Introduction
Bladder cancer is the second most common malignant
tumor in the urogenital tract in the USA, and the seventh most
common cancer worldwide (1). In
the USA, there was an estimated 72,500 newly diagnosed cancer cases
and 17,960 cases of cancer-associated mortality in 2013 (2). The most common type of bladder cancer
is comprised of transitional cell carcinoma that arises from
transitional epithelium (3).
Bladder cancer is classified as having either non-muscle-invasive
or muscle-invasive tumors. Upon initial diagnosis, ~75% cases are
classified as non-muscle-invasive and ~25% of cases as
muscle-invasive (4). Patients with
non-muscle-invasive bladder cancer have a high rate of recurrence,
and in certain cases become muscle-invasive (5). Despite the development of various
surgical and chemotherapeutic methods for the treatment of bladder
cancer, it remains a highly prevalent and lethal malignancy
(1). Therefore, there is a
requirement for sensitive and reliable biomarkers, therapeutic
targets and approaches for the treatment of bladder cancer.
Previous studies have demonstrated that microRNAs
(miRNAs) are expressed in numerous types of human cancer (6–8).
miRNAs are a class of small non-coding RNAs whose mature products
are 17–27 nucleotides long (9).
miRNAs negatively regulate protein expression by inducing
degradation or impairing the translation of target mRNAs, by
specifically binding to the 3 prime untranslated region (3′-UTR) of
target mRNAs (10,11). Although the biological functions of
the miRNAs are unknown, previous studies demonstrated that they
serve a role as regulators, involved in all hallmarks of cancer
(12–14). miRNAs may regulate diverse
biological processes, including cell proliferation, development,
differentiation, apoptosis and tumorigenesis of cancer (9). Increasing evidence has indicated that
deregulation of miRNAs may function as either a tumor suppressor or
an oncogene in the tumorigenesis of numerous types of human cancer
(7). Thus, identifying the targets
of the miRNAs is important to understand their function in cancer
development and progression, as miRNAs may be a target for cancer
therapy.
Previous studies demonstrated that the expression of
miR-335 is downregulated in multiple tumor types (15–18).
However, miR-335 expression remains to be investigated in bladder
cancer. The current study demonstrated that miR-335 was
downregulated in tumor tissues from human bladder cancer compared
with their normal adjacent tissues (NATs). Furthermore, results
demonstrated that miR-335 inhibited cell proliferation, migration
and invasion by directly targeting Rho-associated protein kinase 1
(ROCK1). The data of the present study have diagnostic and
therapeutic implications, and may be exploited for the development
of further treatment strategies in bladder cancer.
Materials and methods
Clinical specimens
Bladder cancer and adjacent normal tissues (27
samples) were obtained from patients diagnosed histopathologically
with bladder cancer and who had received radical cystectomy at The
Fourth Affiliated Hospital of Nantong Medical College (Yancheng,
China), between 2008 and 2012. None of the patients had received
other therapies, such as chemotherapy and radiotherapy, prior to
radical cystectomy. All samples were immediately placed in liquid
nitrogen following excision from patients, and were subsequently
frozen at −80°C for RNA extraction. The present study was approved
by the Hospital's Protection of Human Subjects Committee, and
informed consent was obtained from all patients. The
clinicopathological features of the patients are summarized in
Table I.
 | Table ISummary of clinicopathological
features of bladder cancer. |
Table I
Summary of clinicopathological
features of bladder cancer.
| Patient No. | Sex | Age | Grade | Stage |
|---|
| 1 | M | 61 | 2 | T2N0M0 |
| 2 | F | 59 | 1 | T2N0M0 |
| 3 | M | 55 | 1 | T2N0M0 |
| 4 | M | 55 | 3 | T1N0M0 |
| 5 | M | 65 | 2 | T1N0M0 |
| 6 | M | 45 | 3 | T4N0M0 |
| 7 | M | 58 | 1 | T4N0M0 |
| 8 | F | 62 | 2 | T2bN0M0 |
| 9 | M | 62 | 3 | T2N0M0 |
| 10 | M | 77 | 2 | T2N0M0 |
| 11 | M | 82 | 3 | T1N0M0 |
| 12 | F | 69 | 2 | T2bN0M0 |
| 13 | M | 82 | 1 | T4N0M0 |
| 14 | M | 76 | 3 | T1N0M0 |
| 15 | M | 77 | 2 | T1N0M0 |
| 16 | M | 68 | 2 | T4N2M0 |
| 17 | M | 81 | 1 | T2N0M0 |
| 18 | M | 73 | 1 | T1N0M0 |
| 19 | M | 66 | 2 | T1N0M0 |
| 20 | M | 68 | 3 | T3bN0M0 |
| 21 | M | 77 | 1 | TaN0M0 |
| 22 | M | 52 | 3 | T1N0M0 |
| 23 | M | 43 | 2 | T1N0M0 |
| 24 | M | 67 | 2 | T1N0M0 |
| 25 | M | 61 | 1 | T1N0M0 |
| 26 | M | 53 | 3 | T3aN0M0 |
| 27 | F | 64 | 2 | TaN0M0 |
RNA isolation, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's instructions, and RT reactions
were performed using the M-MLV Reverse Transcriptase system
(Promega Corporation, Madison, WI, USA). RT-qPCR was performed
using a standard protocol from the SYBR Green PCR kit (Toyobo Co.,
Ltd., Osaka, Japan). Each sample was analyzed in triplicate. The
primer sequences used were as follows: miR-335, F
5′-TCAAGAGCAATAACGAAAAATGT-3′ and R 5′-GCTGTCAACGATACGCTACGT-3′;
and U6, F 5′-CGCTTCGGCAGCACATATAC-3′ and R
5′-TTCACGAATTTGCGTGTCAT-3′. Data were normalized using the
endogenous U6 snRNA and fold changes were calculated using the
2−ΔΔCq normalization method with the following formula:
∆∆Cq = CqmiR-335-CqU6 (19).
Cell culture
The bladder cancer cell lines, T24 and EJ, were
purchased from the Institute of Biochemistry and Cell Biology
(Shanghai, China). Cells were maintained in Roswell Park Memorial
Institute-1640 medium supplemented with 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.), and incubated at 37°C in a humidified atmosphere
with 5% CO2.
Cell transfection
Mature miR-335 mimics and miRNA negative control
mimics (NC) were obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). For functional analysis, T24 and EJ cells were
seeded into 6-well plates 24 h prior to transfection to ensure
60–70% confluency at the time of transfection. Cells were
transfected with miR-335 mimics or NC using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions.
Cell proliferation assay
Human bladder cancer cells were seeded into 96-well
plates in triplicate at a density of 3×103 cells/well,
24 h subsequent to transfection with miR-335 or NC mimics. Cell
proliferation was assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay at various time points subsequent to transfection. Briefly,
MTT solution (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added
to each well and incubated at 37°C for 4 h. The MTT solution was
then discarded and 200 µl dimethyl sulfoxide was added to
the cells to dissolve the formazan crystals precipitated.
Absorbance was measured at a wavelength of 490 nm using an
enzyme-linked immunosorbent assay reader (Elx800; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All experiments were
performed in triplicate. The suppression rate was calculated using
the following formula: Suppression rate = [1-optical density (OD)
miR-335/ODmiR-NC] × 100%.
Cell migration and invasion assay
Corning Costar transwell chambers (Thermo Fisher
Scientific, Inc.) with 8 µm pore size polycarbonate membrane
were used to assess cell migration and invasion. For the invasion
assay, the membrane was pre-coated with Matrigel (BD Biosciences,
San Jose, CA, USA). Transfected cells (5×104) were
seeded on the upper chamber with serum-free medium. A volume of 0.5
ml of 20% FBS-containing medium was added to the lower chamber as a
chemoattractant. Cells were then incubated for another 12 h for the
migration assay and 24 h for the invasion assay. The membranes were
stained with 0.1% crystal violet (Beyotime Institute of
Biotechnology, Haimen, China), and five visual fields of ×200
magnification of each membrane were randomly selected for cell
counting under an inverted CKX41 microscope (Olympus Corporation,
Tokyo, Japan).
Western blot analysis
Subsequent to a 72-h transfection with miR-335 or NC
mimics, human bladder cancer cells were lysed in ice-cold
radioimmunoprecipitation assay lysis buffer containing 1 mM
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology). Lysates were centrifuged at 12,000 × g for 40 min
at 4°C, and the protein sample was diluted, heated for
denaturation, and then subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis for 20 min at 70 V and
transferred to a polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA) for 70 min at 110 V. Membranes were then
incubated with rabbit anti-human polyclonal anti-ROCK1 (cat no.
4035S; 1:1,000) or anti-β-actin (cat no. 4970L 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) primary antibodies
overnight. Membranes were rinsed in Tris-buffered saline containing
0.05% Tween 20 (Beyotime Institute of Biotechnology) three times,
and then incubated for 2 h with the corresponding goat anti-rabbit
horseradish peroxidase conjugated secondary antibody (sc-2054;
1:10,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Proteins were visualized with an ECL kit (Pierce Biotechnology,
Inc., Rockford, IL, USA) and analyzed using Quantity One 1-D
analysis software (version 4.62; Bio-Rad Laboratories, Inc.).
Luciferase assay
Human bladder cancer cells were seeded into a
12-well plate at ~90% confluency and transfected with the reporter
plasmid, miR-335 or NC mimics. The Renilla and firefly luciferase
activity were measured 48 h subsequent to transfection with the
Dual-Luciferase Reporter Assay System (Promega Corporation) and a
luminometer (Infinite 200 PRO NanoQuant; Tecan Group Ltd.,
Männedorf, Switzerland). The firefly luciferase activity was
normalized to the renilla luciferase activity for each transfected
well. All experiments were performed in triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation,
and were analyzed using SPSS software, version 13.0 (SPSS, Inc.,
Chicago, IL, USA). Data were analyzed using the Chi-square test.
Kaplan-Meier curves were constructed, and the log-rank test was
performed for analysis of survival data. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-335 expression in bladder cancer
tissues and association with clinicopathological factors
A total of 27 bladder cancer samples were included
in the present study. As demonstrated in Fig. 1, miR-335 was significantly
downregulated in bladder cancer tissues compared with the NAT
(P<0.01). These results suggested that miR-335 may serve an
important role in human bladder cancer.
miR-335 suppressed cell proliferation in
the T24 and EJ cells
The MTT assay was conducted to investigate whether
miR-335 has a biological function in cell proliferation. As
demonstrated in Fig. 2,
upregulation of miR-335 markedly inhibited cell proliferation
compared with the NC. The results indicated that after a 120-h
treatment, the suppression rate of miR-335 reached 38.98±4.5% in
T24 cells and 24.97±4.9% in EJ cells (P<0.05; Fig. 2).
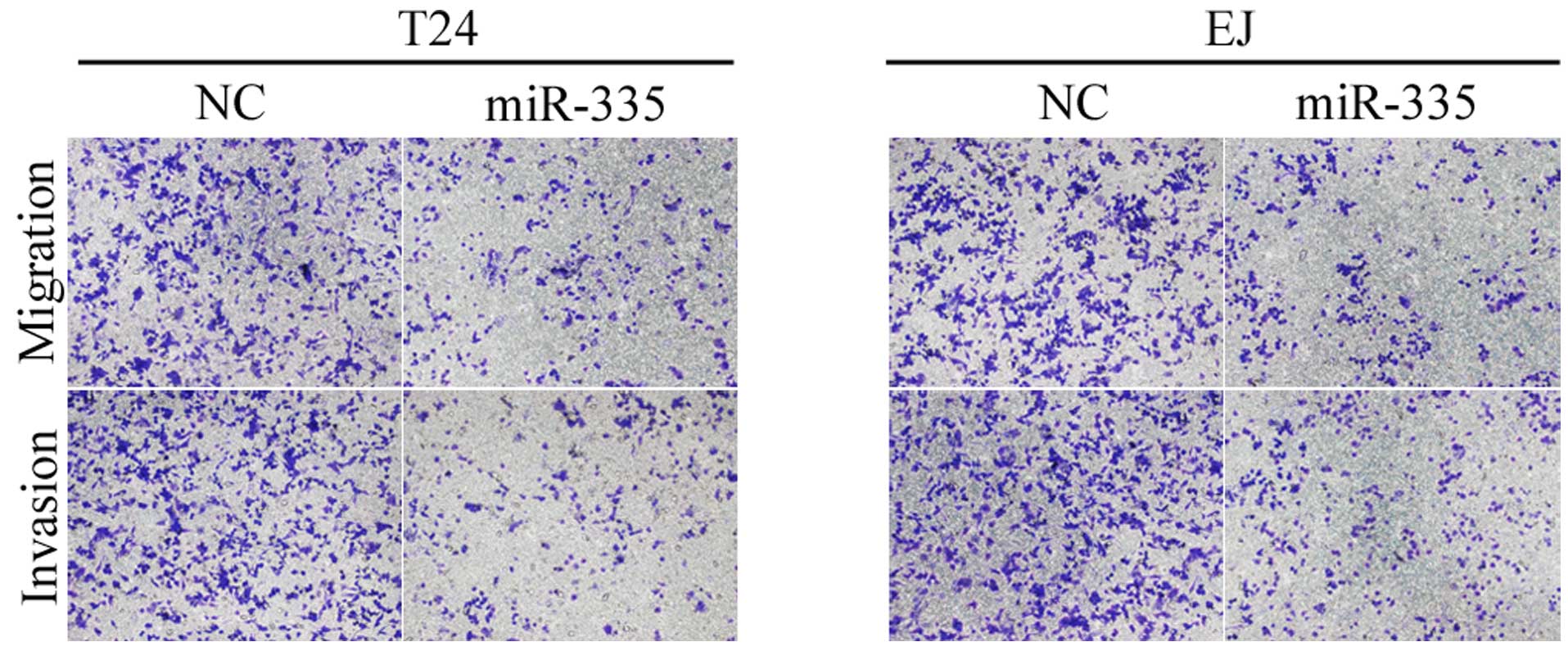
miR-335 suppressed cell migration and
invasion in the T24 and EJ cells
Taking the fact that T24 and EJ cells are metastatic
cells into consideration, it was investigated whether a reduction
in miR-335 had an effect on the capacities of cells to migrate and
invade. As demonstrated in Fig. 3,
the migratory and invasive capacities of T24 and EJ cells
transfected with miR-335 were markedly reduced compared with the
NC. These results indicated that miR-335 reduced cell migration and
invasion in the bladder cancer cells.
ROCK1 is a direct target gene of miR-335
in the T24 and EJ cells
To identify the target gene of miR-335, a public
database (TargetScan; http://www.targetscan.org) was used, and ROCK1 was
predicted to be a target of miR-335 (Fig. 4A). To verify whether miR-335
directly targeted ROCK1, luciferase reporter assays were performed.
As demonstrated in Fig. 4B,
miR-335 significantly inhibited the wild type, and not the mutated
luciferase activity of ROCK1 in the T24 and EJ cells (P<0.05).
Furthermore, western blot analysis was conducted to investigate
whether ROCK1 protein expression levels were reduced following
transfection of miR-335 in the T24 and EJ cells. As demonstrated in
Fig. 4C, ROCK1 protein expression
levels were significantly downregulated following transfection of
miR-335 (P<0.05). The results indicated that ROCK1 is a direct
target gene of miR-335 in the bladder cancer T24 and EJ cells.
 | Figure 4(A) Targetscan assessment indicated
that ROCK1 mRNA contained a miR-335 seven-nucleotide seed match at
position 472–478 of the ROCK1 3′-UTR. (B) ROCK1 may be a direct
target of miR-335 in vitro. Overexpression of miR-335
significantly inhibited the WT, however not the Mut, luciferase
activity of ROCK1 in T24 and EJ cells. *P<0.05 vs.
NC. (C) Downregulation of ROCK1 was confirmed by western blotting,
following transfection, β-actin was used as the control.
*P<0.05 vs. NC. 3′-UTR, 3 prime untranslated region;
hsa-miR-335, human microRNA-335; NC, negative control; ROCK1,
rho-associated protein kinase 1; WT, wild type; Mut, mutated. |
Discussion
The aberrant expression of miRNAs in bladder cancer
has been investigated previously (20). Certain miRNAs were indicated to be
upregulated in bladder cancer tissues and may function as tumor
oncogenes by negatively regulating tumor suppressors (21). miRNAs are small, stable, easy to
deliver and be detected, thus, their abnormal expression may
certify them as potential biomarkers and novel targets for bladder
cancer therapy (22). However,
further studies are required to address the potential diagnostic
and therapeutic roles of these miRNAs in bladder cancer, and
whether this may be beneficial for the treatment of bladder
cancer.
Aberrant expression of miR-335 has been demonstrated
in numerous types of tumor, suggesting a complex role during
tumorigenesis. Previous studies have demonstrated that it is
downregulated in osteosarcoma (15), gastric cancer (16), small cell lung cancer (17), breast cancer (18), ovarian cancer (23), clear cell renal cell carcinoma
(24), hepatocellular carcinoma
(25) and prostate cancer
(26). Furthermore, upregulation
of miR-335 has been demonstrated in colorectal cancer (27), glioma (28) and myeloma (29). However, the expression, function
and mechanism of miR-335 in human bladder cancer remains to be
elucidated. The present study demonstrated that miR-335 was
downregulated in human bladder cancer tissues compared with NATs,
suggesting that miR-335 may have a tumor suppressive role in
bladder cancer development and progression.
Identification of the miR-335 target genes is
important for understanding its role in tumorigenesis, and defining
novel therapeutic targets. Thus far, dishevelled associated
activator of morphogenesis 1 (30), paired box 6 (31,32),
ROCK1 (15), retinoblastoma 1
(33), zinc finger E-box binding
homeobox 2 (34), met
proto-oncogene (35) and OCT4
(36) have been identified as
targets of miR-335. The current study demonstrated that miR-335
transfection resulted in reduced cell proliferation, migration and
invasion in bladder cancer T24 and EJ cells by targeting ROCK1.
These results are in agreement with previous studies in human
osteosarcoma (15). The results of
the present study suggested that miR-335 may be used for the
development of novel molecular markers and therapeutic approaches
to inhibit the metastasis of bladder cancer.
The Rho guanosine triphosphate (GTP)ase family
consists of small proteins that bind to and hydrolyze GTP, and
control a wide variety of cellular processes, such as cell
motility, proliferation, adhesion, differentiation and apoptosis
(37). ROCK is one of the best
characterized Rho effectors (38).
The two ROCK isoforms, ROCK1 and ROCK2, share 65% identity in
amino-acid sequence and 92% identity in their kinase domains, and
display certain similarities in the kinase activity domain
(39). Upregulation of ROCK
promotes invasion and metastasis in numerous solid tumors, such as
bladder (40), hepatocellular
(41), breast (42) and colon cancers (43). In addition, overexpression of ROCK1
has been associated with the progression of bladder cancer
(40). This is consistent with the
results of the present study which demonstrated that exogenetic
overexpression of miR-335 inhibited the migration and invasion of
human bladder cancer cells. These observations provide the evidence
that miR-335 has an effect on cell migration and invasion via
regulating the expression of ROCK1.
Human ROCK1 maps to chromosome 18 (18q11.1)
(44). Previous studies
demonstrated that ROCK1 functioned as an oncogene and was regulated
by a number of miRNAs in human cancer. An et al (45) and Xu et al (46) demonstrated that miR-124 inhibited
cell migration and invasion via directly targeting ROCK1 in bladder
cancer and glioma. In gastric cancer, miR-148a suppressed cell
metastasis by downregulating the expression of ROCK1 (47). ROCK1 was also targeted in other
types of human cancer, including miR-335 in neuroblastoma (48), miR-584 in renal cell carcinoma
(49) and miR-146a in prostate
cancer (50). The current study
demonstrated that miR-335 inhibited bladder cancer cell
proliferation and motility by downregulation of ROCK1. This may be
further investigated as a predictive value for early detection of
tumor recurrence and target therapy drugs to prevent bladder cancer
from becoming invasive.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that miR-335 is downregulated in
bladder cancer. The current study provided evidence that miR-335
suppressed cell proliferation, migration and invasion by directly
targeting ROCK1 in bladder cancer. Identifying the candidate target
genes of miR-335 may provide an understanding of potential
carcinogenic mechanisms in bladder cancer. These observations
indicated that miR-335 may serve an important role as a diagnostic
and prognostic marker in bladder cancer, and may be exploited for
further treatment of bladder cancer.
Further research is required to identify the full
potential of miR-335 in cancer treatment, and its benefits in the
treatment of bladder cancer.
References
|
1
|
Hendricksen K and Witjes JA: Current
strategies for first and second line intravesical therapy for
nonmuscle invasive bladder cancer. Curr Opin Urol. 17:352–357.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pasin E, Josephson DY, Mitra AP, Cote RJ
and Stein JP: Superficial bladder cancer: an update on etiology,
molecular development, classification, and natural history. Rev
Urol. 10:31–43. 2008.PubMed/NCBI
|
|
4
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Bohle A, Palou-Redorta J and Roupret M; European
Association of Urology: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder, the 2011 update. Eur Urol.
59:997–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luke C, Tracey E, Stapleton A and Roder D:
Exploring contrary trends in bladder cancer incidence, mortality
and survival: implications for research and cancer control. Intern
Med J. 40:357–362. 2010. View Article : Google Scholar
|
|
6
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu D, Zhou Y, Pan H, Zhou J, Fan Y and Qu
P: microRNA-99a inhibiting cell proliferation, migration and
invasion by targeting fibroblast growth factor receptor 3 in
bladder cancer. Oncol Lett. 7:1219–1224. 2014.PubMed/NCBI
|
|
8
|
Zheng K, Liu W, Liu Y, Jiang C and Qian Q:
MicroRNA-133a suppresses colorectal cancer cell invasion by
targeting Fascin1. Oncol Lett. 9:869–874. 2015.PubMed/NCBI
|
|
9
|
Liu LY, Wang W, Zhao LY, Guo B, Yang J,
Zhao XG, Hou N, Ni L, Wang AY, Song TS, et al: Mir-126 inhibits
growth of SGC-7901 cells by synergistically targeting the oncogenes
PI3KR2 and Crk, and the tumor suppressor PLK2. Int J Oncol.
45:1257–1265. 2014.PubMed/NCBI
|
|
10
|
Guancial EA, Bellmunt J, Yeh S, Rosenberg
JE and Berman DM: The evolving understanding of microRNA in bladder
cancer. Urol Oncol. 32:41 e31–40. 2014. View Article : Google Scholar
|
|
11
|
Yoshino H, Seki N, Itesako T, Chiyomaru T,
Nakagawa M and Enokida H: Aberrant expression of microRNAs in
bladder cancer. Nat Rev Urol. 10:396–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naito Y, Yasuno K, Tagawa H, Sakamoto N,
Oue N, Yashiro M, Sentani K, Goto K, Shinmei S, Oo HZ, et al:
MicroRNA-145 is a potential prognostic factor of scirrhous type
gastric cancer. Oncol Rep. 32:1720–1726. 2014.PubMed/NCBI
|
|
14
|
Liu Z, Xu Y, Long J, Guo K, Ge C and Du R:
microRNA-218 suppresses the proliferation, invasion and promotes
apoptosis of pancreatic cancer cells by targeting HMGB1. Chin J
Cancer Res. 27:247–257. 2015.PubMed/NCBI
|
|
15
|
Wang Y, Zhao W and Fu Q: miR-335
suppresses migration and invasion by targeting ROCK1 in
osteosarcoma cells. Mol Cell Biochem. 384:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae IH, Park MJ, Yoon SH, Kang SW, Lee SS,
Choi KM and Um HD: Bcl-w promotes gastric cancer cell invasion by
inducing matrix metalloproteinase-2 expression via phosphoinositide
3-kinase, Akt and Sp1. Cancer Res. 66:4991–4995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong M, Ma J, Guillemette R, Zhou M, Yang
Y, Yang Y, Hock JM and Yu X: miR-335 inhibits small cell lung
cancer bone metastases via IGF-IR and RANKL pathways. Mol Cancer
Res. 12:101–110. 2014. View Article : Google Scholar
|
|
18
|
Tavazoie SF, Alarcon C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massague J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Bo L, Zhao X and Chen Q:
MicroRNA-133a inhibits cell proliferation, colony formation
ability, migration and invasion by targeting matrix
metallopeptidase 9 in hepatocellular carcinoma. Mol Med Rep.
11:3900–3907. 2015.PubMed/NCBI
|
|
20
|
Zabolotneva AA, Zhavoronkov A, Garazha AV,
Roumiantsev SA and Buzdin AA: Characteristic patterns of microRNA
expression in human bladder cancer. Front Genet. 3:3102012.
|
|
21
|
Wu D, Ding J, Wang L, Pan H, Zhou Z, Zhou
J and Qu P: microRNA-125b inhibits cell migration and invasion by
targeting matrix metallopeptidase 13 in bladder cancer. Oncol Lett.
5:829–834. 2013.PubMed/NCBI
|
|
22
|
Feng Y, Kang Y, He Y, Liu J, Liang B, Yang
P and Yu Z: microRNA-99a acts as a tumor suppressor and is
down-regulated in bladder cancer. BMC Urol. 14:502014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao J, Cai J, Huang D, Han Q, Yang Q, Li
T, Ding H and Wang Z: miR-335 represents an invasion suppressor
gene in ovarian cancer by targeting Bcl-w. Oncol Rep. 30:701–706.
2013.PubMed/NCBI
|
|
24
|
White NM, Bao TT, Grigull J, Youssef M,
Girgis A, Diamandis M, Fatoohi E, Metias M, Honey JR, Stewart R, et
al: miRNA profiling for clear cell renal cell carcinoma: biomarker
discovery and identification of potential controls and consequences
of miRNA dysregulation. J Urol. 186:1077–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dohi O, Yasui K, Gen Y, Takada H, Endo M,
Tsuji K, Konishi C, Yamada N, Mitsuyoshi H, Yagi N, et al:
Epigenetic silencing of miR-335 and its host gene MEST in
hepatocellular carcinoma. Int J Oncol. 42:411–418. 2013.
|
|
26
|
Wang L, Alcon A, Yuan H, Ho J, Li QJ and
Martins-Green M: Cellular and molecular mechanisms of pomegranate
juice-induced anti-metastatic effect on prostate cancer cells.
Integr Biol (Camb). 3:742–754. 2011. View Article : Google Scholar
|
|
27
|
Vickers MM, Bar J, Gorn-Hondermann I,
Yarom N, Daneshmand M, Hanson JE, Addison CL, Asmis TR, Jonker DJ,
Maroun J, et al: Stage-dependent differential expression of
microRNAs in colorectal cancer: potential role as markers of
metastatic disease. Clin Exp Metastasis. 29:123–132. 2012.
View Article : Google Scholar
|
|
28
|
Shu M, Zhou Y, Zhu W, Zhang H, Wu S, Chen
J and Yan G: MicroRNA 335 is required for differentiation of
malignant glioma cells induced by activation of cAMP/protein kinase
A pathway. Mol Pharmacol. 81:292–298. 2012. View Article : Google Scholar
|
|
29
|
Ronchetti D, Lionetti M, Mosca L, Agnelli
L, Andronache A, Fabris S, Deliliers GL and Neri A: An integrative
genomic approach reveals coordinated expression of intronic
miR-335, miR-342, and miR-561 with deregulated host genes in
multiple myeloma. BMC Med Genomics. 1:372008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shu M, Zheng X, Wu S, Lu H, Leng T, Zhu W,
Zhou Y, Ou Y, Lin X, Lin Y, Xu D, et al: Targeting oncogenic
miR-335 inhibits growth and invasion of malignant astrocytoma
cells. Mol Cancer. 10:592011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng Q, Cao H, Chen Z, Ma Z, Wan X, Peng
R and Jiang B: PAX6, a novel target of miR-335, inhibits cell
proliferation and invasion in glioma cells. Mol Med Rep.
10:399–404. 2014.PubMed/NCBI
|
|
32
|
Meng Y, Zou Q, Liu T, Cai X, Huang Y and
Pan J: microRNA-335 inhibits proliferation, cell-cycle progression,
colony formation, and invasion via targeting PAX6 in breast cancer
cells. Mol Med Rep. 11:379–385. 2015.
|
|
33
|
Shi L, Jiang D, Sun G, Wan Y, Zhang S,
Zeng Y, Pan T and Wang Z: miR-335 promotes cell proliferation by
directly targeting Rb1 in meningiomas. J Neurooncol. 110:155–162.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun Z, Zhang Z, Liu Z, Qiu B, Liu K and
Dong G: MicroRNA-335 inhibits invasion and metastasis of colorectal
cancer by targeting ZEB2. Med Oncol. 31:9822014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao Y, Zeng F, Wu JY, Li HY, Fan JJ, Mai
L, Zhang J, Ma DM, Li Y and Song FZ: MiR-335 inhibits migration of
breast cancer cells through targeting oncoprotein c-Met. Tumour
Biol. 36:2875–2883. 2015. View Article : Google Scholar
|
|
36
|
Gao L, Yang Y, Xu H, Liu R, Li D, Hong H,
Qin M and Wang Y: MiR-335 functions as a tumor suppressor in
pancreatic cancer by targeting OCT4. Tumour Biol. 35:8309–8318.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsui T, Amano M, Yamamoto T, Chihara K,
Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A and Kaibuchi K:
Rho-associated kinase, a novel serine/threonine kinase, as a
putative target for small GTP binding protein Rho. EMBO J.
15:2208–2216. 1996.PubMed/NCBI
|
|
39
|
Montalvo J, Spencer C, Hackathorn A,
Masterjohn K, Perkins A, Doty C, Arumugam A, Ongusaha PP,
Lakshmanaswamy R, Liao JK, et al: ROCK1&2 perform overlapping
and unique roles in angiogenesis and angiosarcoma tumor
progression. Curr Mol Med. 13:205–219. 2013. View Article : Google Scholar :
|
|
40
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
41
|
Xue F, Takahara T, Yata Y, Xia Q, Nonome
K, Shinno E, Kanayama M, Takahara S and Sugiyama T: Blockade of
Rho/Rho-associated coiled coil-forming kinase signaling can prevent
progression of hepatocellular carcinoma in matrix
metalloproteinase-dependent manner. Hepatol Res. 38:810–817. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lane J, Martin TA, Watkins G, Mansel RE
and Jiang WG: The expression and prognostic value of ROCK I and
ROCK II and their role in human breast cancer. Int J Oncol.
33:585–593. 2008.PubMed/NCBI
|
|
43
|
Vishnubhotla R, Sun S, Huq J, Bulic M,
Ramesh A, Guzman G, Cho M and Glover SC: ROCK-II mediates colon
cancer invasion via regulation of MMP-2 and MMP-13 at the site of
invadopodia as revealed by multiphoton imaging. Lab Invest.
87:1149–1158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lock FE, Ryan KR, Poulter NS, Parsons M
and Hotchin NA: Differential regulation of adhesion complex
turnover by ROCK1 and ROCK2. PLoS One. 7:e314232012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
An L, Liu Y, Wu A and Guan Y: microRNA-124
inhibits migration and invasion by down-regulating ROCK1 in glioma.
PLoS One. 8:e694782013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu X, Li S, Lin Y, Chen H, Hu Z, Mao Y, Xu
X, Wu J, Zhu Y, Zheng X, et al: MicroRNA-124-3p inhibits cell
migration and invasion in bladder cancer cells by targeting ROCK1.
J Transl Med. 11:2762013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng B, Liang L, Wang C, Huang S, Cao X,
Zha R, Liu L, Jia D, Tian Q, Wu J, et al: MicroRNA-148a suppresses
tumor cell invasion and metastasis by downregulating ROCK1 in
gastric cancer. Clin Cancer Res. 17:7574–7583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lynch J, Fay J, Meehan M, Bryan K, Watters
KM, Murphy DM and Stallings RL: MiRNA-335 suppresses neuroblastoma
cell invasiveness by direct targeting of multiple genes from the
non-canonical TGF-beta signalling pathway. Carcinogenesis.
33:976–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ueno K, Hirata H, Shahryari V, Chen Y,
Zaman MS, Singh K, Tabatabai ZL, Hinoda Y and Dahiya R: Tumour
suppressor microRNA-584 directly targets oncogene Rock-1 and
decreases invasion ability in human clear cell renal cell
carcinoma. Br J Cancer. 104:308–315. 2011. View Article : Google Scholar :
|
|
50
|
Lin SL, Chiang A, Chang D and Ying SY:
Loss of mir-146a function in hormone-refractory prostate cancer.
RNA. 14:417–424. 2008. View Article : Google Scholar : PubMed/NCBI
|