Introduction
Oxidative stress is important in mediating brain
injury caused by different pathological conditions, such as
cerebral ischemia/reperfusion, head trauma, epilepsy and
neurodegenerative diseases (1–4). A
previous study demonstrated that neurons, glial cells, and cerebral
microvasculature could be damaged during the process of oxidative
stress (5). The crucial feature of
oxidative stress is excessive production of reactive oxygen species
(ROS), such as superoxide anions (O2−),
hydroxyl radicals and hydrogen peroxide
(H2O2), which are regarded as being the
result of disrupted equilibrium between their formation and
clearance (5). ROS may attack
intracellular biological macromolecules, including nuclear acids,
proteins and lipids, induce dysfunction of mitochondria, and
activate signaling pathways leading to apoptosis (6). Among the various ROS,
H2O2 easily diffuses in and out of cells and
tissues (6), and is readily
converted into highly reactive hydroxyl radicals by the Fenton
reaction (7). Thus,
H2O2 has been considered to be an appropriate
molecule to induce oxidative stress and is extensively used to
investigate the protective effect of certain agents on cellular
injury (8).
Lycopene, a lipid-soluble carotenoid compound, is
often present in tomatoes and red fruits, including watermelon,
pink grapefruit and guava (9).
Although it has multiple biological functions, such as inhibition
of inflammation, and suppression of cellular carcinogenesis and
tumor growth (10,11), accumulating evidence has indicated
that lycopene exerts a strong protective effect against brain
damage. A population-based follow-up study demonstrated that males
in the highest quartile of serum lycopene concentrations exhibited
59 and 55% lower risks of ischemic stroke when compared with males
in the lowest quartile (12).
Furthermore, animal experiments using rat models demonstrated that
lycopene prevents brain injury caused by focal or global ischemia
and reperfusion (13,14), and alleviates cognition dysfunction
induced by colchicine and rote-none (15,16).
In vitro research indicated that lycopene protects against
neuronal apoptosis induced by different neurotoxic compounds,
including 1-methyl-4-phenylpyridinium (MPP+),
methylmercury, amyloid β, trimethyltin and 6-hydroxydopamine
(17–21). Despite previous data, which
revealed that lycopene possesses a potent antioxidant capacity
(22) and inhibition of oxidative
stress is the common mechanism responsible for its neuroprotection,
the effect of lycopene on activation of neuronal apoptosis pathways
remains unclear. SH-SY5Y cells are human neuroblastoma cells, which
are comparable to neurons with regards to their morphological,
neurochemical and electrophysiological properties and have been
extensively applied to evaluate neuronal injury or death in
neurodegenerative disease, cerebral ischemia/reperfusion and
epilepsy (23–25). Therefore, the present study used
H2O2-induced apoptosis in SH-SY5Y cells as a
model to investigate the effect of lycopene on the activation of
apoptotic pathways induced by oxidative stress.
Materials and methods
Drugs and chemicals
Lycopene was purchased from Sigma-Aldrich (St.
Louis, MO, USA) and was dissolved in tetrahydrofuran
(Sigma-Aldrich) prior to each experiment.
H2O2 was purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China). Superoxide Dismutase
(SOD) activity and Catalase assay kits were obtained from BioVision
Inc. (Milpitas, CA, USA). A lactate dehydrogenase (LDH) assay was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
Rabbit anti-caspase-3 polyclonal antibody (ab44976), rabbit
anti-apoptosis inducing factor (AIF) polyclonal antibody ab1998)
and mouse anti-β-actin monoclonal antibody (ab8226) were purchased
from Abcam (Cambridge, MA, USA). Rabbit anti-Bcl-2-like protein 4
(Bax) polyclonal antibody (cat. no. 2774) and rabbit anti-B-cell
lymphoma 2 (Bcl-2; cat. no. 4223), rabbit anti-lamin B1 (cat. no.
13435), rabbit anti-cytochrome c (Cyt c; cat. no.
4280) and mouse anti-cytochrome c oxidase IV (COX IV; cat.
no. 11967) monoclonal antibodies were from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin (Ig) G and horse
anti-mouse IgG were from Cell Signaling Technology, Inc. (cat. nos.
7074 and 7076, respectively). Enhanced chemiluminescence (ECL)
western blotting detection reagents were purchased from GE
Healthcare Life Sciences, and polyvinylidene difluoride (PVDF)
membranes were from EMD Millipore (Billerica, MA, USA).
Cell culture and groups
Human SH-SY5Y neuroblastoma cells were obtained from
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum, 2
mmol/l glutamine (Sigma-Aldrich), 100 U/ml penicillin and 100
µg/ml streptomycin (Sigma-Aldrich), and maintained in a
humid environment at 37°C and 5% CO2 atmosphere. The
medium was replaced twice per week.
The cells were randomized to the following groups:
Control (normal SH-SYSY cells), lycopene (the cells were treated
only with lycopene at the indicated concentrations),
H2O2 (the cells were treated alone with
H2O2 at indicated concentration), and
H2O2 + lycopene (the cells were treated for 2
h with lycopene followed by a 24-h incubation with
H2O2).
Cellular viability assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay (Sigma-Aldrich) was performed to determine cell
viability. SH-SY5Y cells were seeded at a density of
4×104 cells per well on collagen-coated 96-well plates
for 24 h. After being treated as described in each experimental
group, the absorbance value at 570 nm was read using an automatic
multi-well spectrophotometer (xMark™; Bio-Rad Laboratories, Inc.,
Richmond, CA, USA). The results are expressed as the percentage of
MTT reduction relative to the absorbance of control cells.
LDH release assay
SH-SY5Y cells were seeded into 96-well culture
plates at a density of 4×104 cells/well. The cells, with
or without lycopene pretreatment, were incubated with 400
µmol/l H2O2 for 24 h. According to the
manufacturer's instructions, the supernatant from each sample was
used in the LDH assay. The LDH activity was measured by monitoring
the reduction of pyruvic acid. The absorbance of each sample was
determined at 490 nm with a microplate reader (xMark™). LDH release
was expressed as the percentage (%) of the total LDH activity (LDH
in the medium + LDH in the cell), according to the following
equation: % LDH release = (LDH activity in the medium / total LDH
activity) ×100.
Detection of apoptosis by flow
cytometry
Apoptosis was examined by analysis of DNA
fragmentation using flow cytometry. After a 24-h incubation with
400 µmol/l H2O2 following a 2-h
treatment with or without lycopene, SH-SY5Y cells were collected by
0.25% trypsin (Sigma-Aldrich) and washed once with
phosphate-buffered saline (PBS). The cells were then fixed in 70%
ethanol at 4°C overnight, treated with 100 mg/l RNase
(Sigma-Aldrich) at 37°C for 30 min and stained with 50 mg/l
propidium iodide (Sigma-Aldrich) for 30 min. Finally, the cells
were analyzed using flow cytometry (FACScan; BD Biosciences, San
Jose, CA, USA). The rate of apoptosis was analyzed using CellQuest
software, version 5.1 (BD Biosciences). Data acquisition was
conducted by collecting 20,000 cells per tube, and the numbers of
viable and apoptotic cells were determined for each experimental
condition.
Transmission electron microscopy
SH-SY5Y cells from the control group and 400
µmol/l H2O2 group were harvested using
0.25% trypsin, washed with PBS, collected by centrifugation for 10
min at 1,500 × g and treated as previously described by Watkins and
Cullen (26). Briefly, the cells
were fixed in ice-cold 2.5% glutaraldehyde (Sigma-Aldrich) in PBS
(pH 7.3), rinsed with PBS, post-fixed in 1% osmium tetroxide
(Sigma-Aldrich) with 0.1% potassium ferricyanide (Sigma-Aldrich),
dehydrated through a graded series of ethanol (30–90%) and embedded
in Epon (Energy Beam Sciences, Agawam, MA, USA). Semi-thin (300 nm)
sections were sliced using a Reichart Ultracut E Ultra-Microtome
(Leica Microsystems, Inc., Buffalo Grove, IL, USA), stained with
0.5% Toluidine Blue (Sigma-Aldrich) and examined under a light
microscope (Olympus IX71; Olympus Corporation, Tokyo, Japan).
Ultra-thin sections (65 nm) were stained with 1% uranyl acetate and
0.1% lead citrate (both Sigma-Aldrich), and examined on a
JEM-2000EX transmission electron microscope (JEOL USA, Inc.,
Pleasanton, CA, USA).
Hoechst 33342 staining
SH-SY5Y cells were seeded into 6-well plates at a
density of 2×105 cells/well. After pretreatment with or
without lycopene, the cells were exposed to 400 µmol/l
H2O2 for 24 h, washed with PBS solution and
loaded with 10 µg/ml Hoechst 33342 dye (Sigma-Aldrich) for
15 min. The cells were subsequently visualized under a fluorescence
microscope (Olympus IX71). Individual nuclei were visualized at a
magnification of ×400 to distinguish the normal uniform nuclear
pattern from the characteristic, condensed coalesced chromatin
pattern of apoptotic cells. To quantify apoptosis, 400 nuclei from
six random microscopic fields were analyzed by an observer blinded
to the treatment groups. The total number of apoptotic cells in
each section was summed and expressed as the percentage of the
total cell number. A minimum of 10 individual sections were
evaluated per slide.
Measurement of intracellular ROS
levels
The average density of intracellular ROS was
evaluated in cells loaded with the redox-sensitive dye,
dichloro-dihydro-fluorescein diacetate (Molecular Probes DCFH-DA;
Thermo Fisher Scientific, Inc.). SH-SY5Y cells, with or without
lycopene pretreatment, were seeded into 96-well culture plates at a
density of 4×104 cells/well, exposed for 24 h to 400
µmol/l H2O2, washed twice with PBS,
stained with 20 µmol/l DCFH-DA in the dark for 30 min and
harvested. The cells were then dissolved with 1% Triton X-100
(Sigma-Aldrich). Fluorescence was observed under a fluorescent
light microscope, and measured at excitation and emission
wavelengths of 485 and 530 nm, respectively using a fluorescence
spectrometer (HTS 7000; PerkinElmer, Inc., Waltham, MA, USA). The
ROS levels were expressed as arbitrary unit/mg protein and as a
percentage of the control.
Measurement of mitochondrial membrane
potential
Mitochondrial membrane potential was determined by
the retention of the dye, rhodamine 123 (Sigma-Aldrich). SH-SY5Y
cells, with or without lycopene pretreatment, were exposed for 24 h
to 400 µmol/l H2O2. The cells were
collected using 0.25% trypsin and washed twice with PBS, followed
by incubation with 10 µg/ml rhodamine 123 at 37°C for 30
min. The cells were washed again with PBS, and fluorescence
intensities of rhodamine 123 in cells were analyzed by flow
cytometry (FACScan).
Determination of mitochondrial
permeability transition pore (MPTP) opening
The opening of MPTP was determined using a
calcein-cobalt assay kit (Genmed Scientifics, Inc., Wilmington, DE,
USA) as described previously (20). Briefly, SH-SY5Y cells were seeded
into 24-well plates at a density of 5×104 cells/well.
After pretreatment with or without lycopene, the cells were exposed
to 400 µmol/l H2O2 for 24 h, washed
twice with 1 ml Reagent A, incubated for 20 min at 37°C with 250
µl each of Reagents B (calcein AM) and C (cobalt dichloride;
1:50; 500 µl per well). The cells were subsequently washed
twice with pre-warmed Reagent A. The fluorescence intensity was
measured on a microplate reader with excitation and emission
wavelengths of 488 and 505 nm, respectively. After the fluorescence
intensity was determined, the protein concentration for each well
was measured using a Bradford protein assay kit (Bio-Rad
Laboratories, Inc.). The fluorescent signals were normalized to
total protein content in the corresponding cell extract and data
were presented as normalized relative fluorescence units.
Measurement of cellular anti-oxidative
enzymes
The activity of antioxidant enzymes, SOD and
catalase were measured according to the manufacturer's
instructions. After incubation for 24 h with 400 µmol/l
H2O2 following pretreatment with or without
lycopene, SH-SY5Y cells were collected from the culture dishes
using a scraper, centrifuged at 1,000 × g for 10 min at 4°C, and
the cell pellets were washed with PBS. For the SOD activity assay,
the cells were suspended in ice-cold lysis buffer [0.1 M Tris/HCl
(pH 7.4), 0.25 mol/l sucrose, 5 mmol/l β-mercaptoethanol and 0.1
mg/ml phenylmethylsulfonyl fluoride (PMSF); Sigma-Aldrich],
homogenized with a glass Pyrex microhomogenizer (20 strokes;
Beyotime Institute of Biotechnology), and centrifuged at 1,500 × g
for 5 min at 4°C. The supernatant was then collected for assaying.
For the catalase activity assay, the cells were suspended in
ice-cold assay buffer (Sigma-Aldrich), homogenized with a glass
Pyrex microhomogenizer (20 strokes), centrifuged at 10,000 × g for
15 min at 4°C. The supernatant was then collected for assaying. The
catalase was spectrophotometrically determined by measuring
decreased absorbance at 570 nm, using the catalase assay kit, and
SOD was measured spectrophotometrically by monitoring the
absorbance at 450 nm using the SOD assay kit. Catalase and SOD
activities were expressed as U/mg protein.
Immunocytochemistry
The collected SH-SY5Y cells were washed with PBS and
fixed in 4.0% paraformaldehyde (Sigma-Aldrich) for 30 min at 4°C.
After being washed with PBS three times for 5 min each time, cells
were incubated with 1% Triton X-100 for 10 min, and blocked at
nonspecific antibody binding sites by incubating with 5% bovine
serum albumin (BSA; Sigma-Aldrich) in PBS containing 0.3% Triton
X-100 for 1 h at room temperature. The cells were then incubated
with a polyclonal antibody against AIF (1:100) at 4°C overnight
followed by incubation in cy3-conjugated goat anti-rabbit IgG
(1:200) for 50 min at room temperature. After washing with PBS,
cells were incubated with Hoechst 33342 for 30 min. After three
washes, the cells were mounted on slides and visualized under a
fluorescence microscope.
Differential centrifugation and cellular
fraction
The collected cells were washed with PBS, and
centrifuged for 10 min at 1,000 × g, after which the cell pellets
were suspended in ice-cold lysis buffer containing 15 mmol/l Tris,
pH 7.6, 250 mmol/l sucrose, 1 mmol/l MgCl2, 2.5 mmol/l
EDTA, 1 mmol/l ethylene glycol-bis (β-aminoethyl ether) tetraacetic
acid, 1 mmol/l dithiothreitol, 1.25 mg/ml pepstatin A, 10 mg/ml
leupeptin, 2.5 mg/ml aprotinin, 1.0 mmol/l PMSF, 0.1 mmol/l
Na3VO4, 50 mmol/l NaF, and 2 mmol/l
Na4P2O7 (Sigma-Aldrich) and homogenized with
a glass Pyrex microhomogenizer (20 strokes). The homogenates were
centrifuged at 800 × g at 4°C for 10 min to obtain the pellet
containing the nuclear fraction. The supernatants were then
centrifuged at 15,000 × g at 4°C for 10 min to obtain the pellet
containing the mitochondria and the supernatant containing the
cytosolic fraction. All the pellets were resuspended in lysis
buffer. The protein content of each cellular fraction was
determined using the Bradford protein assay kit.
Gel electrophoresis and western
blotting
Equal protein quantities were electrophoresed on 10%
sodium dodecyl sulfate-polyacrylamide gels and then transferred to
PVDF membranes. The membranes were blocked with 3% BSA in
Tris-buffered saline for 30 min and then incubated overnight at 4°C
with primary antibodies against AIF (1:1,000), Bax (1:1,000), Bcl-2
(1:1,000), caspase-3 (1:1,000), Cyt c (1:1,000), COX IV
(1:1,000), β-actin (1:3,000) and lamin B1 (1:1,000). After being
incubated with HRP-conjugated goat anti-rabbit IgG (1:2,000) or
horse anti-mouse IgG (1:2,000), blots were washed and
immunoreactive proteins were visualized on a Kodak X-omat LS film
(Eastman Kodak Co., New Haven, CT, USA) with ECL. Densitometry was
performed using Kodak ID Image Analysis Software, version 3.4.5
(Eastman Kodak Co.).
Statistical analysis
All data represent at least four independent
experiments and are expressed as means ± standard deviation.
Statistical comparisons were conducted using one-way analysis of
variance and P<0.05 was considered to indicate a statistically
significant difference.
Results
Lycopene inhibited
H2O2-induced cell death in SH-SY5Y cells
H2O2-induced changes in the
viability of SH-SY5Y cells were firstly examined by MTT assay. When
compared with the control group, the viability of the cells exposed
for 24 h to H2O2 at the indicated
concentrations decreased significantly (Fig. 1A; P<0.01). Furthermore, the
H2O2-induced reduction in cellular viability
was concentration-dependent. Given that the viability of the cells
treated with 400 µmol/l H2O2 reduced
to 52.1±2.3% in comparison with that in the control group, the
400-µmol/l concentration was used in subsequent experiments
to examine the effect of lycopene on
H2O2-induced changes in SH-SY5Y cells.
As shown in Fig.
1B, no obvious differences were identified in the cellular
viability between the cells in the control group and the cells
treated lycopene alone at the indicated concentrations. By
contrast, decreased cellular viability caused by 400 umol/l
H2O2 was improved significantly by
pretreatment with lycopene at the concentration of 2.0, 4.0 and 8.0
µmol/l lycopene (P<0.01), despite no significant
difference being observed in the protective effect of lycopene
between the 4.0 and 8.0 µmol/l groups.
Additionally, the effect of lycopene on
H2O2-induced toxicity was examined by
measuring the quantity of LDH in the culture medium. As Fig. 1C shows, the leakage of LDH
significantly increased to 1,250.6±85.8% in the group exposed to
400 µmol/l H2O2 when compared with
that in the control group (P<0.01). However, the elevated LDH
leakage caused by H2O2 was suppressed to
962.5±79.8 and 378.9±36.7% by pretreatment with 2.0 and 4.0
µmol/l lycopene, respectively (Fig. 1C; P<0.01). These results
indicate that lycopene exerts a protective effect on cells exposed
to a lethal concentration of H2O2.
Lycopene inhibited
H2O2-induced apoptosis in SH-SY5Y cells
Apoptosis is a form of programmed neuronal death
caused by H2O2 (27); therefore, the present study
examined the effect of lycopene on
H2O2-induced apoptosis in SH-SY5Y cells.
Fluorescence microscopy in combination with Hoechst 33342 staining
showed that the nuclei in the H2O2 group were
polygonal, condensed and bright blue in color when compared with
those in the control group (Fig.
2A). Cell counting under a florescence microscope demonstrated
that the percentage of cells with apoptotic features was 32.6±4.8%
in the H2O2 group, which was significantly
higher than in the control group (5.2±0.7%; P<0.01). However,
this reduced to 25.1±1.9 and 15.2±1.7% in the cells pretreated with
2.0 and 4.0 µmol/l lycopene, respectively (Fig. 2B). Fig. 2C demonstrates the transmission
electronic microscopy images of the cells exposed to
H2O2, demonstrating membrane blebbing,
chromatin accumulation beneath the nucleus membrane and nucleus
condensation, which are consistent with the morphological features
of apoptotic cells (Fig. 2C).
Furthermore, flow cytometry indicated that the apoptosis rate in
the control and H2O2 groups was 3.21 and
42.04%, respectively. By contrast, 2.0 and 4.0 µmol/l
lycopene decreased the apoptosis rate to 26.55 and 17.87%,
respectively (Fig. 2D). These
results indicate that lycopene protects SH-SY5Y cells against
H2O2-induced death via inhibition of
apoptosis.
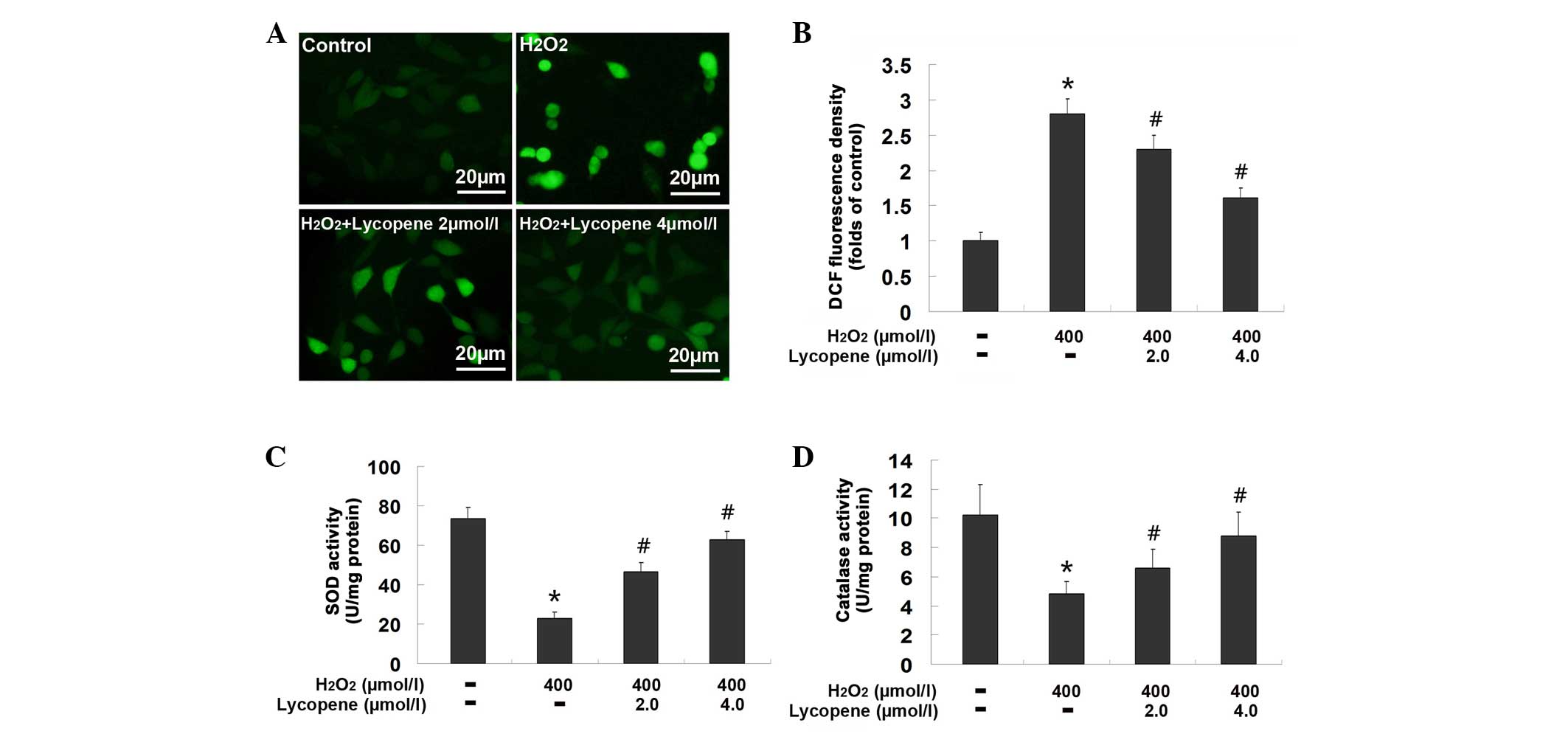
Lycopene mitigated oxidative stress
caused by H2O2
ROS is an important indicator of oxidative stress,
which is also a trigger of apoptosis. Therefore, ROS production
caused by H2O2 was examined in cells
pretreated with or without lycopene. The green fluorescence in the
group exposed to 400 µmol/l H2O2 was
observed to be markedly stronger than that in the control group,
however, was attenuated by pretreatment with either 2.0 or 4.0
µmol/l lycopene (Fig. 3A).
Additionally, the spectrometric assay showed that the fluorescence
intensity in the 400 µmol/l H2O2 group
was 2.83±0.21 times higher than that in the control group
(P<0.01), but reduced to 2.34±0.19 times and 1.67±0.15 times in
the cells pretreated with 2.0 and 4.0 µmol/l lycopene,
respectively (Fig. 3B; P<0.01
vs. the H2O2 group). These results indicate
that pretreatment with lycopene inhibits
H2O2-induced production of ROS in SH-SY5Y
cells.
As the antioxidant enzymes, SOD and catalase may
inhibit the ROS level within cells, the effect of lycopene on the
enzymatic activities of SOD and catalase were examined in the
present study. As shown in Fig. 3C and
D, the activity of SOD in the cells exposed to 400
µmol/l H2O2 was 22.8±3.23 U/mg
protein, significantly lower than 73.66±5.39 U/mg protein in the
control group (P<0.01). By contrast, it was improved to
46.72±4.53 and 62.77±5.36 U/mg protein in the cells pretreated with
2.0 and 4.0 µmol/l lycopene, respectively (P<0.01 vs. the
H2O2 group). Similarly, the catalase activity
reduced from 10.22±2.11 to 4.81±0.88 U/mg protein when SH-SY5Y
cells were exposed to 400 µmol/l H2O2
(P<0.01), whereas pretreatment with 2.0 and 4.0 µmol/l
lycopene restored its activity to 6.58±1.28 and 8.76±1.69 U/mg
protein, respectively (P<0.01 vs. the H2O2
group). These results indicate that lycopene facilitates the
maintenance of SOD and catalase activities in the cells exposed to
H2O2.
Lycopene protected against
H2O2-induced mitochondrial dysfunction
Mitochondrial dysfunction due to ROS attack is
proposed to participate in cellular apoptosis. Therefore,
depolarization of the mitochondrial membrane and opening of the
MPTP, which are sensitive indicators of mitochondrial function,
were assayed in the present study. As shown in Fig. 4A, the mitochondrial membrane
potentials reduced from 91.25 to 72.56% after the SH-SY5Y cells
were exposed to H2O2. However, the
mitochondrial membrane potentials were restored to 81.27 and 88.98%
in the groups pretreated with 2.0 and 4.0 µmol/l lycopene,
respectively. This indicates that pretreatment with lycopene
inhibits H2O2-induced depolarization of the
mitochondrial membrane in SH-SY5Y cells.
Similar changes were observed in the MPTP opening
(Fig. 4B). Compared with the
control group, the fluorescence intensity of cells in the
H2O2 group was significantly weakened (from
17.22±1.51 to 7.83±1.12; P<0.01), which was indicative of the
opening of the MPTP. When the cells were pretreated with lycopene
at concentrations of 2.0 or 4.0 µmol/l, the fluorescence
intensity was significantly strengthened to 12.18±1.33 (P<0.01)
and 14.49±1.47 (P<0.01), respectively, indicating closure of the
MPTP.
Since it has been reported that Bax and Bcl-2
regulate the opening of MPTP (28), the mitochondrial fraction was
isolated via differential centrifugation, and the levels of Bax and
Bcl-2 were examined by western blotting (Fig. 4C). The protein expression level of
Bax was significantly increased from 0.05±0.01 to 0.72±0.08
(P<0.01), and the expression level of Bcl-2 was significantly
decreased from 0.61±0.07 to 0.38±0.04 (P<0.01), in the
mitochondria of the cells exposed to H2O2
when compared with those in the control group (Fig. 4D). By contrast, the changes in the
expression levels of Bax and Bcl-2 within the mitochondria were
reversed by pretreatment with 2.0 and 4.0 µmol/l lycopene.
Following pretreatment with 2.0 µmol/l lycopene, the
expression levels of Bax were decreased to 0.51±0.06 and the
expression levels of Bcl-2 were increased to 0.48±0.04. Notably,
4.0 µmol/l demonstrated stronger effects compared with 2.0
µmol/l lycopene; the expression levels of Bax were decreased
to 0.35±0.04 and those of Bcl-2 were increased to 0.57±0.07
following pretreatment with 4.0 µmol/l lycopene (Fig. 4D). These results indicate that
lycopene is able to modulate the level of mitochondrial Bax and
Bcl-2, which may clarify the molecular mechanism underlying the
inhibitory effect of lycopene on H2O2-induced
MPTP opening.
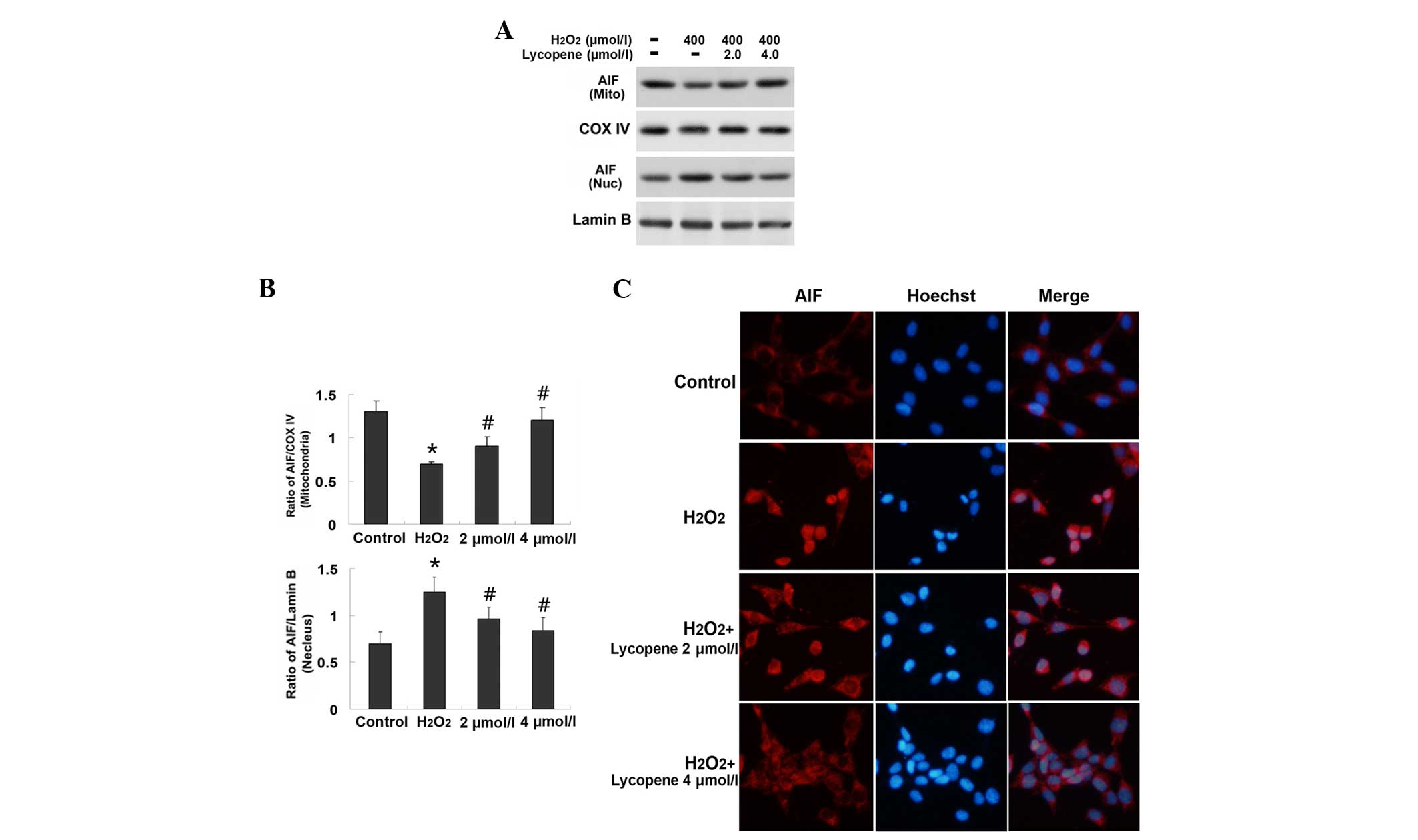
Lycopene inhibited the
mitochondrial-associated apoptosis pathway
Mitochondrial proteins, including Cyt c and
AIF, are critical in the initiation of intrinsic apoptosis
signaling pathways. Thus, the sub-cellular mitochondrial, nuclear
and cytoplasm fractions were isolated, and the distribution of Cyt
c (Fig. 5) and AIF
(Fig. 6) were examined by western
blotting. The protein levels of Cyt c and AIF within the
mitochondria were significantly decreased from 0.82±0.13 to
0.23±0.02 (P<0.01) and 1.33±0.15 to 0.73±0.03 (P<0.01),
respectively, in the H2O2 group. Conversely,
the level of Cyt c was elevated from 0.08±0.07 to 0.62±0.04
(P<0.01) in the cytoplasm, and the level of AIF was increased
from 0.71±0.07 to 1.25±0.16 (P<0.01) in the nucleus (Figs. 5 and 6). Cleaved caspase-3 is a downstream
apoptosis executor of the Cyt c signaling pathway. Notably,
the levels of cleaved caspase-3 were increased from 0.02±0.0018 to
0.13±0.015 (P<0.01), and the activity of caspase-3 was elevated
from 1.02±0.11 to 3.83±0.42 (P<0.01), in the cells exposed to
H2O2 (Fig.
5). In addition, immunocytochemistry demonstrated that a
proportion of mitochondrial AIF translocated into the nuclei of the
cells treated with H2O2 (Fig. 6). By contrast, pretreatment with
lycopene at either 2.0 or 4.0 µmol/l suppressed these
alterations in levels of Cyt c, cleaved caspase-3 and AIF
that were caused by H2O2 (Figs. 5 and 6). These results indicate that lycopene
inhibits H2O2-induced apoptosis in SH-SY5Y
cells via inhibition of caspase and AIF apoptotic pathways.
Discussion
The present study demonstrated that pretreatment
with lycopene significantly inhibited
H2O2-induced apoptosis in SH-SY5Y cells via
attenuation of ROS production, protection against mitochondrial
dysfunction and mitigation of the activation of
mitochondria-associated apoptotic pathways. The underlying
mechanisms of the inhibitory effects of lycopene on
H2O2-induced apoptosis in SH-SY5Y cells are
the inhibition of oxidative stress and suppression of
mitochondria-associated apoptosis pathways.
Apoptosis is an important form of programmed
neuronal death, which has been identified under various
pathological conditions, such as cerebral ischemia, neurotrauma,
and Parkinson's disease (29–31).
H2O2-induced cell death in human SH-SY5Y
cells is often used as an in vitro model to investigate
neuronal apoptosis caused by oxidative stress (27,32,33).
In the current study, transmission electronic and fluorescence
microscopy revealed that SH-SY5Y cells treated with
H2O2 presented morphological features of
apoptosis, including membrane blebbing, chromatin accumulation
beneath the nucleus membrane and nucleus condensation. By contrast,
pretreatment with lycopene significantly attenuated
H2O2-induced apoptosis in SH-SY5Y cells,
which was demonstrated by Hoechst 33342 staining and flow
cytometric analysis. As previous reports demonstrated that lycopene
prevented apoptosis (induced by cerebral ischemia and reperfusion)
in gerbil hippocampal neurons and in cardiomyocytes (where
apoptosis was induced in vitro by hypoxia and
re-oxygenation) (14,34), it was hypothesized that lycopene
inhibits H2O2-induced death in SH-SY5Y cells
via suppression of apoptosis.
Oxidative stress, characterized by accumulation of
ROS, is proposed to be an initiator of neuronal apoptosis (35). The brain is an organ that is prone
to producing reactive oxidative species (such as
O2−, H2O2 and hydroxyl
radicals) as it requires a large quantity of oxygen to maintain its
normal function, but is deficient in anti-oxidants (36). Furthermore, neuronal cells are more
sensitive to oxidative stress than the cells in other types of
tissue (37). Therefore,
inhibition of oxidative stress is considered to be a strategy to
prevent neuronal apoptosis. Previous studies have shown that
lycopene is a potent agent that inhibits oxidative stress, which
occurs within human cells. Tang et al (38) found that lycopene protected human
endothelial cell against H2O2-induced
oxidative injury. Palozza et al (39) reported that lycopene prevented
7-ketocholesterol-induced oxidative stress in human macrophages.
Consistent with these previous findings, the present study found
that lycopene significantly inhibited
H2O2-induced accumulation of ROS in SH-SY5Y
cells. Lycopene is a chemical found to inhibit oxidative stress by
quenching singlet oxygen and trapping peroxyl radicals (10), which may elucidate why pretreatment
with lycopene in the present study inhibited the ROS levels in the
SH-SY5Y cells that were exposed to H2O2.
Furthermore, it was demonstrated that lycopene mitigated the
H2O2-induced reduction in SOD and catalase
activities. Consistently, Srinivasan et al (40) reported that lycopene was able to
recover the damaged activities of SOD and catalase in human
lymphocytes caused by gamma-radiation. SOD and catalase are the
predominant members of the intracellular enzymes that have the
ability to clear ROS. SOD converts O2− into
H2O2, which is subsequently transformed into
water and oxygen via catalase. Therefore, maintaining the
activities of the anti-oxidative enzymes, SOD and catalase is
another method by which lycopene clears
H2O2-indcued excessive ROS.
The mitochondrion is an organelle that may activate
apoptosis when its function is damaged. ROS, which are produced
during the process of oxidative stress, may target mitochondria,
resulting in the opening of the MPTP and the decline in
mitochondrial membrane potentials (41). Although it was observed that
lycopene is an effective agent that could prevent mitochondrial
dysfunction caused by MPP+ and hypoxia/reoxygenation
(17,34), its underlying molecular mechanism
remains unclear. Previously, Sandhir et al (42) reported that lycopene maintained
mitochondrial function by suppression of mitochondrial oxidative
stress that was caused by 3-nitropropionic acid in the rat brain.
By contrast, the present study demonstrated that the inhibitory
effect of lycopene on the H2O2-induced
decline in mitochondrial membrane potentials and opening of MPTP in
SH-SY5Y cells was associated with the fact that lycopene
significantly counteracted the H2O2-induced
increase in pro-apoptotic Bax level and reduction in anti-apoptotic
Bcl-2 level within mitochondria. The Bax and Bcl-2 within
mitochondria has been found to participate in the regulation of
mitochondrial function, where Bax promotes and Bcl-2 blocks the
formation and opening of MPTP (43). Furthermore, inhibition of MPTP
opening has been identified as a promising therapeutic target for
preventing cell damage (44).
Therefore, the present study hypothesized that lycopene mitigated
H2O2-induced mitochondrial dysfunction in
SH-SY5Y cells by counteracting the
H2O2-induced reduction in Bcl-2 and elevation
in Bax expression levels.
It was reported previously that lycopene attenuated
apoptosis via inhibition of the nuclear factor-κB signaling pathway
(45), alleviation of c-Jun
N-terminal kinase activation (46), mitigation of endoplasmic reticulum
stress (47) and induction of
protective autophagy (48). In the
present study, lycopene inhibited mitochondria-associated apoptotic
pathways, which were activated in the SH-SY5Y cells that were
exposed to H2O2. The opening of MPTP results
in the release of pro-apoptotic proteins, Cyt c and AIF,
which are normally located in the space between the mitochondrial
inner and outer membranes (45).
After being released as a result of apoptotic stress, Cyt c
translocates into the cytoplasm to activate the caspase-dependent
apoptotic pathway by cleaving caspase-3. Thus, cleaved caspase-3 is
often regarded as a marker representing the activation of
caspase-3. In the current study, pretreatment with lycopene
mitigated the release of Cyt c from the mitochondria and
suppressed the level of cleaved caspase-3 in SH-SY5Y cells that
were exposed to H2O2. Results from previous
studies indicate that inhibition of the activation of caspase-3 is
one factor that is responsible for the protection of lycopene
against apoptosis in various types of cells (19,38).
Qu et al (19) reported
that lycopene inhibited amyloid β-induced caspase-3 activation in
cultured rat cortical neurons and Tang et al (38) demonstrated that lycopene attenuated
the level of activated caspase-3 caused by
H2O2 exposure in human endothelial cells.
Thus, the inhibitory effect of lycopene on
H2O2-induced apoptosis in SH-SY5Y cells is
associated with its attenuation of caspase-3 apoptosis pathway
activation.
AIF, as another protein that is released from the
mitochondria of cells stressed by apoptotic stimuli, represents a
caspase-independent apoptotic pathway. AIF translocates into the
nucleus to initiate chromatin condensation and DNA fragmentation
(49) and has been found to be
involved in neuronal apoptosis caused by different
neuropathological conditions. Ba et al (50) demonstrated that AIF nuclear
translocation occurred following focal cerebral
ischemia-reperfusion injury in rats. Yang et al (51) found that AIF translocation into
nuclei contributed to epilepsy-induced apoptosis in hippocampal
neurons in vitro. Thus, in vitro and in vivo
studies have demonstrated that AIF is a crucial factor leading to
neuronal injury or apoptosis. By contrast, in vitro and
in vivo studies have demonstrated that inhibition of AIF
translocation into nuclei is responsible for the protection of
certain chemicals against neuronal apoptosis. Huang et al
(52) reported that the protection
of forsythiaside against H2O2-induced
apoptosis in PC12 cells was associated with inhibiting the
accumulation of AIF within the nuclei. Similarly, an in vivo
study revealed that blocking the mitochondrio-nuclear translocation
of AIF by ginsenoside Rd benefited neuronal survival following
focal cerebral ischemia in rats (53). In the present study, the
H2O2-induced elevation of AIF level within
the nuclei of SH-SY5Y cells was observed to be suppressed when
cells were pre-treated with lycopene. Although there was no report
regarding the effect of lycopene on AIF translocation from the
mitochondria into the nuclei, lycopene was demonstrated to inhibit
H2O2-induced apoptosis in SH-SY5Y cells via
mitigation of AIF nuclear translocation.
In conclusion, the present study demonstrated that
lycopene inhibits H2O2-induced excessive
production of ROS in SH-SY5Y cells by maintaining the activity of
cellular anti-oxidative enzymes, SOD and catalase. Lycopene
protects against mitochondrial dysfunction by counteracting
H2O2-induced upregulation of Bax expression
and downregulation of Bcl-2 expression. Furthermore, lycopene
suppressed H2O2-induced activation of
caspase-3 and AIF apoptotic pathways in SH-SY5Y cells. Thus,
lycopene is considered to be a potent agent that exerts a
protective effect against oxidative stress, mitochondrial
dysfunction and apoptosis, and may serve as a therapeutic strategy
for preventing neuronal injury or death.
Acknowledgments
The present study was supported by the National
Nature and Science Foundation of China (grant nos. 81171234 and
81271215), the Nature and Science Foundation of Jilin province
(grant nos. 201115068 and 20121809) and the Bethune project of
Jilin University (grant no. 2012203).
References
|
1
|
Chan PH: Reactive oxygen radicals in
signaling and damage in the ischemic brain. J Cereb Blood Flow
Metab. 21:2–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang T, Kong B, Gu JW, Kuang YQ, Cheng L,
Yang WT, Xia X and Shu HF: Anti-apoptotic and anti-oxidative roles
of quercetin after traumatic brain injury. Cell Mol Neurobiol.
34:797–804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aguiar CC, Almeida AB, Araújo PV, de Abreu
RN, Chaves EM, do Vale OC, Macêdo DS, Woods DJ, Fonteles MM and
Vasconcelos SM: Oxidative stress and epilepsy: Literature review.
Oxid Med Cell Longev. 2012:7952592012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cahill-Smith S and Li JM: Oxidative
stress, redox signalling and endothelial dysfunction in
ageing-related neurodegenerative diseases: A role of NADPH oxidase
2. Br J Clin Pharmacol. 78:441–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aliev G, Smith MA, Seyidov D, Neal ML,
Lamb BT, Nunomura A, Gasimov EK, Vinters HV, Perry G, LaManna JC
and Friedland RP: The role of oxidative stress in the
pathophysiology of cerebrovascular lesions in Alzheimer's disease.
Brain Pathol. 12:21–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barbouti A, Doulias PT, Nousis L,
Tenopoulou M and Galaris D: DNA damage and apoptosis in hydrogen
peroxide-exposed Jurkat cells: Bolus addition versus continuous
generation of H(2) O(2). Free Radic Biol Med. 33:691–702. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian X, Guo LP, Hu XL, Huang J, Fan YH,
Ren TS and Zhao QC: Protective Effects of Arctium lappa L. roots
against hydrogen peroxide-induced cell injury and potential
mechanisms in SH-SY5Y cells. Cell Mol Neurobiol. 35:335–344. 2015.
View Article : Google Scholar
|
|
8
|
Jiang J, Yu S, Jiang Z, Liang C, Yu W, Li
J, Du X, Wang H, Gao X and Wang X: N-acetyl-serotonin protects
HepG2 cells from oxidative stress injury induced by hydrogen
peroxide. Oxid Med Cell Longev. 2014:3105042014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agca CA, Tuzcu M, Gencoglu H, Akdemir F,
Ali S, Sahin K and Kucuk O: Lycopene counteracts the hepatic
response to 7,12-dimethylbenz[a]anthracene by altering the
expression of Bax, Bcl-2, caspases and oxidative stress biomarkers.
Pharm Biol. 50:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marcotorchino J, Romier B, Gouranton E,
Riollet C, Gleize B, Malezet-Desmoulins C and Landrier JF: Lycopene
attenuates LPS-induced TNF-α secretion in macrophages and
inflammatory markers in adipocytes exposed to
macrophage-conditioned media. Mol Nutr Food Res. 56:725–732. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trejo-Solís C, Pedraza-Chaverrí J,
Torres-Ramos M, Jiménez-Farfán D, Cruz Salgado A, Serrano-García N,
Osorio-Rico L and Sotelo J: Multiple molecular and cellular
mechanisms of action of lycopene in cancer inhibition. Evid Based
Complement Alternat Med. 2013:7051212013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karppi J, Laukkanen JA, Sivenius J,
Ronkainen K and Kurl S: Serum lycopene decreases the risk of stroke
in men: A population-based follow-up study. Neurology.
79:1540–1547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsiao G, Fong TH, Tzu NH, Lin KH, Chou DS
and Sheu JR: A potent antioxidant, lycopene, affords
neuroprotection against microglia activation and focal cerebral
ischemia in rats. In Vivo. 18:351–356. 2004.PubMed/NCBI
|
|
14
|
Fujita K, Yoshimoto N, Kato T, Imada H,
Matsumoto G, Inakuma T, Nagata Y and Miyachi E: Lycopene inhibits
ischemia/reperfusion-induced neuronal apoptosis in gerbil
hippocampal tissue. Neurochem Res. 38:461–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prakash A and Kumar A: Lycopene protects
against memory impairment and mito-oxidative damage induced by
colchicine in rats: An evidence of nitric oxide signaling. Eur J
Pharmacol. 721:373–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaur H, Chauhan S and Sandhir R:
Protective effect of lycopene on oxidative stress and cognitive
decline in rotenone induced model of Parkinson's disease. Neurochem
Res. 36:1435–1443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi F, He X and Wang D: Lycopene protects
against MPP(+)-induced cytotoxicity by maintaining mitochondrial
function in SH-SY5Y cells. Neurochem Res. 38:1747–1757. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qu M, Nan X, Gao Z, Guo B, Liu B and Chen
Z: Protective effects of lycopene against methylmercury-induced
neurotoxicity in cultured rat cerebellar granule neurons. Brain
Res. 1540:92–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qu M, Li L, Chen C, Li M, Pei L, Chu F,
Yang J, Yu Z, Wang D and Zhou Z: Protective effects of lycopene
against amyloid β-induced neurotoxicity in cultured rat cortical
neurons. Neurosci Lett. 505:286–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu M, Zhou Z, Chen C, Li M, Pei L, Chu F,
Yang J, Wang Y, Li L, Liu C, et al: Lycopene protects against
trimethyltin-induced neurotoxicity in primary cultured rat
hippocampal neurons by inhibiting the mitochondrial apoptotic
pathway. Neurochem Int. 59:1095–1103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
di Matteo V, Pierucci M, Di Giovanni G,
Dragani LK, Murzilli S, Poggi A and Esposito E: Intake of
tomato-enriched diet protects from 6-hydroxydopamine-induced
degeneration of rat nigral dopaminergic neurons. J Neural Transm
Suppl. 333–341. 2009.PubMed/NCBI
|
|
22
|
Di Mascio P, Kaiser S and Sies H: Lycopene
as the most ef ficient biological carotenoid singlet oxygen
quencher. Arch Biochem Biophys. 274:532–538. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alberio T, Bondi H, Colombo F, Alloggio I,
Pieroni L, Urbani A and Fasano M: Mitochondrial proteomics
investigation of a cellular model of impaired dopamine homeostasis,
an early step in Parkinson's disease pathogenesis. Mol Biosyst.
10:1332–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang J, Yu Y, Wang B, Lu B, Zhang J,
Zhang H and Ge P: Ginsenoside Rb1 attenuates oxygen-glucose
deprivation-induced apoptosis in SH-SY5Y cells via protection of
mitochondria and inhibition of AIF and cytochrome c release.
Molecules. 18:12777–12792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han DY, Di XJ, Fu YL and Mu TW: Combining
valosin-containing protein (VCP) inhibition and
suberanilohydroxamic acid (SAHA) treatment additively enhances the
folding, trafficking and function of epilepsy-associated
γ-aminobutyric acid, type A (GABAA) receptors. J Biol Chem.
290:325–337. 2015. View Article : Google Scholar :
|
|
26
|
Watkins SC and Cullen MJ: A qualitative
and quantitative study of the ultrastructure of regenerating muscle
fibres in Duchenne muscular dystrophy and polymyositis. J Neurol
Sci. 82:181–192. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwon SH, Kim JA, Hong SI, Jung YH, Kim HC,
Lee SY and Jang CG: Loganin protects against hydrogen
peroxide-induced apoptosis by inhibiting phosphorylation of JNK,
p38 and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 58:533–541.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
James D, Parone PA, Terradillos O,
Lucken-Ardjomande S, Montessuit S and Martinou JC: Mechanisms of
mitochondrial outer membrane permeabilization. Novartis Found Symp.
287:170–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakka VP, Gusain A, Mehta SL and Raghubir
R: Molecular mechanisms of apoptosis in cerebral ischemia: Multiple
neuroprotective opportunities. Mol Neurobiol. 37:7–38. 2008.
View Article : Google Scholar
|
|
30
|
Springer JE: Apoptotic cell death
following traumatic injury to the central nervous system. J Biochem
Mol Biol. 35:94–105. 2002. View Article : Google Scholar
|
|
31
|
Alves da Costa C and Checler F: Apoptosis
in Parkinson's disease: Is p53 the missing link between genetic and
sporadic Parkinsonism? Cell Signal. 23:963–968. 2011. View Article : Google Scholar
|
|
32
|
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan
C, Cao G and Wang Z: Protective effects of salidroside on hydrogen
peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells.
Eur J Pharmacol. 564:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SY, Seo M, Kim Y, Lee YI, Oh JM, Cho
EA, Kang JS and Juhnn YS: Stimulatory heterotrimeric GTP-binding
protein inhibits hydrogen peroxide-induced apoptosis by repressing
BAK induction in SH-SY5Y human neuroblastoma cells. J Biol Chem.
283:1350–1361. 2008. View Article : Google Scholar
|
|
34
|
Yue R, Hu H, Yiu KH, Luo T, Zhou Z, Xu L,
Zhang S, Li K and Yu Z: Lycopene protects against
hypoxia/reoxygenation-induced apoptosis by preventing mitochondrial
dysfunction in primary neonatal mouse cardiomyocytes. PLoS One.
7:e507782012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Méndez-Armenta M, Nava-Ruíz C,
Juárez-Rebollar D, Rodríguez-Martínez E and Gómez PY: Oxidative
stress associated with neuronal apoptosis in experimental models of
epilepsy. Oxid Med Cell Longev. 2014:2936892014. View Article : Google Scholar
|
|
36
|
Sugawara T and Chan PH: Reactive oxygen
radicals and pathogenesis of neuronal death after cerebral
ischemia. Antioxid Redox Signal. 5:597–607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidant therapeutic options. Curr
Neuropharmacol. 7:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang X, Yang X, Peng Y and Lin J:
Protective effects of lycopene against H2O2-induced oxidative
injury and apoptosis in human endothelial cells. Cardiovasc Drugs
Ther. 23:439–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Palozza P, Simone R, Catalano A,
Boninsegna A, Böhm V, Fröhlich K, Mele MC, Monego G and Ranelletti
FO: Lycopene prevents 7-ketocholesterol-induced oxidative stress,
cell cycle arrest and apoptosis in human macrophages. J Nutr
Biochem. 21:34–46. 2010. View Article : Google Scholar
|
|
40
|
Srinivasan M, Devipriya N, Kalpana KB and
Menon VP: Lycopene: An antioxidant and radioprotector against
gamma-radiation-induced cellular damages in cultured human
lymphocytes. Toxicology. 262:43–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sugawara T, Fujimura M, Noshita N, Kim GW,
Saito A, Hayashi T, Narasimhan P, Maier CM and Chan PH: Neuronal
death/survival signaling pathways in cerebral ischemia. NeuroRx.
1:17–25. 2004. View Article : Google Scholar
|
|
42
|
Sandhir R, Mehrotra A and Kamboj SS:
Lycopene prevents 3-nitropropionic acid-induced mitochondrial
oxidative stress and dysfunctions in nervous system. Neurochem Int.
57:579–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harris MH and Thompson CB: The role of the
Bcl-2 family in the regulation of outer mitochondrial membrane
permeability. Cell Death Differ. 7:1182–1191. 2000. View Article : Google Scholar
|
|
44
|
Javadov S, Karmazyn M and Escobales N:
Mitochondrial permeability transition pore opening as a promising
therapeutic target in cardiac diseases. J Pharmacol Exp Ther.
330:670–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He Q, Zhou W, Xiong C, Tan G and Chen M:
Lycopene attenuates inflammation and apoptosis in post-myocardial
infarction remodeling by inhibiting the nuclear factor-κB signaling
pathway. Mol Med Rep. 11:374–378. 2015.
|
|
46
|
Lv JC, Wang G, Pan SH, Bai XW and Sun B:
Lycopene protects pancreatic acinar cells against severe acute
pancreatitis by abating the oxidative stress through JNK pathway.
Free Radic Res. 49:151–163. 2015. View Article : Google Scholar
|
|
47
|
Gao Y, Jia P, Shu W and Jia D: The
protective effect of lycopene on hypoxia/reoxygenation-induced
endoplasmic reticulum stress in H9C2 cardiomyocytes. Eur J
Pharmacol. 774:71–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen F, Sun ZW, Ye LF, Fu GS, Mou Y and Hu
SJ: Lycopene protects against apoptosis in
hypoxia/reoxygenation-induced H9C2 myocardioblast cells through
increased autophagy. Mol Med Rep. 11:1358–1365. 2015.
|
|
49
|
Polster BM: AIF, reactive oxygen species
and neurodegeneration: A 'complex' problem. Neurochem Int.
62:695–702. 2013. View Article : Google Scholar :
|
|
50
|
Ba XH, Cai LP and Han W: Effect of
cilostazol pretreatment on the PARP/AIF-mediated apoptotic pathway
in rat cerebral ischemia-reperfusion models. Exp Ther Med.
7:1209–1214. 2014.PubMed/NCBI
|
|
51
|
Yang X, Wang S, Lin Y, Han Y, Qiu X, Zhao
X, Cao L, Wang X and Chi Z: Poly (ADP-ribose) polymerase inhibition
protects epileptic hippocampal neurons from apoptosis via
suppressing Akt-mediated apoptosis-inducing factor translocation in
vitro. Neuroscience. 231:353–362. 2013. View Article : Google Scholar
|
|
52
|
Huang C, Lin Y, Su H and Ye D:
Forsythiaside protects against hydrogen peroxide-induced oxidative
stress and apoptosis in PC12 cell. Neurochem Res. 40:27–35. 2015.
View Article : Google Scholar
|
|
53
|
Hu G, Wu Z, Yang F, Zhao H, Liu X, Deng Y,
Shi M and Zhao G: Ginsenoside Rd blocks AIF mitochondrio-nuclear
translocation and NF-κB nuclear accumulation by inhibiting poly
(ADP-ribose) polymerase-1 after focal cerebral ischemia in rats.
Neurol Sci. 34:2101–2106. 2013. View Article : Google Scholar : PubMed/NCBI
|