Introduction
Prostate cancer (PCa) is one of the most frequently
diagnosed malignant tumors in men worldwide, and its incidence
continues to rise in numerous countries. According to a recent
American Cancer Society report, there were 233,000 new cases and
29,480 PCa-associated mortalities in the United States in 2014
(1). Currently, the early
detection of PCa predominantly depends on serum prostate-specific
antigen (PSA) testing. Although the routine use of PSA testing
increased the detection of PCa, the greatest deficiency of this
method is its lack of specificity, as elevated PSA levels may also
be detected in men with benign prostatic hyperplasia (BPH) and
prostatitis. Although various preventive therapies, such as radical
prostatectomy, androgen- deprivation taxane- based chemotherapy,
drugs targeting the androgen receptor (enzalutamide) or androgen
synthesis (abiraterone acetate), and 5α-reductase inhibitors, may
prevent or manage early disease stages, there is no effective
therapeutic target for aggressive PCa; this results in a tumor
progression of 40–50% patients with castration resistant prostate
cancer (2). Thus, it is necessary
to elucidate novel biomarkers that may be used for the development
of more sensitive diagnosis and treatment (3).
MicroRNAs (miRNAs/miRs) are small, non-coding RNAs
that regulate gene expression at the post-transcriptional and
translational levels. The complementarity between miRNAs and target
mRNAs causes mRNA cleavage and/or translational suppression,
resulting in reduced gene expression levels (4). Multiple miRNAs have been implicated
in PCa progression, including the miRNA-17-92 cluster, which has
been demonstrated to contribute to tumorigenesis (5–8). In
addition, miR-17-5p and miR-17-3p, which are part of the
miRNA-17-92 cluster, target TIMP metallopeptidase inhibitor 3 and
coordinately function as an oncogene in PCa (9). Furthermore, Yang et al
(10) confirmed the inhibitory
effect of miR-19a-3p in breast cancer progression and metastasis.
In serum samples from patients with colorectal adenocarcinoma, the
miR-19a-3p level was significantly correlated with the different
clinical stages of colorectal adenocarcinoma (11). The high expression of miR-19a-3p
was also significantly associated with poor survival of patients
with astrocytoma (12). However,
there is currently limited knowledge regarding the role of
miR-19a-3p in PCa tumorigenesis and metastasis. Thus, the present
study investigated the function of miR-19a-3p in PCa. It was
observed that miR-19a-3p may promote the progression of PCa
progression through the targeting prostate transmembrane protein
androgen induced 1 (PMEPA1), which is abundant in the prostate
abundant and can negatively regulate the growth of refractory PCa
cells.
Materials and methods
Clinical specimens
A total of 22 PCa samples and 20 BPH tissues were
obtained from patients who underwent surgery at Beijing Chao-Yang
Hospital, Capital Medical University (Beijing, China) from May 2014
to January 2015. The tissue of each patient was acquired from
transrectal biopsy or prostatectomy, and the tumor stage of PCa was
assessed according to the modified tumor, node, metastasis system
suggested by the UICC International Union Against Cancer (13). All the tissue samples were stored
at −80°C following flash freezing in liquid nitrogen. The present
study was approved by the ethics committee of Beijing Chao-Yang
Hospital, Capital Medical University, and written informed consent
was obtained from each patient.
Cell cultures
The BPH cell line, BPH1, and the PCa cell lines,
LNCaP and PC3, were obtained from the American Type Culture
Collection (Manassas, VA, USA). Cells were routinely cultured in
RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 50 µg/ml streptomycin, all purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). All cell
lines were cultured in a humidified incubator at 37°C and 5%
CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Small RNA was extracted from tissues or cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), in
accordance with the manufacturer's instructions. To analyze
miR-19a-3p expression levels, an miRcute miRNA First-Strand cDNA
Synthesis kit and miRcute miRNA qPCR Detection kit (SYBR Green),
purchased from Tiangen Biotech Co., Ltd. (Beijing, China), were
used, according to the manufacturer's protocol. All reactions were
run on an ABI7500 Sequence Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The PCR conditions included an
initial holding period of 2 min at 94°C, followed by a two-step PCR
program consisting of 20 sec at 94°C and 34 sec at 60°C for 40
cycles. The forward primer was designed based on the mature
sequence of human miR-19a-3p. The forward primer sequence for
miR-19a-3p was 5′-CGC ATC CCA GTG TGC AAA TCT-3′ and the forward
primer sequence of U6 was 5′-GCA AGG ATG ACA CGC AAA TTC-3′. The
universal reverse primer was provided in the miRcute miRNA qPCR
Detection kit. All samples were normalized against the reference
gene (U6 small nuclear RNA) and analyzed using the
2−ΔΔCq method (14).
Transfection of miR-19a-3p
mimics/inhibitors
miRNA mimics are small, chemically modified
double-stranded RNAs that mimic the function of an endogenous
miRNA. miRNA inhibitors are single-stranded chemically modified
RNAs containing a sequence complementary to the seed region or the
complete sequence of the targeted miRNA. They inhibit the
incorporation of the endogenous miRNA into the RNA-induced
silencing complex, therefore, blocking mRNA cleavage or
translational repression. The negative control (NC) used was a
scramble miRNA, which is a random sequence of precursor miRNA
molecules validated by the manufacturer to not produce any
identifiable effects on known miRNA function (15). In the present study,
4×104 PC3 cells were transfected with miR-19a-3p mimics
(miR10002869- 1- 5) and inhibitors (miR20000073- 1- 5) in 24-well
plates using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For the
miRNA mimic experiments, miR-19a-3p mimics and NC (miR01101-1- 5)
(Guangzhou RiboBio Co., Ltd., Guangzhou, China) were used at 50 nM.
For the miRNA inhibitors experiments, miR-19a-3p inhibitors and NC
(Guangzhou RiboBio Co., Ltd.) were used at 100 nM. In the vehicle
group, cells were cultured in medium only. The cells were harvested
24 h after transfection and subjected to various assays.
Small interfering RNA (siRNA) assay
PC3 cells were seeded at a density of
2×105 cells per well in 6-well plates in 2 ml of culture
medium containing 10% FBS. The cells were transfected with siRNA
against PMEPA1 (siG141117152735) and its negative control
(siN05815122147) (both purchased from Guangzhou RiboBio Co., Ltd.)
using Lipofectamine 2000. The cells were harvested 48 h after
transfection and subjected to various assays.
Cell proliferation assay
Cell proliferation was determined by
5-ethynyl-2′-deoxyuridine (EdU) incorporation assay, which was
performed using a Cell-Light EdU imaging detecting kit, according
to the manufacturer's instructions (Guangzhou RiboBio Co., Ltd.).
Incorporation of the EdU thymidine analogue was used to label cells
undergoing DNA replication. Images of the EdU positive cells were
captured using a light microscope (Leica DM4000B; Leica
Microsystems, Wetzlar, Germany) and the cell numbers were counted
using Image-Pro Plus version 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Cell invasion and migration assay
Cell invasion was measured using a 24-well Transwell
assay (Corning Incorporated, Corning, NY, USA) on a polycarbonate
filter pre-coated with Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) at 1:10 dilution. Cells (2×104) suspended in
0.2 ml of serum-free medium were added to the upper well of the
chamber and 500 µl of medium supplemented with 10% FBS was
added to the lower well. Following incubation at 37°C in 5%
CO2 for 24 h, the cells remaining in the upper chamber
were removed using a cotton swab. Invaded cells on the bottom of
the membranes were fixed with 90% ethanol and stained with 0.1%
crystal violet solution (Sigma-Aldrich, St. Louis, MO, USA). Images
of the fixed cells were captured with a light microscope (Leica
DM4000B; Leica Microsystems) and the cell numbers were counted
using Image-Pro Plus version 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA) in five randomly selected fields. Cell
migration assays were conducted as described for invasion assays,
excluding the use of Matrigel.
miRNA target prediction
Predicted miR-19a-3p target genes were obtained
using the miRWalk atlas (zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/),
which collates data from the following multiple prediction
programs: Targetscan (www.targetscan.org), miRanda (www.microrna.org) and picTar (pictar.mdcberlin.de).
Luciferase activity assay
A luciferase activity assay was performed using a
Dual-Luciferase Reporter System developed by Promega Corporation
(Madison, WI, USA). In brief, PC3 cells were seeded onto 96-well
plates at a density of 2×104 cells/well in medium
containing 10% FBS and cultured for 24 h. The cells were
co-transfected with the luciferase reporter constructs (E1910),
corresponding miRNA mimics and a Renilla luciferase
construct (pmiR- RB- REPORTTM dual luciferase reporter constructs;
Promega Corporation) using Lipofectamine 2000. The cells were then
lysed using 100 µl passive lysis buffer (Promega
Corporation), (Roche Diagnostics, Basel, Switzerland) and the
lysates were centrifuged at 3,000 × g for 10 min for supernatant
collection. Supernatant (20 µl) was mixed with 100 µl
luciferase assay buffer II and the luciferase activities were
measured using a Varioskan Flash (Thermo Fisher Scientific, Inc.).
For the internal control, 100 µl Stop & Glo reagent,
which diluted 1.0 µl Dual- Glo Stop & Glo substrate into
0.1 ml of Dual- Glo Stop & Glo buffer, was added to the
samples. Luciferase activities between different treatments were
compared following normalization to Renilla luciferase
activities. The miR-19a-3p binding sites included positions 359–366
(5′-AAC UUU AAA GAC AUA---UUU GCA CA-3′) and 611–617 (5′-UAU AAA
UGU UGA GAU UUU GCA CU-3′) of the PMEPA1 mRNA 3′-UTR.
Western blot analysis
Cell lysates (1×106 cells) were prepared
from cells cultured in 6-well plates by adding 100 µl lysis
buffer containing protease inhibitors to each well. Protein
concentrations were measured using a Bio-Rad Protein Assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins (30
µg) from each sample were loaded onto 10% SDS-polyacrylamide
gels and separated by electrophoresis, then transferred onto
polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life
Sciences, Chalfont, UK) at 4°C. Following several washes with
Tris-buffered saline with Tween 20 (TBS-T; 20 mM pH 7.5 Tris-Cl,
0.15 M NaCl and 0.05% Tween 20), the PVDF membrane was blocked with
10% non-fat milk in TBS-T for 1 h and then incubated with primary
goat polyclonal anti-PMEPA1 antibody (dilution, 1:1,000; sc-85829;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
The following day, the membrane was washed and incubated with IRDye
800CW donkey anti-goat IgG secondary antibody (dilution, 1:10,000;
P/N 926- 32214; LI-COR, Inc., Lincoln, NE, USA) at room temperature
for 2 h followed by detection with the Odyssey CLx Infrared Imaging
System (LI-COR, Inc.). To verify equal loading of protein, the
blots were reprobed with primary monoclonal antibody against
β-actin (ProteinTech Group, Inc., Chicago, IL, USA).
Statistical analysis
SPSS version 19.0 (IBM SPSS, Amronk, NY, USA) was
used to perform all statistical analyses. Quantitative analysis for
immunoblotting was performed using Quantity One software and a Gel
Doc 2000 imaging system (version 4.62; Bio-Rad Laboratories, Inc.).
For protein quantification, the ratio of PMEPA1 (band density of
PMEPA1:band density of β-actin) was expressed as 100% in the NC
group. The data from the other groups was expressed as a percentage
of that of the NC group. Values are presented as the mean ±
standard error of the mean. Statistical analysis was conducted by
one-way analysis of variance followed by Bonferroni test all
pairwise multiple comparison procedures and Student's t test.
Spearman's rank correlation was used to perform univariate
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Upregulation of miR-19a-3p in PCa
tissues
Table I presents
the clinical and pathological characteristics of the patients in
the present study. To determine the expression levels of miR-19a-3p
in PCa patients, miR-19a-3p was detected in all the 22 PCa and 20
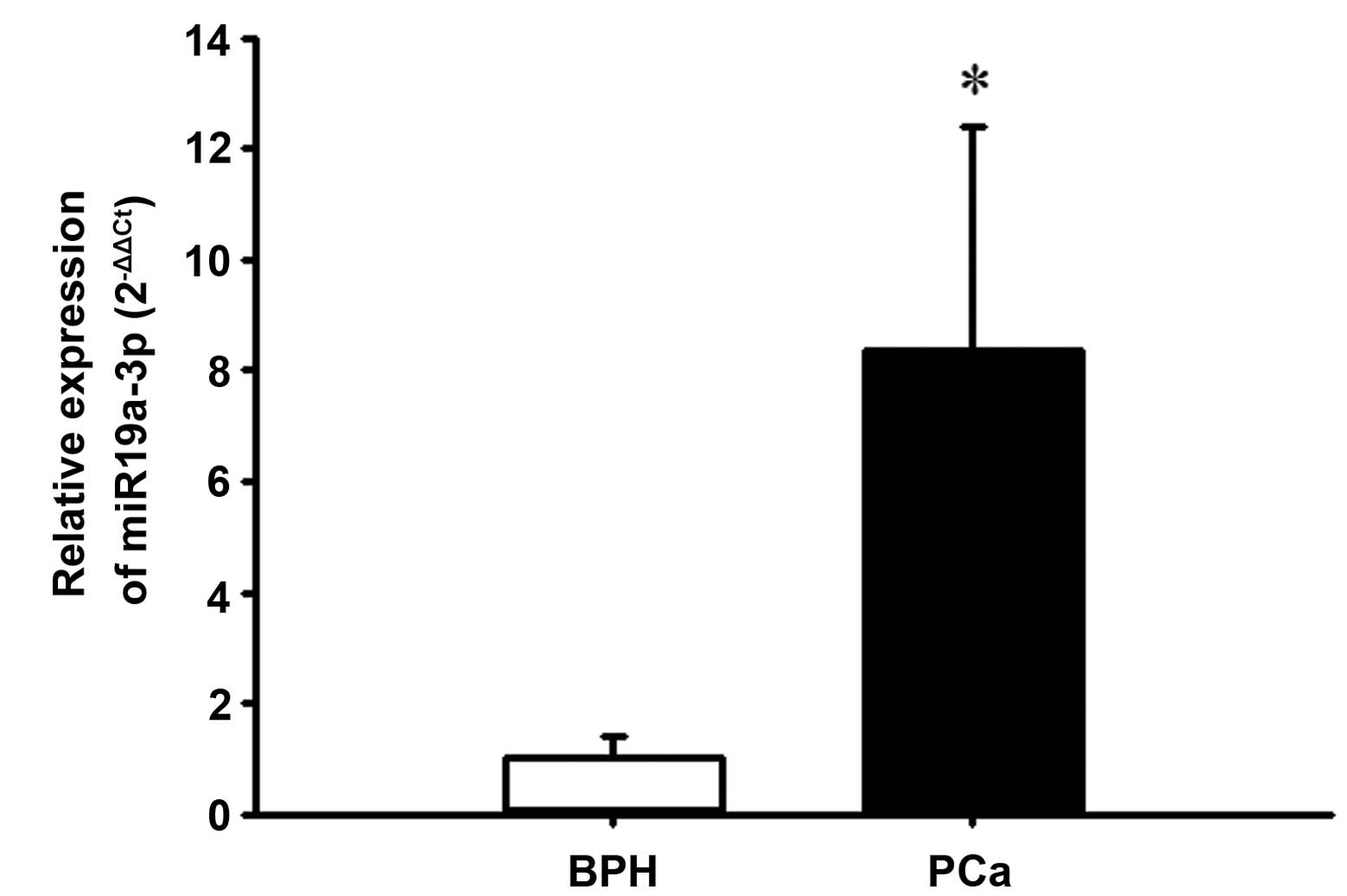
BPH tissues using RT-qPCR. As demonstrated in Fig. 1, PCa tissues exhibited
significantly increased expression of miR-19a-3p compared with that
of the BPH tissues (P<0.05). Spearman's rank correlation
analysis demonstrated that high expression of miR-19a-3p was not
correlated with Gleason grade (r=−0.3376, P=0.1244) or serum PSA
level (r=−0.0766, P=0.735; data not shown).
 | Table IClinicopathological characteristics of
patients with PCa or BPH. |
Table I
Clinicopathological characteristics of
patients with PCa or BPH.
| Characteristic | PCa | BPH |
|---|
| Patients, n (%) | 22 (52.4) | 20 (47.6) |
| Age at diagnosis | | |
| Mean age ± SD,
years | 68±5.8 | 68±7.1 |
| ≤65 years, n
(%) | 6 (27.3) | 7 (35.0) |
| >65 years, n
(%) | 16 (72.7) | 13 (65.0) |
| Mean serum PSA, ng/ml
(range) | 70.701
(0.002–1000) | 5.769
(0.343–17.420) |
| Stage, n (%) | | |
| I–II | 7 (31.8) | NA |
| II–IV | 15 (68.2) | NA |
| Primary tumor
stage, n (%) | | |
| pT1–pT2 | 9 (40.9) | NA |
| pT3–pT4 | 13 (59.1) | NA |
| Lymph node
metastasis, n (%) | | |
| N0 | 18 (81.8) | NA |
| N1–N4 | 4 (12.8) | NA |
| Gleason grade, n
(%) | | |
| ≥6 | 6 (27.3) | NA |
| 7 | 11(50.0) | NA |
| ≤8 | 5 (22.7) | NA |
Overexpression of miR-19a-3p in the PC3
cell line
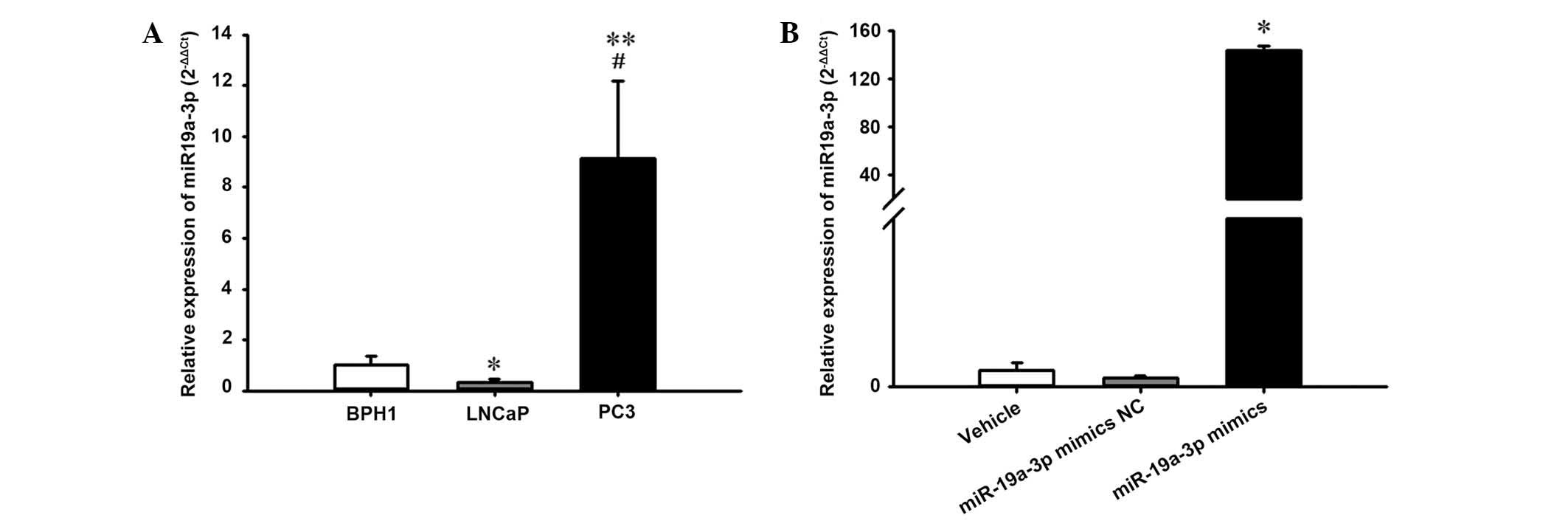
The current study further evaluated the expression
level of miR-19a-3p in human BPH (BPH1) and PCa (LNCaP and PC3)
cell lines. Notably, it was observed that the expression level of
miR-19a-3p was increased significantly in human PC3 cells compared
with the BPH1 and LNCaP cell lines (P<0.05; Fig. 2A).
Additionally, to investigate the effect of
miR-19a-3p on PCa cell growth, the PC3 cells were transiently
transfected with miR-19a-3p mimics and corresponding NC. RT-qPCR
analysis demonstrated that transfection with the miR-19a-3p mimics
caused a significant increase in miR-19a-3p expression levels in
PC3 cells compared with cells transfected with NC (P<0.05;
Fig. 2B).
miR-19a-3p promotes PC3 cell
proliferation
The EdU proliferation assay was conducted in PC3
cells that were transiently transfected with miR-19a-3p mimics. The
results in Fig. 3 demonstrate that
proliferation was significantly increased in cells transfected with
the miR-19a-3p mimics compared with cells transfected with NC
(P<0.05).
miR-19a-3p enhances PC3 cell migration
and invasion
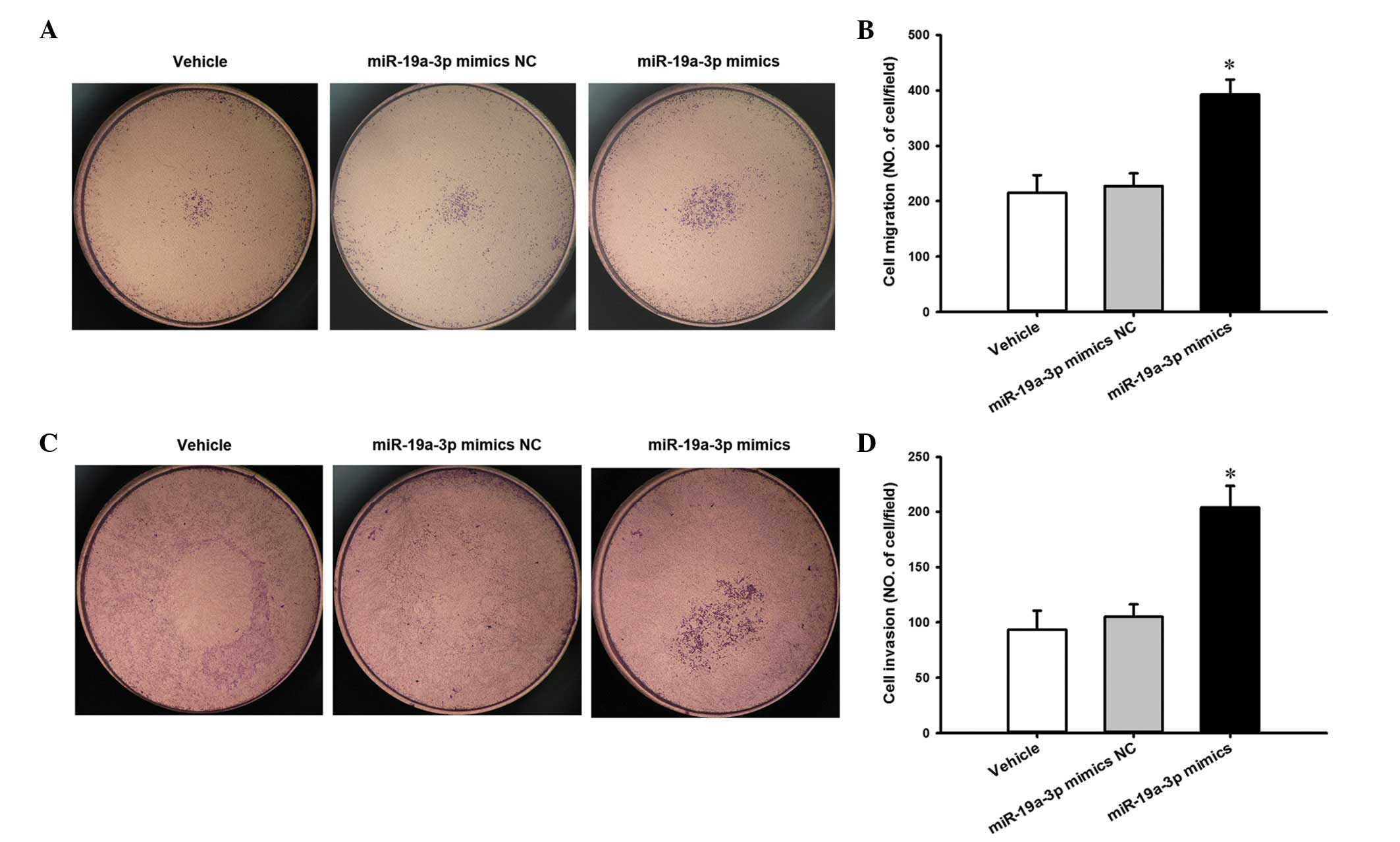
To evaluate the effect of miR-19a-3p overexpression
on the migratory potential of PC3 cells, a Transwell migration
assay was performed in vitro. The results presented in
Fig. 4A and B reveal that
miR-19a-3p overexpression increased the migration of PC3 cells
compared with cells transfected with NC (P<0.05). Similarly, a
Transwell invasion assay was performed to investigate the effect of
miR-19a-3p on the invasive ability of PC3 cells. Compared with the
NC cells, miR-19a-3p overexpression resulted in an increase in the
invasion of PC3 cells (P<0.05; Fig.
4C and D).
PMEPA1 is a direct target of
miR-19a-3p
To explore the potential function of miR-19a-3p, the
current study used bioinformatic analysis to predict putative
miR-19a-3p targets. Targetscan, miRanda and picTar systems
predicted that PMEPA1 was one of the putative targets of
miR-19a-3p. Considering that PMEPA1 is highly expressed in the
prostate and is involved in androgen receptor (AR)-mediated
signaling, which is important during PCa development, the present
study investigated the function of PMEPA1 in PCa progression. To
verify whether miR-19a-3p directly targets PMEPA1 mRNA in PC3
cells, two wild-type (WT) PMEPA1 luciferase reporter constructs,
PMEPA1-WT1 and PMEPA1-WT2, were generated, containing a miR-19a-3p
binding site at nucleotides 359–366 or 611–617 of the PMEPA1
3′-UTR, respectively (Fig. 5A).
Two mutant (Mut) constructs were also generated in which the
binding sites were mutated (Fig.
5B). As demonstrated in Fig.
5C, compared with PMEPA1-WT1 + NC transfection, luciferase
activity was significantly repressed when miR-19a-3p mimics were
co-transfected with PMEPA1-WT1 (P<0.05). However, no effect was
observed upon co-transfection with miR-19a-3 + PMEPA1-WT2 (Fig. 5D). By contrast, co-transfection of
miR-19a-3p mimics + PMEPA1-Mut1 abolished the inhibitory effects of
miR-19a-3p mimics + PMEPA1-WT1, whereas PMEPA1-Mut2 exhibited no
effect.
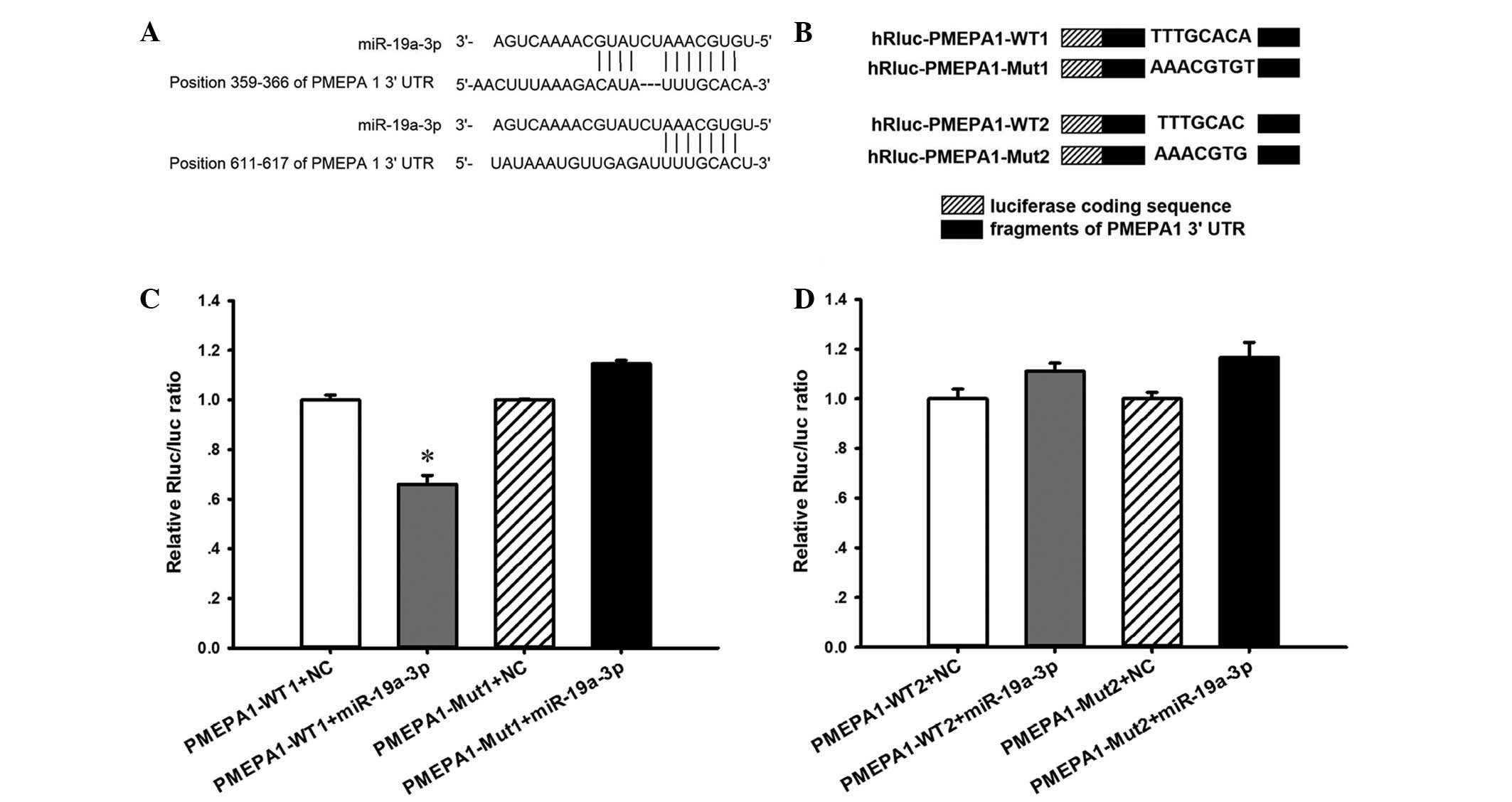 | Figure 5PMEPA1 is a direct target of
miR-19a-3p. (A) Bioinformatic sequencing analysis indicated that
miR-19a-3p potentially targeted the PMEPA1 3′-UTR at nucleotides
359–366 and 611–617. (B) Two PMEPA1 luciferase reporter constructs,
PMEPA1-WT1 and PMEPA1-WT2, were generated, each containing a
fragment harboring the miR-19a-3p target sites of the PMEPA1
3′-UTR. Mutations were also generated on the potential target
sequence, resulting in two Mut constructs, PMEPA1-Mut1 and
PMEPA1-Mut2. PC3 cells were co-transfected with miR-19a-3p mimic
and the luciferase reporter constructs. (C and D) Luciferase
activity was significantly repressed when miR-19a-3p mimics were
co-transfected with PMEPA1-WT1, but not when co-transfected with
PMEPA1-WT2. By contrast, co-transfection of miR-19a-3p mimics and
PMEPA1-Mut1 abolished the inhibitory effects of miR-19a-3p, while
co-transfection with miR-19a-3p mimics and PMEPA1-Mut2 did not.
*P<0.05 vs. PMEPA1-WT1 + NC. Data are presented as
the mean ± standard error. n=3 per group. miR, microRNA; PMEPA1,
prostate transmembrane protein androgen induced 1; UTR,
untranslated region; WT, wild-type; Mut, mutant; Rluc,
Renilla luciferase; NC, negative control. |
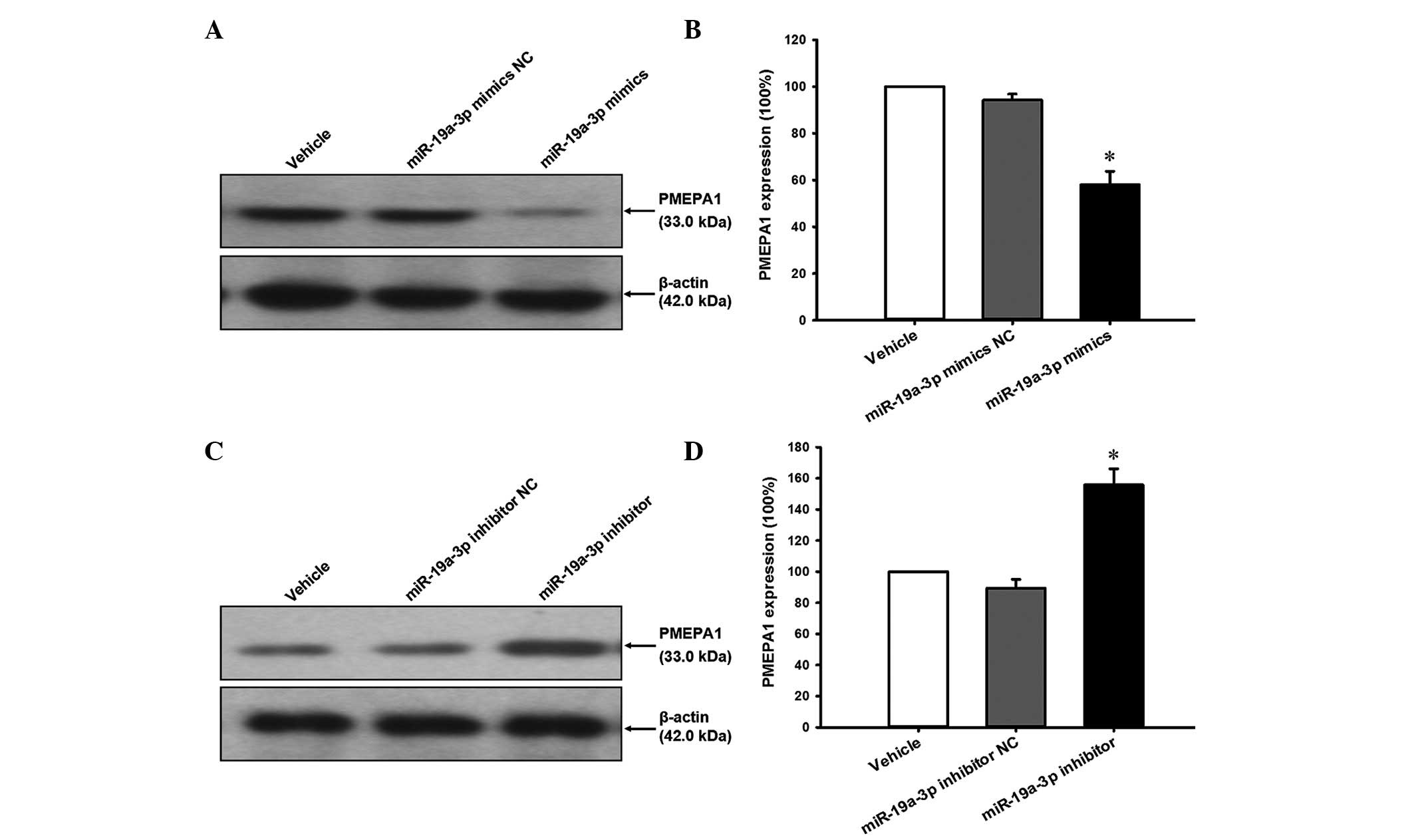
To investigate whether PMEPA1 was repressed by
miR-19a-3p overexpression, cell lysates prepared from cells treated
with the miR-19a-3p mimics or inhibitor were analyzed by western
blotting. The results indicate that the PMEPA1 expression level was
reduced by miR-19a-3p mimics (Fig. 6A
and B) and increased by miR-19a-3p inhibitors (Fig. 6C and D) compared with the NC
transfection (P<0.05).
Confirmation of PMEPA1 mediation of
miR-19a-3p function
To clarify the importance of PMEPA1 in mediating
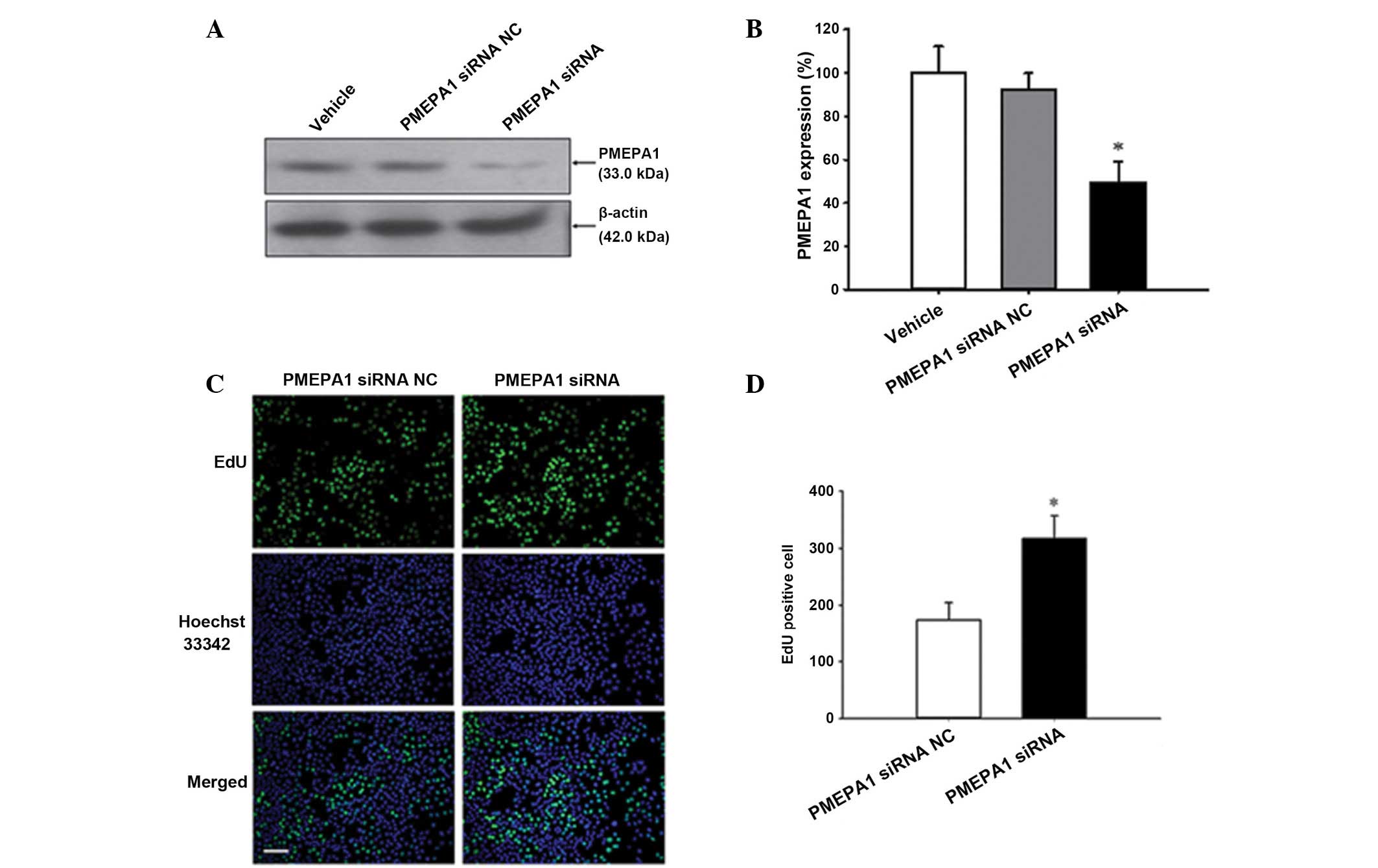
miR-19a-3p functions, PMEPA1 siRNA was transfected into PC3 cells.
Silencing of PMEPA1 expression was confirmed by western blot
analysis (P<0.05; Fig. 7A and
B). Furthermore, the current study examined whether
downregulation of PMEPA1 expression levels mimic the effect of
miR-19a-3p overexpression. Cell proliferation was increased by
PMEPA1 silencing compared with NC transfection (P=0.008; Fig. 7C and D). Additionally, silencing of
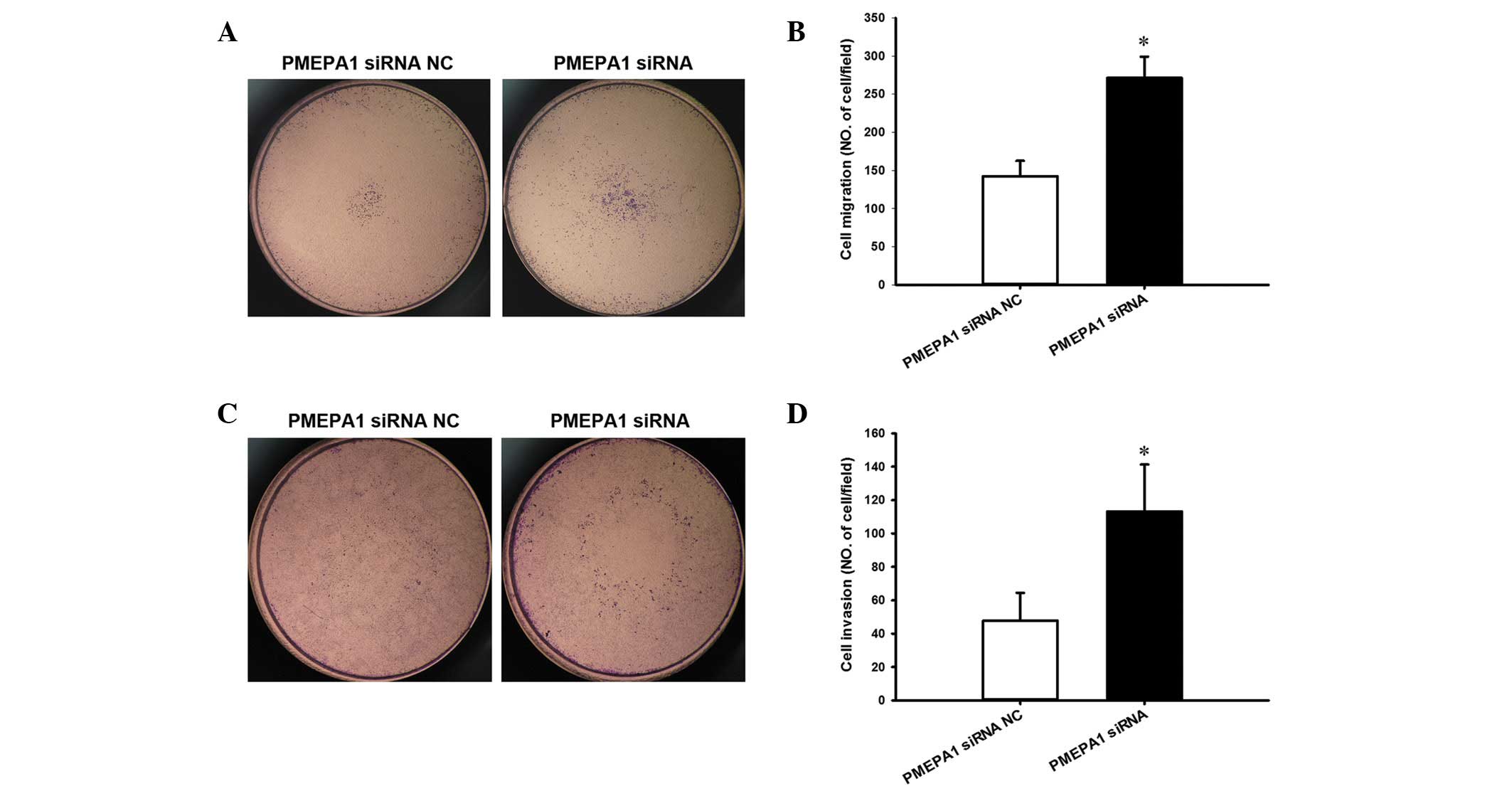
PMEPA1 increased the migration (P=0.003; Fig. 8A and B) and invasion of PC3 cells
compared with NC transfection (P=0.026; Fig. 8C and D), similar to the effect of
miR-19a-3p overexpression.
Discussion
The invasion and metastatic potential of PCa is
highly significant, thus, various methods have been used to manage
PCa; for example, PSA-based screening is increasingly used for PCa
detection at the early, curable stage of the disease. However, as
the prevalence of non-lethal, non-progressive PCa is high,
screening leads to a substantial risk of overdiagnosis and
over-treatment (16). Therefore,
there is a great need for improved diagnostic and predictive
biomarkers to further identify which patients with aggressive
disease require treatment and which can be safely observed.
Dysregulation of numerous miRNAs have been
demonstrated to influence cellular processes associated with
prostate tumorigenesis, including cell proliferation, migration,
invasion, apoptosis and the androgen signaling pathway. In PCa
cells, the expression levels of the miR-17-92 cluster are
upregulated; upregulated miR-17-92 transcripts bind and suppress
E2F transcription factors 1–3, facilitating escape from apoptosis
(17). Additionally, the
expression level of miR-20a was demonstrated to be progressively
increased in benign, low-malignant and high-grade malignant
prostate tissue, respectively, supporting the oncogenic role of
miR-20a in the carcinogenesis of PCa (18). Fuse et al (19) demonstrated that the downregulation
of miR-145 leads to enhanced cell proliferation, migration and
invasion through the direct downregulation of Fascin homolog 1 in
the PC3 and DU145 cell lines. Also, the upregulation or
downregulation of several miRNAs are associated with androgen
signaling in PCa development. For example, Ribas et al
(20) observed that transfection
of LNCaP cells with a miR-21-expressing retrovirus stimulated
androgen-dependent cell growth and rescued cells from
androgen-deficient growth arrest (20). Overexpression of miR-221 and
miR-222 was also demonstrated to attenuate androgen-induced growth
in LNCaP cells and promote androgen-independent growth in
LNCaP-derived castration-resistant PCa cells (21). These previous findings provide
evidence of the importance of miRNAs in PCa development.
miR-19a-3p has been reported to be dysregulated in
various types of cancer. Yang et al (10) reported that miR-19a-3p inhibited
breast cancer progression and metastasis by inducing macrophage
polarization through the downregulation of FOS-related antigen 1
expression levels. Furthermore, exposing MDA-MB-361 breast cancer
cells to multi-fractionated radiation inhibited the expression of
miR-19a-3p (22). In patients with
astrocytoma, high expression of miR-19a-3p was demonstrated to be
significantly associated with poor patient survival (12). Additionally, serum miR-19a-3p
levels may act as a biomarker for the early diagnosis of colorectal
adenocarcinoma (11). Although
abnormal miR-19a-3p expression is frequently observed in various
types of cancer, the importance of miR-19a-3p in the regulation of
PCa remains unclear. Thus, the current study investigated the
expression pattern and functions of miR-19a-3p in PCa specimens and
cell lines. The results obtained in the current study confirmed the
oncogenic effect of miR-19a-3p in PCa.
PMEPA1, also termed TMEPA1, STAG1, ERG1.2 or N4WBP4,
is a subcellular protein localized in the cytoplasm. It was
originally identified as an androgen-induced protein in LNCaP cells
and is highly expressed in prostate epithelial cells. Functional
analysis of PMEPA1 has revealed that it is a neural precursor cell
expressed developmentally dowregulated 4 E3 ubiquitin protein
ligase binding protein and is important for AR downregulation
through a negative feedback loop (23). Several studies have implicated
PMEPA1 in the development of various types of human cancer,
including PCa (24), renal cell
carcinoma, stomach adenocarcinoma (25), and breast, ovarian (26) and colon cancer (27), due to gene amplification, mRNA
expression alterations, and induction by androgens and other growth
factors. Although it is known that PMEPA1 is highly expressed in
the prostate in comparison with other tissues, the role and
mechanism of PMEPA1 in PCa and other types of cancer are not well
characterized. Liu et al (28) reported that the overexpression of
PMEPA1 in PC3 AR-negative cells promotes progression through the
cell cycle and increases cell proliferation via suppression of the
SMAD 3/4-c-Myc-p21 signaling pathway. Additionally, Hirokawa et
al (29) demonstrated that a
high expression level of PMEPA1 can cause tumors to develop at a
increased rate in nude mice injected with a PC3 subclone. However,
cell proliferation is inhibited by PMEPA1 overexpression in PCa
cell lines, suggesting that PMEPA1 downregulation is correlated
with human PCa progression (23).
Accordingly, in the present study, it was observed that when PMEPA1
was downregulated by siRNA, the proliferation, migration and
invasion potential of PC3 cells were enhanced. These contradictory
observations suggest that PMEPA1 may not only be an oncogene in
PCa, it may also act as a tumor suppressor, with its function
dependent on which proteins it interacts with or the pathways it
mediates.
In summary, the results of the present study
elucidated the role of miR-19a-3p in PCa progression. The current
study demonstrated that the levels of miR-19a-3p were upregulated
in vivo and in vitro in PCa. Functional analysis also
revealed that miR-19a-3p overexpression promotes the proliferation,
migration and invasion of PCa cells. Additionally, PMEPA1 was
identified as a direct target of miR-19a-3p and was demonstrated to
partially mediate the effect of miR-19a-3p in tumor metastasis.
Considering that PMEPA1 is an androgen-induced protein, further
investigation is required to determine the regulatory mechanisms of
miR-19a-3p and PMEPA1-associated androgen signaling, and their
importance in mediating the development and progression of PCa.
Acknowledgments
The present study was supported by a grant from the
Beijing Municipal Administration of Hospitals Clinical Medicine
Development of Special Funding Support (no. ZYLX201408).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loblaw DA, Walker- Dilks C, Winquist E and
Hotte SJ; Genitourinary Cancer Disease Site Group of Cancer Care
Ontario's Program in Evidence- Based Care: Systemic therapy in men
with metastatic castration- resistant prostate cancer: A systematic
review. Clin Oncol (R Coll Radiol). 25:406–430. 2013. View Article : Google Scholar
|
|
3
|
Hessels D and Schalken JA: Urinary
biomarkers for prostate cancer: A review. Asian J Androl.
15:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gong AY, Eischeid AN, Xiao J, Zhao J, Chen
D, Wang ZY, Young CY and Chen XM: miR-17-5p targets the
p300/CBP-associated factor and modulates androgen receptor
transcriptional activity in cultured prostate cancer cells. BMC
Cancer. 12:4922012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayashita Y, Osada H, Tatematsu Y, Yamada
H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y and
Takahashi T: A polycistronic microRNA cluster, miR-17-92, is
overexpressed in human lung cancers and enhances cell
proliferation. Cancer Res. 65:9628–9632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He L, Thomson JM, Hemann MT,
Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe
SW, Hannon GJ and Hammond S: A microRNA polycistron as a potential
human oncogene. Nature. 435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tagawa H and Seto M: A microRNA cluster as
a target of genomic amplification in malignant lymphoma. Leukemia.
19:2013–2016. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bosch DA: Stereotactic rostral
mesencephalotomy in cancer pain and deafferentation pain. A series
of 40 cases with follow-up results. J Neurosurg. 75:747–751. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang X, Du WW, Li H, Liu F, Khorshidi A,
Rutnam ZJ and Yang BB: Both mature miR-17-5p and passenger strand
miR-17-3p target TIMP3 and induce prostate tumor growth and
invasion. Nucleic Acids Res. 41:9688–9704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Zhang Z, Chen C, Liu Y, Si Q,
Chuang TH, Li N, Gomez-Cabrero A, Reisfeld RA, Xiang R and Luo Y:
MicroRNA-19a-3p inhibits breast cancer progression and metastasis
by inducing macrophage polarization through downregulated
expression of Fra-1 proto-oncogene. Oncogene. 33:3014–3023. 2014.
View Article : Google Scholar
|
|
11
|
Zheng G, Du L, Yang X, Zhang X, Wang L,
Yang Y, Li J and Wang C: Serum microRNA panel as biomarkers for
early diagnosis of colorectal adenocarcinoma. Br J Cancer.
111:1985–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhi F, Shao N, Wang R, Deng D, Xue L, Wang
Q, Zhang Y, Shi Y, Xia X, Wang S, et al: Identification of 9 serum
microRNAs as potential noninvasive biomarkers of human astrocytoma.
Neuro Oncol. 17:383–391. 2015.
|
|
13
|
Ohori M1, Wheeler TM and Scardino PT: The
New American Joint Committee on Cancer and International Union
Against Cancer TNM classification of prostate cancer.
Clinicopathologic correlations. Cancer. 74:104–114. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Bali KK and Kuner R: Noncoding RNAs: Key
molecules in understanding and treating pain. Trends Mol Med.
20:437–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schröder FH, Hugosson J, Roobol MJ,
Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H,
Zappa M, et al: Screening and prostate-cancer mortality in a
randomized European study. N Engl J Med. 360:1320–1328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sylvestre Y, De Guire V, Querido E,
Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G and Chartrand P:
An E2F/miR-20a autoregulatory feedback loop. J Biol Chem.
282:2135–2143. 2007. View Article : Google Scholar
|
|
18
|
Pesta M, Klecka J, Kulda V, Topolcan O,
Hora M, Eret V, Ludvikova M, Babjuk M, Novak K, Stolz J and Holubec
L: Importance of miR-20a expression in prostate cancer tissue.
Anticancer Res. 30:3579–3583. 2010.PubMed/NCBI
|
|
19
|
Fuse M, Nohata N, Kojima S, Sakamoto S,
Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, Naya Y, Ichikawa T
and Seki N: Restoration of miR-145 expression suppresses cell
proliferation, migration and invasion in prostate cancer by
targeting FSCN1. Int J Oncol. 38:1093–1101. 2011.PubMed/NCBI
|
|
20
|
Ribas J, Ni X, Haffner M, Wentzel EA,
Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J,
Rodriguez R, et al: miR-21: An androgen receptor-regulated microRNA
that promotes hormone-dependent and hormone-independent prostate
cancer growth. Cancer Res. 69:7165–7169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun T, Wang Q, Balk S, Brown M, Lee GS and
Kantoff P: The role of microRNA-221 and microRNA-222 in
androgen-independent prostate cancer cell lines. Cancer Res.
69:3356–3363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leung CM, Chen TW, Li SC, Ho MR, Hu LY,
Liu WS, Wu TT, Hsu PC, Chang HT and Tsai KW: MicroRNA expression
profiles in human breast cancer cells after multifraction and
single-dose radiation treatment. Oncol Rep. 31:2147–2156.
2014.PubMed/NCBI
|
|
23
|
Xu LL, Shi Y, Petrovics G, Sun C, Makarem
M, Zhang W, Sesterhenn IA, McLeod DG, Sun L, Moul JW and Srivastava
S: PMEPA1, an androgen-regulated NEDD4-binding protein, exhibits
cell growth inhibitory function and decreased expression during
prostate cancer progression. Cancer Res. 63:4299–4304.
2003.PubMed/NCBI
|
|
24
|
Ishkanian AS, Mallof CA, Ho J, Meng A,
Albert M, Syed A, van der Kwast T, Milosevic M, Yoshimoto M, Squire
JA, et al: High-resolution array CGH identifies novel regions of
genomic alteration in intermediate-risk prostate cancer. Prostate.
69:1091–1100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rae FK, Hooper JD, Nicol DL and Clements
JA: Characterization of a novel gene, STAG1/PMEPA1, upregulated in
renal cell carcinoma and other solid tumors. Mol Carcinog. 2:44–53.
2001. View
Article : Google Scholar
|
|
26
|
Tanner MM, Tirkkonen M, Kallioniemi A,
Collins C, Stokke T, Karhu R, Kowbel D, Shadravan F, Hintz M, Kuo
WL, et al: Increased copy number at 20q13 in breast cancer:
Defining the critical region and exclusion of candidate genes.
Cancer Res. 54:4257–4260. 1994.PubMed/NCBI
|
|
27
|
Reichling T, Goss KH, Carson DJ, Holdcraft
RW, Ley-Ebert C, Witte D, Aronow BJ and Groden J: Transcriptional
profiles of intestinal tumors in Apc(Min) mice are unique from
those of embryonic intestine and identify novel gene targets
dysregulated in human colorectal tumors. Cancer Res. 65:166–176.
2005.PubMed/NCBI
|
|
28
|
Liu R, Zhou Z, Huang J and Chen C: PMEPA1
promotes androgen receptor-negative prostate cell proliferation
through suppressing the Smad3/4-c-Myc-p21 Cip1 signaling pathway. J
Pathol. 223:683–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirokawa YS, Takagi A, Uchida K, Kozuka Y,
Yoneda M, Watanabe M and Shiraishi T: High level expression of
STAG1/PMEPA1 in an androgen-independent prostate cancer PC3
subclone. Cell Mol Biol Lett. 12:370–377. 2007. View Article : Google Scholar : PubMed/NCBI
|