected to one-way analysis of variance. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Isolation and identification of
osteoblasts
Primary osteoblasts were obtained from newborn
rabbit calvaria and, following culture for 24 h, the osteoblasts
adhered to the walls, and exhibited a spindle-like, triangular or
polyangular appearance (Fig. 1A and
B). The cells exhibited a typical cobblestone morphology at
confluence, and were positively stained for ALP (Fig. 1C and D). Following five passages,
the osteoblasts exhibited a positivity of >95%.
hCGRP induces increases in cAMP, but has
no effect on intracellular Ca2+
Intracellular Ca2+ measurements were
performed following treatment of the osteoblasts with hCGRP, and
the fluorescence intensity and distribution of Ca2+
fluorescence signal in single cells were measured using LSCM. The
cells were successfully loaded with the Fluo-3/AM
Ca2+-sensitive dye; exhibiting a bright cytoplasm and
dark nucleus under immunofluorescence microscopy and LSCM,
respectively (Fig. 2A and B).
hCGRP caused no significant effect on transient intracellular
Ca2+ in the osteoblasts (data not shown). Compared with
the control group, intracellular levels of cAMP in the cells
treated with hCGRP were significantly increased, and peaked at a
hCGRP concentration of 10−9 mol/l (P<0.01). This
hCGRP-stimulated increase in cAMP content was inhibited by the
selective antagonist of CGRP receptors, hCGRP (8-37), as shown in
Fig. 2C.
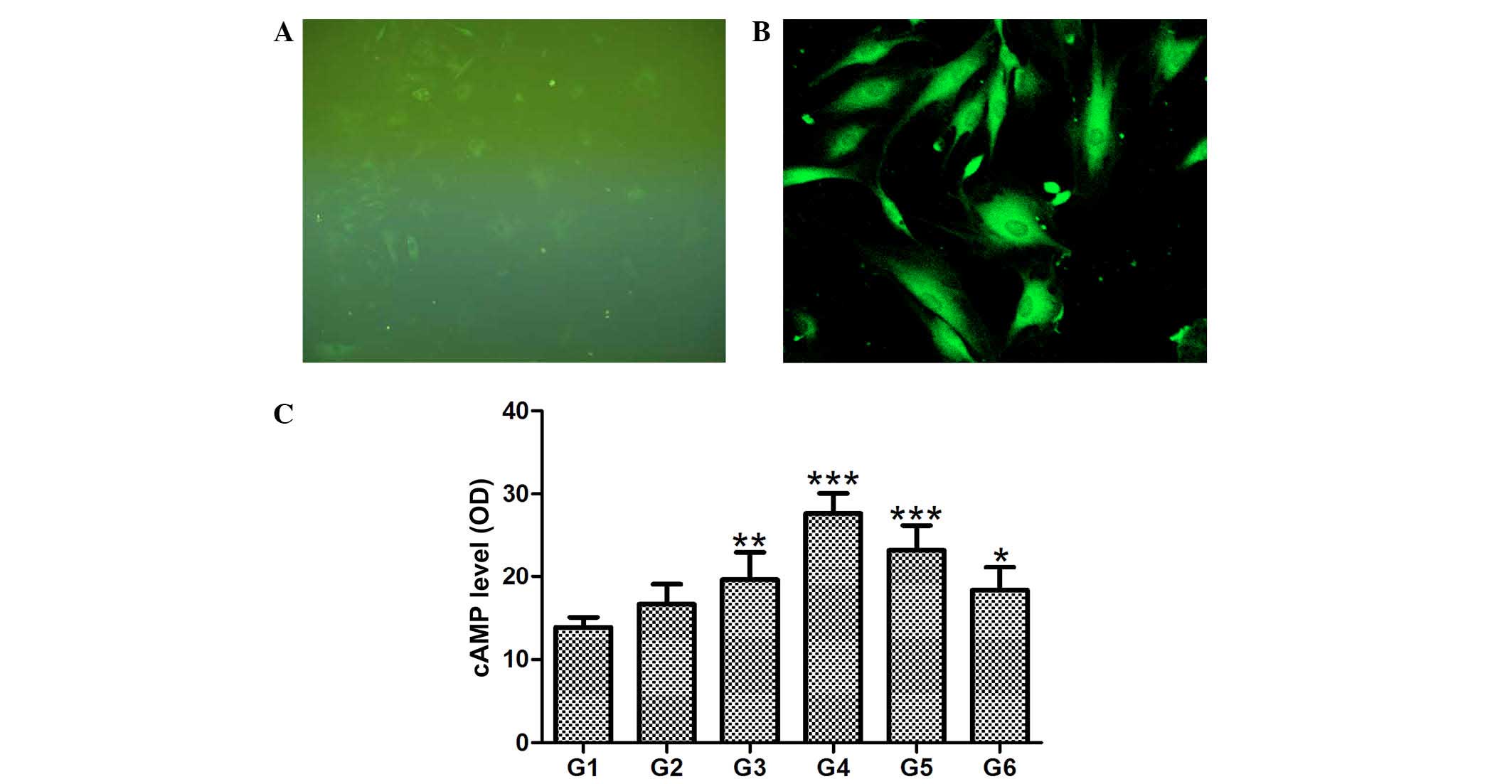 | Figure 2Effect of
chumanalcitonin-gene-related peptide (hCGRP) treatment on
intracellular levels of Ca2+ and cAMP in isolated
osteoblasts. Fluo-3 AM was successfully loaded into the osteoblast,
as demonstrated by (A) immunofluorescence microscopy
(magnification, ×100) and (B) laser scanning confocal microscopy
(magnification, ×200). (C) hCGRP treatment upregulated the levels
of cAMP in a dose-dependent manner, and this effect was reversed by
the selective antagonist of CGRP receptors, hCGRP (8-37). Data are
expressed as the mean ± standard deviation (n=4).
*P<0.05, **P<0.001 and
***P<0.0001 vs. control. G1, osteoblasts cultured in
Dulbecco's modified Eagle's medium (DMEM) only as a negative
control; G2, osteoblasts cultured in DMEM with 10−9
mol/l hCGRP and 10−6 mol/l hCGRP (8-37); G3, osteoblasts
cultured in DMEM with 10−9 mol/l hCGRP; G4, osteoblasts
cultured in DMEM with 10−8 mol/l hCGRP; G5, osteoblasts
cultured in DMEM with 10−7 mol/l hCGRP; G6, osteoblasts
cultured in DMEM with 10−10 mol/l hCGRP; cAMP, cyclic
adenosine monophosphate; OD, optical density. |
hCGRP induces the activation of ATF4
As shown in Fig. 3,
treatment of osteoblasts with hCGRP led to the accumulation of ATF4
in the nuclei of the cells, in a dose-dependent manner, and the
maximum activation of ATF4 was obtained at the hCGRP concentration
of 10−8 mol/l. This effect was markedly reversed by the
addition of hCGRP (8-37), as shown in Fig. 3A (P<0.05). Similarly, the
protein expression of ATF4 in the osteoblasts was also
significantly enhanced following hCGRP treatment, and treatment
with hCGRP (8-37) compromised this hCGRP-induced upregulation of
ATF4 (P<0.05; Fig. 3B).
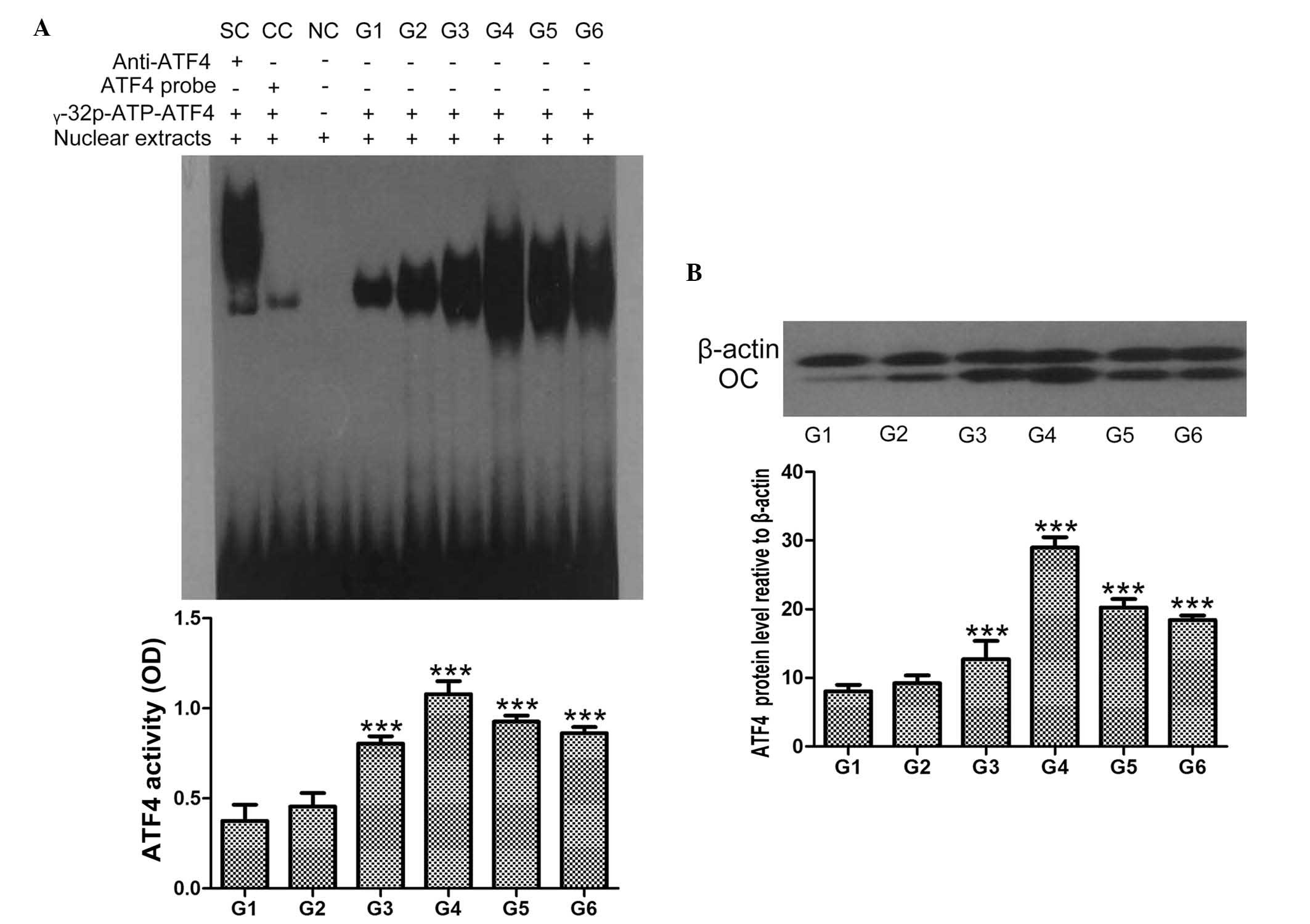 | Figure 3Effect of
chumanalcitonin-gene-related peptide (hCGRP) treatment on the
expression of ATF4 in isolated osteoblasts. (A) EMSA assay of ATF4
activity following treatment with hCGRP and column diagram of ATF4
activity. (B) Western blot analysis of the expression of ATF4
following treatment with hCGRP and column diagram of the expression
of ATF4. Data are expressed as the mean ± standard deviation.
***P<0.001, vs. control group. G1, osteoblasts
cultured in Dulbecco's modified Eagle's medium (DMEM) only as a
negative control; G2, osteoblasts cultured in DMEM with
10−9 mol/l hCGRP and 10−6 mol/l hCGRP (8-37);
G3, osteoblasts cultured in DMEM with 10−9 mol/l hCGRP;
G4, osteoblasts cultured in DMEM with 10−8 mol/l hCGRP;
G5, osteoblasts cultured in DMEM with 10−7 mol/l hCGRP;
G6, osteoblasts cultured in DMEM with 10−10 mol/l hCGRP;
ATF4, activating transcription factor-4; OD, optical density; OC,
osteocalcin; SC, supershift using anti-ATF4 antibody; CC,
competition control using unlabeled ATF4 probe; NC, negative
control without nuclear extracts. |
hCGRP induces the levels of OC and OPG,
and inhibits the level of RANKL in osteoblasts
Northern blot and western blot analyses were
performed to determine the expression levels of OC, OPG and RANKL
in the osteoblasts following treatment with hCGRP. The results
showed that the expression levels of OC and OPG were significantly
upregulated by hCGRP at the mRNA and protein levels, whereas the
expression of RANKL was markedly downregulated. These effects were
significantly counteracted by hCGRP (8-37) administration (Figs. 4 and 5).
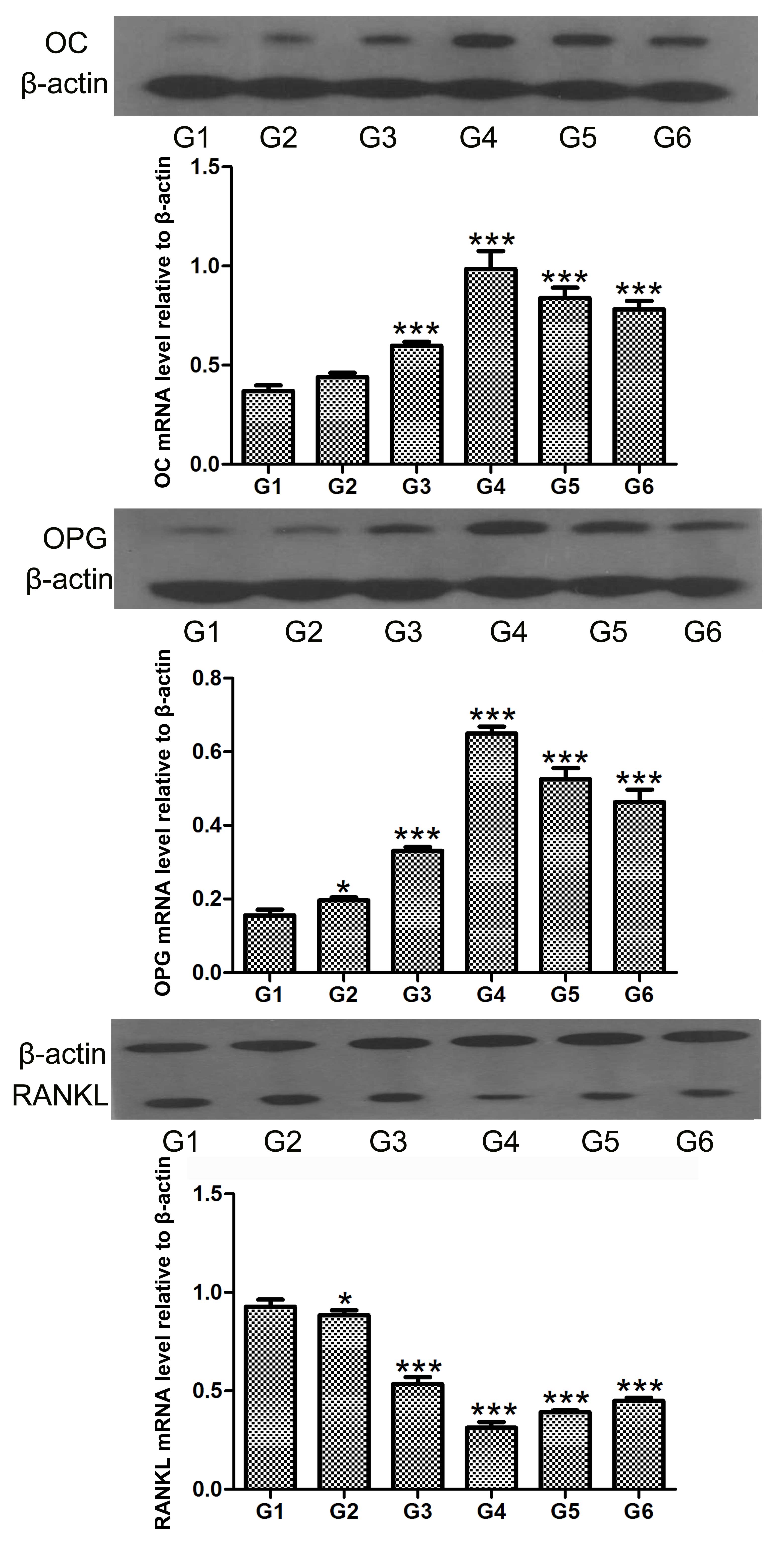 | Figure 4Northern blot analysis of the effect
of chumanalcitonin-gene-related peptide (hCGRP) treatment on the
mRNA expression levels of osteocalcin, OPG and RANKL in isolated
osteoblasts. G1, osteoblasts cultured in Dulbecco's modified
Eagle's medium (DMEM) only as a negative control; G2, osteoblasts
cultured in DMEM with 10−9 mol/l hCGRP and
10−6 mol/l hCGRP (8-37); G3, osteoblasts cultured in
DMEM with 10−9 mol/l hCGRP; G4, osteoblasts cultured in
DMEM with 10−8 mol/l hCGRP; G5, osteoblasts cultured in
DMEM with 10−7 mol/l hCGRP; G6, osteoblasts cultured in
DMEM with 10−10 mol/l hCGRP. Data are expressed as the
mean ± standard deviation. *P<0.05 and
***P<0.001, vs. control group. OC, osteocalcin; OPG,
osteoprotegerin; RANKL, receptor activator of nuclear factor κB
ligand. |
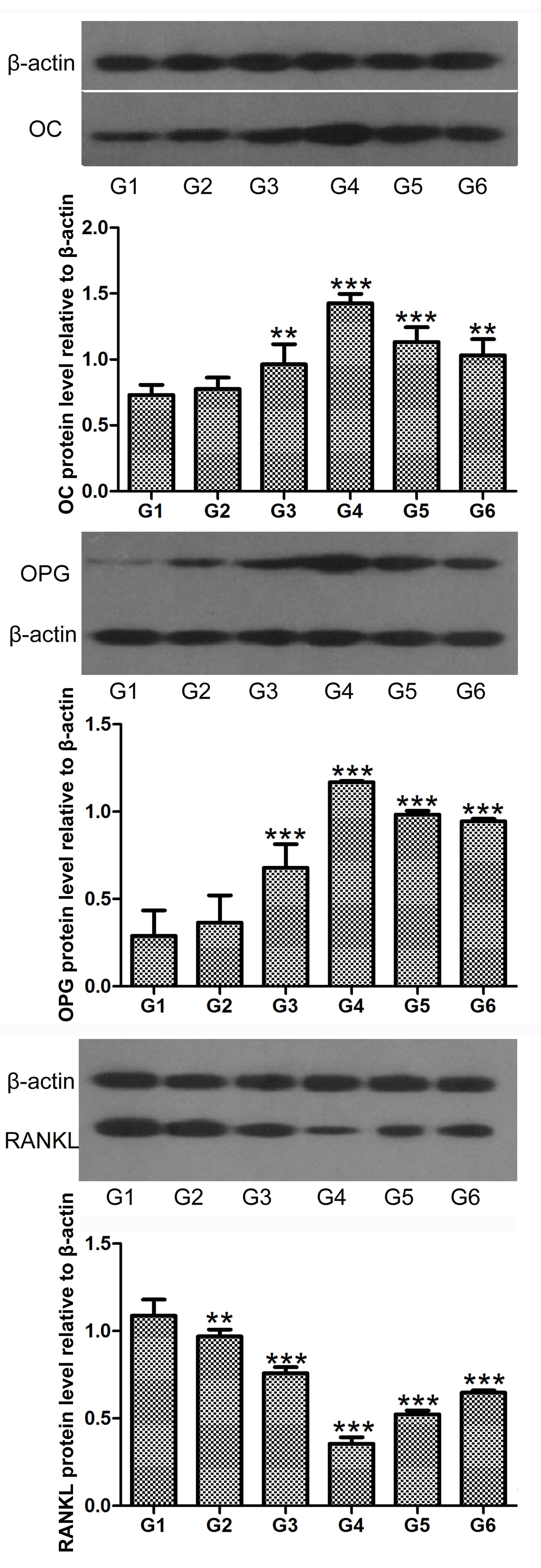 | Figure 5Western blot analysis of the effect
of chumanalcitonin-gene-related peptide (hCGRP) treatment on the
protein expression levels of OC, OPG and RANKL in isolated
osteoblasts. Data are expressed as the mean ± standard deviation.
**P<0.01 and ***P<0.001, vs. control
group. G1, osteoblasts cultured in Dulbecco's modified Eagle's
medium (DMEM) only as a negative control; G2, osteoblasts cultured
in DMEM with 10−9 mol/l hCGRP and 10−6 mol/l
hCGRP (8-37); G3, osteoblasts cultured in DMEM with 10−9
mol/l hCGRP; G4, osteoblasts cultured in DMEM with 10−8
mol/l hCGRP; G5, osteoblasts cultured in DMEM with 10−7
mol/l hCGRP; G6, osteoblasts cultured in DMEM with 10−10
mol/l hCGRP; OC, osteocalcin; OPG, osteoprotegerin; RANKL, receptor
activator of nuclear factor κB ligand. |
Discussion
As a multifunctional regulatory neuropeptide, CGRP
is known to be involved in bone formation, metabolism, healing and
remodeling. The potential mechanisms have been examined
extensively, and cAMP-related pathways have been reported to be
involved in CGRP-regulated bone metabolism, and CGRP-induced cAMP
accumulation in osteoblastic cells (22). Pretreatment with the cAMP pathway
inhibitor, H89, can eliminate the CGRP-induced increases in the
level of cAMP and expression of bone morphogenetic protein-2
(23). By contrast, the absence of
cAMP formation has also been reported following CGRP treatment
(12). The findings of the present
study showed that hCGRP treatment caused a significant accumulation
of cAMP, in a dose-dependent manner, indicating that cAMP was
involved in CGRP-regulated bone metabolism, and detailed
investigations were performed to clarify this further.
CGRP has been reported to increase the intracellular
levels of Ca2+ (24,25),
whereas lower plasma levels of Ca2+ were identified in
another study (26). A previous
study indicated that CGRP may elevate Ca2+ in MG-63
cells through cAMP-independent and cAMP-dependent mechanisms
(27). To closely evaluate the
effect of hCGRP on intracellular levels of Ca2+ in
primary osteoblasts, LSCM was used to detect the intracellular
concentrations of Ca2+ in the osteoblasts following
hCGRP treatment. Unlike the results described above, these results
showed no significant effect of CGRP on intracellular
Ca2+ in the osteoblasts. These conflicting results can
be partially explained by the specie and tissue specificities of
the CGRP receptor, the interaction of receptor activity-modify
proteins with the CGRP receptor (28,29)
or the phase of the cell cycle selected for investigation. In the
present study, the osteoblasts were synchronized to the G0 phase by
serum starvation, whereas cells in the S and G2 phases were used in
the previous study reported by Drissi et al (12).
ATF4, a member of the ATF/CREB family, was
originally identified as an osteoblast-specific transcription
factor, required for the transcription of OC, as in an
osteoblast-specific marker routinely used as an important indicator
of late-stage osteoblast differentiation (30). The expression of ATF4 is known to
be required for osteoblast terminal differentiation and for
maintaining mature osteoblast function (31). Activation of the cAMP-CREB
signaling pathway by G-protein-coupled receptor 48 has been
reported to regulate the expression levels of ATF4 and OC in
osteoblasts (32). CGRP has been
previously shown to upregulate the expression of OC (11). Consistent with these results, the
present study showed enhanced mRNA and protein expression levels of
ATF4 and OC following treatment of the osteoblasts with hCGRP,
suggesting the inducing effect of CGPR on osteoblast
differentiation.
As members of the tumor necrosis factor family,
RANKL and OPG are critical in bone remodeling by directly
controlling osteoclast differentiation and osteolysis (33). Previous studies have presented
conflicting data regarding the effect of CGRP on the expression
levels of OPG and RANKL in osteoblast-like cells. Villa et
al found that CGRP favored osteoclastogenesis by inhibiting the
production of OPG in human osteoblast-like cells via the
cAMP/PKA-dependent pathway, without detectable effects on the
expression of RANKL (8). By
contrast, a study by Kauther et al showed a significant
depressive effect of CGRP on the expression of RANKL, but not on
the expression of OPG, in primary human osteoblasts (16). It has also been previously
indicated that CGRP may suppress bone resorption through OPG
stimulation and RANKL inhibition (15,34).
These results are consistent with the data obtained in the present
study, which indicated that CGRP treatment altered the balance
between the expression levels of OPG and RANKL towards a decline in
RANKL, thus inhibiting osteoclast formation and function.
In conclusion, the findings of the present study
presented evidence to suggest that CGRP administration not only
stimulated osteoblast differentiation, as demonstrated by
upregulated expression levels of ATF4 and OC in osteoblasts treated
with hCGRP, but it also inhibited OPG/RANKL-regulated
osteoclastogenesis. CGRP may act as a modulator of bone metabolism
through osteoblast- and osteoclast-associated mechanisms, which
favor osteoblast formation and the subsequent activation of bone
formation.
Acknowledgments
The current study was financially supported by the
National Natural Science Foundation of China (grant no.
30772436).
References
|
1
|
Offley SC, Guo TZ, Wei T, Clark JD, Vogel
H, Lindsey DP, Jacobs CR, Yao W, Lane NE and Kingery WS:
Capsaicin-sensitive sensory neurons contribute to the maintenance
of trabecular bone integrity. J Bone Miner Res. 20:257–267. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Irie K, Hara-Irie F, Ozawa H and Yajima T:
Calcitonin gene-related peptide (CGRP)-containing nerve fibers in
bone tissue and their involvement in bone remodeling. Microsc Res
Tech. 58:85–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sample SJ, Heaton CM, Behan M, Bleedom JA,
Racette MA, Hao Z and Muir P: Role of calcitonin gene-related
peptide in functional adaptation of the skeleton. PLoS One.
9:e1139592014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villa I, Melzi R, Pagani F, Ravasi F,
Rubinacci A and Guidobono F: Effects of calcitonin gene-related
peptide and amylin on human osteoblast-like cells proliferation.
Eur J Pharmacol. 409:273–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ballica R, Valentijn K, Khachatryan A,
Guerder S, Kapadia S, Gundberg C, Gilligan J, Flavell RA and
Vignery A: Targeted expression of calcitonin gene-related peptide
to osteoblasts increases bone density in mice. J Bone Miner Res.
14:1067–1074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valentijn K, Gutow AP, Troiano N, Gundberg
C, Gilligan JP and Vignery A: Effects of calcitonin gene-related
peptide on bone turnover in ovariectomized rats. Bone. 21:269–274.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schinke T, Liese S, Priemel M, Haberland
M, Schilling AF, Catala-Lehnen P, Blicharski D, Rueger JM, Gagel
RF, Emeson RB and Amling M: Decreased bone formation and osteopenia
in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner
Res. 19:2049–2056. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Villa I, Mrak E, Rubinacci A, Ravasi F and
Guidobono F: CGRP inhibits osteoprotegerin production in human
osteoblast-like cells via cAMP/PKA-dependent pathway. Am J Physiol
Cell Physiol. 291:C529–C537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Villa I, Dal Fiume C, Maestroni A,
Rubinacci A, Ravasi F and Guidobono F: Human osteoblast-like cell
proliferation induced by calcitonin-related peptides involves PKC
activity. Am J Physiol Endocrinol Metab. 284:E627–E633. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naot D and Cornish J: The role of peptides
and receptors of the calcitonin family in the regulation of bone
metabolism. Bone. 43:813–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bo Y, Yan L, Gang Z, Tao L and Yinghui T:
Effect of calcitonin gene-related peptide on osteoblast
differentiation in an osteoblast and endothelial cell co-culture
system. Cell Biol Int. 36:909–915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drissi H, Lieberherr M, Hott M, Marie PJ
and Lasmoles F: Calcitonin gene-related peptide (CGRP) increases
intracellular free Ca2+ concentrations but not cyclic
AMP formation in CGRP receptor-positive osteosarcoma cells (OHS-4).
Cytokine. 11:200–207. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuo K and Irie N: Osteoclast-osteoblast
communication. Arch Biochem Biophys. 473:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoo YM, Kwag JH, Kim KH and Kim CH:
Effects of neuropeptides and mechanical loading on bone cell
resorption in vitro. Int J Mol Sci. 15:5874–5883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kauther MD, Xu J and Wedemeyer C:
Alpha-calcitonin gene-related peptide can reverse the catabolic
influence of UHMWPE particles on RANKL expression in primary human
osteoblasts. Int J Biol Sci. 6:525–536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Sun X, Sun G, Liu S and Wang L:
DNA damage induced by fluoride in rat osteoblasts. Fluoride.
39:191–194. 2006.
|
|
18
|
Li SH, Guo DZ, Li B, Yin HB, Li JK, Xiang
JM and Deng GZ: The stimulatory effect of insulin-like growth
factor-1 on the proliferation, differentiation and mineralisation
of osteoblastic cells from Holstein cattle. Vet J. 179:430–436.
2009. View Article : Google Scholar
|
|
19
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu HT, Yu M, Li CY, Zhan YQ, Xu WX, Li YH,
Li W, Wang ZD, Ge CH and Yang XM: Specific expression and
regulation of hepassocin in the liver and down-regulation of the
correlation of HNF1alpha with decreased levels of hepassocin in
human hepatocellular carcinoma. J Biol Chem. 284:13335–13347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vignery A and McCarthy TL: The
neuropeptide calcitonin gene-related peptide stimulates
insulin-like growth factor I production by primary fetal rat
osteoblasts. Bone. 18:331–335. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian G, Zhang G and Tan YH: Calcitonin
gene-related peptide stimulates BMP-2 expression and the
differentiation of human osteoblast-like cells in vitro. Acta
Pharmacol Sin. 34:1467–1474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawase T, Howard GA, Roos BA and Burns DM:
Diverse actions of calcitonin gene-related peptide on intracellular
free Ca2+ concentrations in UMR 106 osteoblastic cells.
Bone. 16(Suppl 4): S379–S384. 1995. View Article : Google Scholar
|
|
25
|
Bjurholm A, Kreicbergs A, Brodin E and
Schultzberg M: Substance P- and CGRP-immunoreactive nerves in bone.
Peptides. 9:165–171. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tippins JR, Morris HR, Panico M, Etienne
T, Bevis P, Girgis S, MacIntyre I, Azria M and Attinger M: The
myotropic and plasma-calcium modulating effects of calcitonin
gene-related peptide (CGRP). Neuropeptides. 4:425–434. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burns DM, Stehno-Bittel L and Kawase T:
Calcitonin gene-related peptide elevates calcium and polarizes
membrane potential in MG-63 cells by both cAMP-independent
and-dependent mechanisms. Am J Physiol Cell Physiol. 287:C457–C467.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katafuchi T, Kikumoto K, Hamano K, Kangawa
K, Matsuo H and Minamino N: Calcitonin receptor-stimulating
peptide, a new member of the calcitonin gene-related peptide family
its isolation from porcine brain, structure, tissue distribution
and biological activity. J Biol Chem. 278:12046–12054. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Born W, Fischer JA and Muff R: Receptors
for calcitonin gene-related peptide, adrenomedullin and amylin: The
contributions of novel receptor-activity-modifying proteins.
Receptors Channels. 8:201–209. 2002. View Article : Google Scholar
|
|
30
|
Lian N, Lin T, Liu W, Wang W, Li L, Sun S,
Nyman JS and Yang X: Transforming growth factor β suppresses
osteoblast differentiation via the vimentin activating
transcription factor 4 (ATF4) axis. J Biol Chem. 287:35975–35984.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang X, Matsuda K, Bialek P, Jacquot S,
Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes
TM, et al: ATF4 is a substrate of RSK2 and an essential regulator
of osteoblast biology: Implication for Coffin-Lowry Syndrome. Cell.
117:387–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo J, Zhou W, Zhou X, Li D, Weng J, Yi Z,
Cho SG, Li C, Yi T, Wu X, et al: Regulation of bone formation and
remodeling by G-protein-coupled receptor 48. Development.
136:2747–2756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Angelopoulos NG, Goula A, Katounda E,
Rombopoulos G, Kaltzidou V, Kaltsas D, Malaktari S, Athanasiou V
and Tolis G: Circulating osteoprotegerin and receptor activator of
NF-kappaB ligand system in patients with beta-thalassemia major. J
Bone Miner Metab. 25:60–67. 2007. View Article : Google Scholar
|
|
34
|
Wang L, Shi X, Zhao R, Halloran BP, Clark
DJ, Jacobs CR and Kingery WS: Calcitonin-gene-related peptide
stimulates stromal cell osteogenic differentiation and inhibits
RANKL induced NF-kappaB activation, osteoclastogenesis and bone
resorption. Bone. 46:1369–1379. 2010. View Article : Google Scholar :
|



















