Introduction
Hepatic fibrosis represents a wound-healing response
to liver injury, resulting from a number of chronic liver diseases,
including viral and autoimmune hepatitis, biliary obstruction and
alcohol consumption. Globally, hepatitis B, hepatitis C and alcohol
consumption have been the leading causes of hepatic fibrosis,
although recently, the increasing prevalence of obesity and
metabolic syndrome has further increased the incidence of liver
fibrosis (1). At the end-stage of
chronic liver diseases, hepatic fibrosis may result in cirrhosis,
portal hypertension, liver failure and hepatocellular carcinoma
(2). Chronic liver diseases and
cirrhosis are important causes of morbidity and mortality worldwide
(1).
The occurrence of hepatic fibrosis is a dynamic
process, which is intimately associated with the abnormal structure
and dysfunctions of the liver, involving cytokines and such cells
as hepatocytes, hepatic stellate cells (HSCs), Kupffer cells and
hepatic sinusoidal endothelial cells. Progression to hepatic
fibrosis is characterized by an excessive deposition of
extracellular matrix (ECM), including such components as collagens,
glycoproteins, mucopolysaccharides and proteoglycans (3). The ECM is predominantly generated by
HSCs, whose activation and proliferation fulfill a major role in
the development of hepatic fibrosis. In normal liver, HSCs exhibit
a star-like morphology, are located in the space of Disse
surrounding the sinusoids as pericytes, and store
vitamin-A-associated lipid droplets. Upon activation, HSCs undergo
a phenotypic transformation into myfibroblast-like cells, as
demonstrated by the expression of α-smooth muscle actin (α-SMA) and
the dysregulation of collagens, matrix metalloproteinases (MMPs)
and tissue inhibitor of metalloproteinase 1 (TIMP1), processes
which are largely attributed to the increased deposition of ECM.
Among the members of the collagen family, collagens I and III are
predominantly associated with fibrogenesis, and increases in their
expression levels occur concomitantly with the progression of liver
fibrosis.
An emerging body of evidence has indicated that
increasing levels of transforming growth factor (TGF)-β1, a major
profibrogenic cytokine, induce intracellular signaling events,
resulting in excess matrix protein deposition and an inhibition of
the synthesis of ECM-degradation enzymes (4–6).
Bone morphogenetic proteins (BMPs) are members of the TGF-β family
(7), which exert their biological
functions via interaction with types I and II serine/threonine
kinase receptors. Signals from the types I and II receptors are
subsequently transmitted to the downstream substrates, including
Smads (8,9). Once BMP ligands are stimulated,
R-Smads are phosphorylated and form a complex with Co-Smads
(Smad4), which are subsequently translocated into the nucleus to
trigger the expression of target genes (9).
However, BMP-7 possesses anti-inflammatory and
anti-fibrotic properties in the fibrotic model (10). BMP-7 has been demonstrated to
inhibit TGF-β1/Smad signaling (11,12).
BMP-7 was revealed to enhance the phosphorylation of Smad1/5/8
[phospho (p)-Smad1/5/8], resulting in the suppression of the
profibrogenic effect of TGF-β1 (13). Therefore, BMP-7/Smad signaling has
attracted special attention for its anti-profibrogenic effects via
the negative regulation of TGF-β1/Smad signaling.
Currently, multiple treatment strategies aimed at
alleviating the inflammatory necrosis of hepatocytes and
ameliorating the severity of hepatic fibrosis have been employed,
including the inhibition of inflammation, targeting the host immune
response, HSC activation and ECM synthesis. Herbal compound 861
(Cpd 861) is an extract of several mixed Chinese herbs, including
red sage (Salvia miltiorrhiza), Astragalus
membranaceus, Radix Bupleuri, Spatholobus suberectus,
Szechwan Lovage Rhizome, nut grass (Cyperus rotundus)
and red paeonia. Clinical trials have revealed that Cpd 861 is able
to markedly ameliorate clinical symptoms in patents with hepatic
fibrosis, and experimental observations demonstrated that the
formula reverses the progression of liver fibrosis in rat models
(14). Further studies have lent
support to the hypothesis that Cpd 861 inhibits the expression of
TGF-β1, the proliferation and activation of HSCs and the
progression of hepatic fibrosis (15,16).
However, the anti-fibrotic molecular mechanism which underpins the
action of Cpd 861 has yet to be fully elucidated. The present study
aimed to further determine whether Cpd 861 attenuates hepatic
fibrosis via the upregulation of BMP-7/Smad signaling.
Materials and methods
Animal grouping and treatment
Animal care and experimental procedures were
approved by the Ethics Committee of Beijing Friendship Hospital,
Capital Medical University (Beijing, China). A total of 18 male SPF
Wistar rats, weighing 180–220 g, were obtained from the Laboratory
Animal Center of Chinese Academy of Medical Sciences (Beijing,
China). All animals were maintained under pathogen-free conditions
and allowed unlimited access to food and water. Following
acclimation, the rats were randomly assigned to three groups
containing six rats each: The sham-operation group, the bile duct
ligation (BDL) model group and the Cpd 861-treated group. Bile duct
ligation (BDL) and sham operation were performed as described
previously (17,18) under general anesthesia induced by
intraperitoneal injection of sodium pentobarbital (40 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA). Following midline laparotomy,
the common bile duct was identified, twice ligated with 6.0 silk
sutures (19) and sectioned
between the ligatures. The abdomen was subsequently closed, and
rats were allowed to recover under a heating lamp. The animals in
the Cpd 861-treated group were intragastrically administered 9 g/kg
Cpd 861 (Beijing Tcmages Pharmaceutical Co., Ltd., Beijing, China)
daily for 28 days, and the remaining two groups were
intragastrically administered with an equal volume of physiological
saline. Following the treatment, animals were sacrificed. Following
anesthesia by intraperitoneal injection of sodium pentobarbital (40
mg/kg), midline laparotomy was performed and the right lobe of the
liver was identified and collected from the animals. Prior to
sacrification of the rats, blood samples were collected from the
heart.
Histological examination
Liver tissues were fixed in 4% paraformaldehyde,
paraffin-embedded, and sectioned at 4 μm. Histological
changes were assessed using hematoxylin-eosin (H&E) and Masson
staining (Bestbio Co., Shanghai, China). Images from the different
groups were captured using a microscope (Leica DM6000B; Leica
Microsystems GmbH, Wetzlar, Germany).
Detection of serum biochemical
indicators
Serum was isolated by centrifugation at 1,811 × g
for 5 min, and subsequently used for alanine transaminase (ALT),
aspartate transaminase (AST), total bilirubin (TBIL) and direct
bilirubin (DBIL) analyses using an automatic biochemistry analyzer
(Abbott Laboratories, North Chicago, IL, USA), according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the preserved liver
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Waltham, MA, USA), according to the manufacturer's
protocol. RNase-free deoxynuclease (Promega Corp., Madison, WI,
USA) was used to eliminate any contaminating genomic DNA. The first
cDNA strand was synthesized by reverse transcription reaction in a
20 μl sample containing 4 μl MgCl2, 3
μl 10X RT buffer, 2 μl deoxynucleotides mixture, 0.5
μl recombinant ribonuclease inhibitor, 0.6 μl avian
myeloblastosis virus reverse transcriptase, 1 μl Oligo
dT-Adaptor primer and 5 μg RNA sample (A3500; Promega
Corp.). The RT reaction product was subsequently used for the
amplification of cDNA using the SYBR® Mastermix reaction
system (Thermo Fisher Scientific) comprising, in a total sample of
10 μl, 1 μl upstream primers, 1 μl downstream
primers, 1 μl cDNA and 7 μl
diethyl-pyrocarbonate-treated water. The primer sequences are
listed in Table I, and the
housekeeper gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH),
was used as an internal control.
 | Table IPCR primer sequences of BMP-7, Smad1,
Smad5, and GAPDH. |
Table I
PCR primer sequences of BMP-7, Smad1,
Smad5, and GAPDH.
| Gene | Forward sequence | Reverse sequence |
|---|
| BMP-7 |
5′-ATCCCCAATGTCTCACCACCTA-3′ |
5′-AAGTATGCTGCTTATCAACCACG-3′ |
| Smad1 |
5′-ATGAACTAAAGCCTCTGGAAT-3′ |
5′-GGTTGTACTCGCTGTGCC-3′ |
| Smad5 |
5′-AACCATGGGTTTGAGGCTGTG-3′ |
5′-AGAGGCCCATGGAGGTGAATC-3′ |
| GAPDHa |
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
The PCR protocol was as follows: The mixture was
first denatured at 95°C for 10 min, followed by 40 cycles at 95°C
for 15 sec and 64°C for 30 sec, and subsequently followed by 95°C
for 15 sec, 60°C for 1 min, 95°C for 15 sec and a final extension
step at 60°C for 15 sec. The expression levels of the target genes
were determined following subtraction of the expression levels of
GAPDH, with the value for the control group designated as 1 using
the 2−ΔΔCq method (20).
Immunohistochemistry assay
Histological sections were heated for 60 min at
60°C, deparaffinized through xylene and rinsed in a decreasing
gradient of alcohol (100, 95 and 70%). Subsequently, antigens were
retrieved with citrate buffer (0.01 M; pH 6) in a microwave oven.
Following treatment with hydrogen peroxide at 37°C for 30 min to
block endogenous peroxidase activity, the sections were incubated
with a primary antibody overnight at 4°C and subsequently incubated
with a peroxidase-conjugated affiniPure monoclonal goat anti-mouse
or anti-rabbit immunoglobulin G secondary antibody (PV-9001 or
PV-9002; Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing,
China) for 30 min at room temperature. Finally, sections were
stained with diaminobenzidine (Zhongshan Goldenbridge Biotechnology
Co., Ltd.) for 1 min, and subsequently counterstained with H&E
stain for a further 20 sec. The following primary antibodies were
used: Monoclonal mouse anti-rat BMP-7 (1:200 dilution; cat. no.
ab54904; Abcam, Cambridge, UK), polyclonal rabbit anti-rat collagen
I (1:200 dilution; cat. no. ab34710; Abcam), polyclonal rabbit
anti-rat α-SMA (1:200 dilution; cat. no. ab5694; Abcam), polyclonal
rabbit anti-rat TIMP-1 (1:200 dilution; cat. no. ab61224; Abcam)
and monoclonal rabbit anti-rat p-Smad 1/5 (1:100 dilution; cat. no.
13820; Cell Signaling Technology, Inc., Danvers, MA, USA).
Western blotting
The total protein was extracted by homogenizing
liver tissues in RIPA Lysis and Extraction buffer (Thermo Fisher
Scientific) supplemented with a protease inhibitor cocktail from
Roche (Summerville, NJ, USA). Subsequently, equal quantities of
proteins were separated by 8–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a nitrocellulose membrane. After blocking with 5%
milk for 2 h at room temperature, the membranes were incubated with
primary antibody (monoclonal mouse anti-rat BMP-7 (1:300 dilution;
cat. no. ab54904; Abcam), monoclonal mouse anti-rat Smad 1/5
(1:300; cat. no. ab75273; Abcam), monoclonal rabbit anti-rat p-Smad
1/5 (1:300 dilution; cat. no. 13820; Cell Signaling Technology,
Inc.), polyclonal rabbit anti-rat collagen I (1:300 dilution; cat.
no. ab34710; Abcam), monoclonal mouse anti-rat collagen III (1:300
dilution; cat. no. ab6310; Abcam), polyclonal rabbit anti-rat α-SMA
(1:300 dilution; cat. no. ab5694; Abcam), polyclonal rabbit
anti-rat TIMP-1 (1:300 dilution; cat. no. ab61224; Abcam) and
polyclonal rabbit anti-rat β-actin (1:5,000 dilution; cat. no.
ab25894; Abcam) overnight at 4°C, and subsequently with secondary
with secondary antibodies [peroxidase-conjugated affiniPure goat
Anti-mouse (1:5,000 dilution; cat. no. ZB-2305) or anti-rabbit
immunoglobulin G (1:5,000 dilution; cat. no. ZB-2301); both from
Zhongshan Goldenbridge Biotechnology Co., Ltd.] at room temperature
for 60 min. The blots were visualized using an enhanced
chemiluminescence kit (Merck Millipore, Billerica, MA, USA). All
primary antibodies were diluted 1:300, with the exception of
β-actin (1:5,000).
Statistical analysis
The data are presented as the mean ± standard
deviation, unless otherwise specified. All statistical analyses
were performed with SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA). The differences between the means were analyzed using one-way
analysis of variance and the least significant difference was used
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
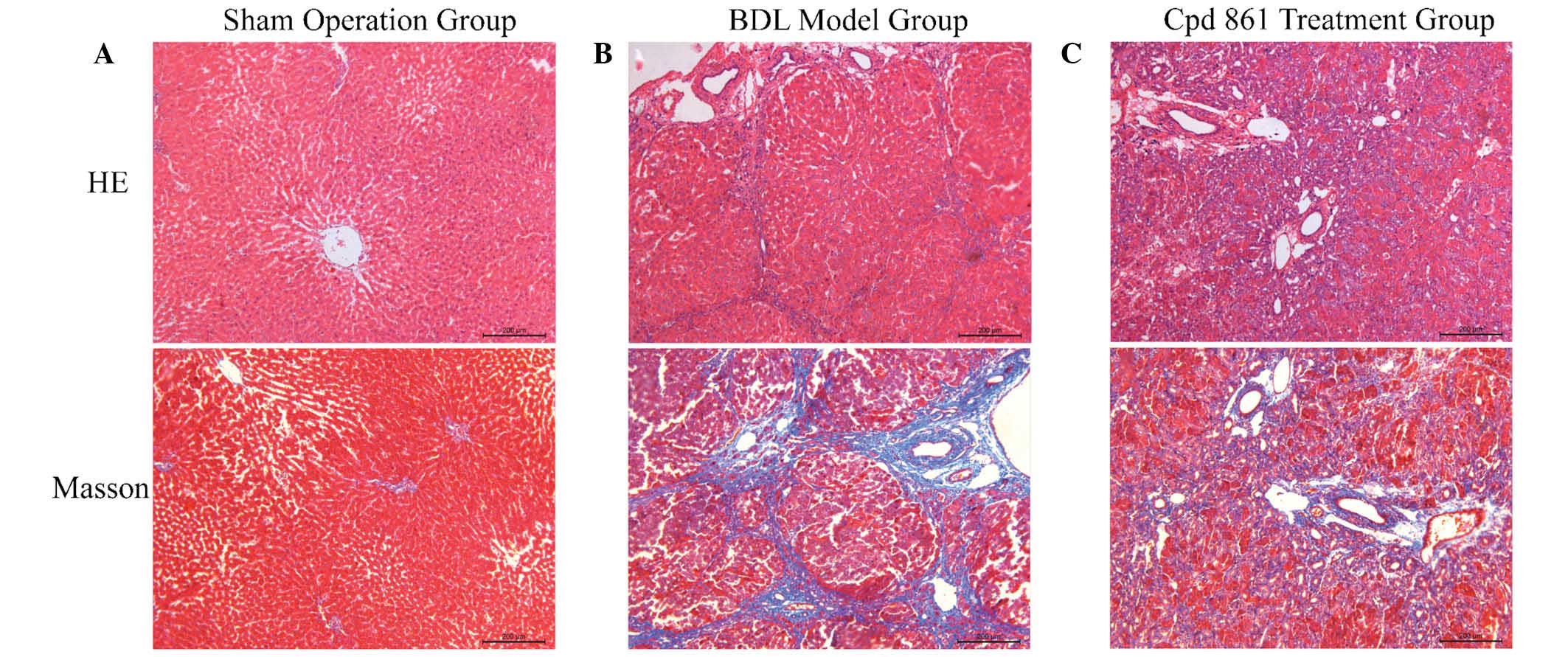
Cpd 861 reduces collagen deposition and
tissue damage in the livers of rats induced by BDL
To evaluate the effects of Cpd 861 on BDL-induced
hepatic fibrosis in rats, the histological changes of liver tissues
were detected using H&E and Masson staining. As shown in
Fig. 1, the sham-operation group
exhibited normal H&E and Masson staining around the vessels,
and a normal lobular architecture, with central veins and radiating
hepatic cords. However, the BDL model group exhibited disturbed
liver lobules characterized by a mess of deposition of fibrous
tissue, which formed membrane-like intervals in the lobules of the
liver, resulting in the formation of pseudo-lobules. By contrast,
collagenous fibers were decreased, and pathological changes were
markedly ameliorated, in the Cpd 861 treatment group. In addition,
intrahepatic small bile duct proliferation was observed in the
portal area in the BDL model group, although this was reduced in
the Cpd 861 treatment group, indicating a role for Cpd 861 in the
improvement of hepatic fibrosis.
Cpd 861 improves serum biochemical
parameters in the BDL model rat
As shown in Fig. 2,
when compared with the sham-operation group, BDL treatment
significantly increased the serum levels of ALT, AST, TBIL and DBIL
(all P<0.05). Although the addition of Cps 861 exerted no
significant effect on ALT and AST compared with the BDL treatment,
it did elicit a significant reduction in the serum levels of TBIL
and DBIL in the BDL model group (P<0.05), suggesting that Cpd
861 attenuates cholestasis in the BDL model group.
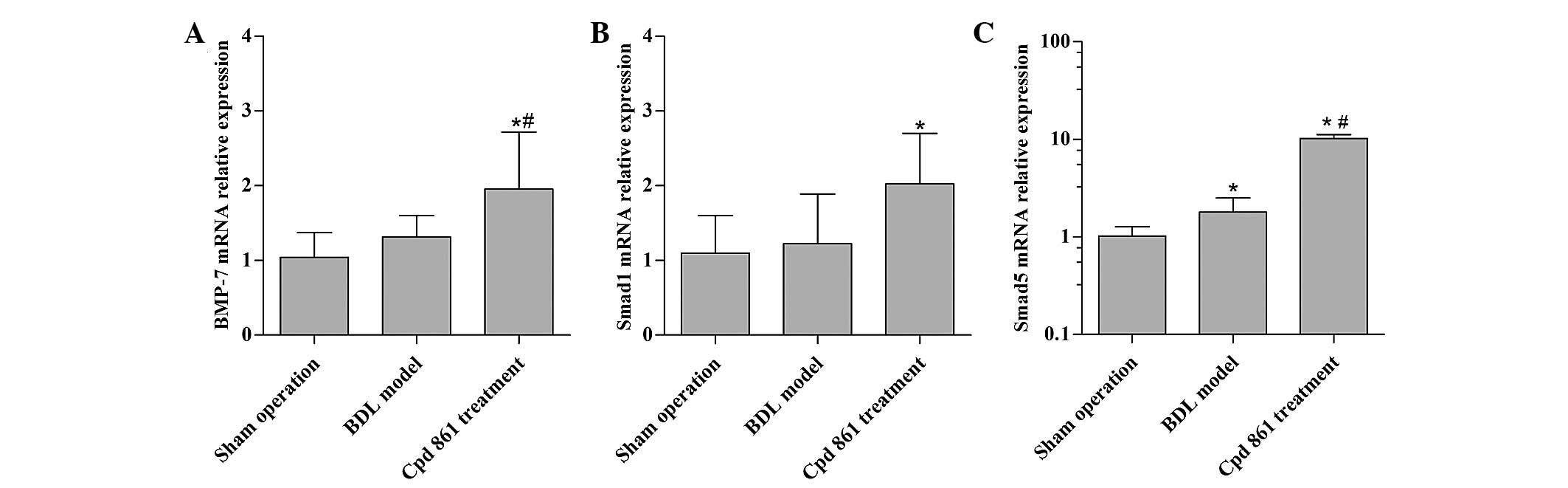
Cpd 861 upregulates BMP-7/Smad
signaling-associated genes in the BDL model rats
To assess the anti-fibrotic mechanism of Cpd 861,
the effect of Cpd 861 on the expression of BMP-7/Smad
signaling-associated genes was first assessed. When compared with
the sham operation control group, the BDL model group revealed an
increased gene expression of Smad5, although no significant
influence on the expression levels of BMP-7 and Smad1 were
observed. When compared with the BDL model group, Cpd 861 treatment
significantly increased the gene expression of BMP-7 and Smad5
(P<0.05), and modestly increased the expression of Smad1
(P>0.05; Fig. 3).
It should be noted that the expression of Smad8 in
the Cpd 861 treatment group was lower compared with that in the BDL
model group (data not shown).
Cpd 861 reduces the expression of
fibrosis-associated proteins, although it increases BMP-7/smad
signaling, as detected by immunohistochemistry
To assess the mechanism by which Cpd 861 treatment
attenuates hepatic fibrosis in BDL model rats, the expression and
localization of several proteins involved in fibrosis formation and
BMP-7/Smad signaling were further investigated. The expression
levels of fibrosis-associated proteins, including collagen I, α-SMA
and TIMP1, were revealed to be negligible in the sham-operation
group (Fig. 4A), although these
proteins were observed in the BDL fibrosis model group (Fig. 4B). However, the expression levels
of these fibrosis-associated proteins were downregulated on
treatment with Cpd 861 (Fig. 4C),
suggesting that Cpd 861 inhibits the progression of hepatic
fibrosis. Furthermore, a negligible expression of BMP-7 and
p-Smad1/5/8 was observed in the sham-operation and BDL model groups
(Fig. 4A and B), which were,
however, upregulated in the Cpd 861 treatment group (Fig. 4C), indicating upregulation of Cpd
861 in the BMP-7/Smad signaling pathway. Additionally, collagen I
and TIMP1 were shown to exhibit positive immunostaining,
predominantly in the hepatocytes and biliary epithelial cells,
whereas α-SMA was predominantly expressed in the mesen-chymal
region of the liver. Positive immunostaining of BMP-7 and
p-Smad1/5/8 were observed in the cytoplasm and nuclei of
hepatocytes, but predominantly in the cytoplasm, indicating that
Cpd 861 activates the BMP-7/Smad signaling pathway.
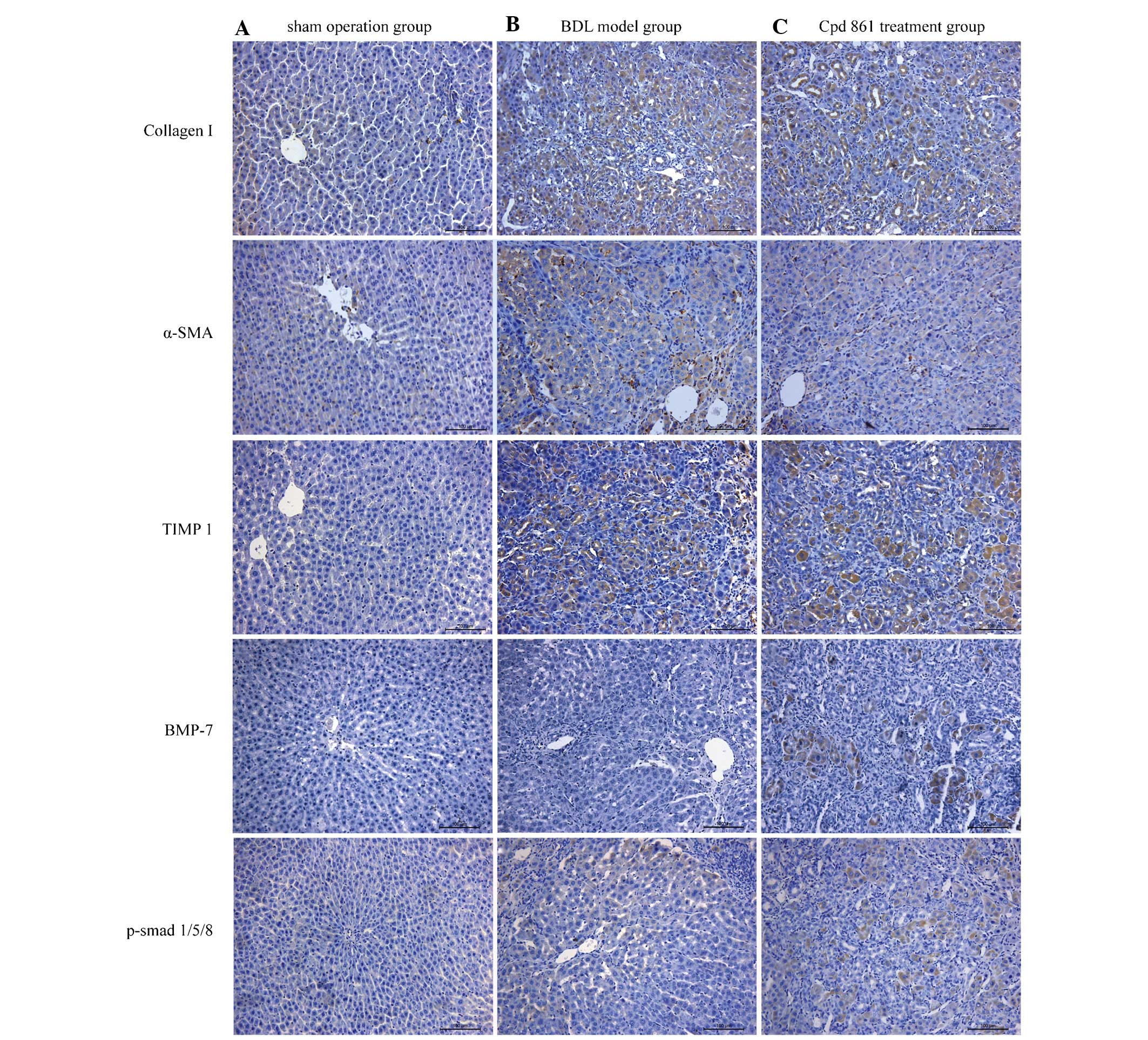 | Figure 4Effect of Cpd 861 on the protein
expression of collagen I, α-SMA, TIMP1, BMP-7 and phosphorylated
Smad1/5/8 analyzed by immunohistochemistry assay (magnification,
×200). Representative images are shown for the (A) sham operation
group, (B) BDL model group and (C) Cpd 861 treatment group. α-SMA,
α-smooth muscle actin; TIMP1, tissue inhibitor of metalloproteinase
1; BMP-7, bone morphogenetic protein 7; BDL, bile duct ligation;
Cpd 861, compound 861. |
Cpd 861 reduces the expression of
fibrosis-associated proteins, but enhances the BMP-7/Smad signaling
pathway, as detected by western blotting
As shown in Fig.
5A–E, BDL induced the expression of collagen I, collagen III,
α-SMA, and TIMP1, although Cpd 861 reversed the BDL effect,
indicating that Cpd 861 was effective at completely inhibiting the
development of cholestatic hepatic fibrosis. In addition, BDL
induced the expression of Smad1/5 and p-Smad1/5/8, although no
significant differences in terms of the protein expression levels
of BMP-7 were observed for the BDL model group (Fig. 5F–H). Cpd 861 treatment further
upregulated the expression of Smad1/5 and p-Smad1/5/8, as well as
increasing the protein expression levels of BMP-7 compared with the
BDL model group. These data further demonstrated that the
BMP-7/Smad signaling pathway is activated during the process of
administering Cpd 861 for hepatic fibrosis treatment.
 | Figure 5Protein expression levels of collagen
I, collagen III, α-SMA, TIMP1, BMP-7 and p-Smad1/5/8 were (A)
evaluated by western blotting and were normalized to the levels of
β-actin. Quantification of the identical data is shown for (B)
collagen I, (C) collagen III, (D) α-SMA, (E) TIMP1, (F) BMP-7, (G)
Smad 1/5 and (H) p-Smad1/5/8. The data are expressed as the mean ±
standard deviation (n=6).*P<0.05 vs. the sham
operation group; #P<0.05 vs. the BDL model group.
α-SMA, α-smooth muscle actin; TIMP1, tissue inhibitor of
metalloproteinase 1; BMP-7, bone morphogenetic protein 7; BDL, bile
duct ligation; Cpd 861, compound 861. |
Discussion
Hepatic fibrosis is a complex pathophysiological
process, involving various growth factors and their underlying
signaling networks. It should be noted that the TGF-β signaling
pathway is considered to be an activator of HSCs, and exerts a
crucial role in fibrogenesis (21). Once activated, HSCs acquire the
capacity to proliferate, and to secrete α-SMA and various other
chemoattractant factors. Additionally, activated HSCs exhibit an
altered expression profile of collagens, MMPs and TIMPs (22). In accordance with our data,
increased protein expression levels of collagen I, collagen III,
α-SMA and TIMP1 were observed during BDL-induced fibrogenesis.
Cpd 861 has been confirmed by liver biopsies to be
an effective compound for the treatment of hepatic fibrosis.
Several studies have revealed that Cpd 861 inhibits the expression
of collagen synthesis-associated genes and the MMP-1 gene (15,23,24),
and that the activation and proliferation of HSCs is inhibited
(25). In the present study, Cpd
861 treatment was observed to reduce the levels of ALT, AST, TBIL
and DBIL, suggesting a protective effect of Cpd 861 in liver
function and a beneficial role in alleviating cholestasis. The
H&E and Masson staining experiments confirmed that Cpd 861
ameliorated the pathological changes of the liver, decreased the
synthesis of collagenous fiber, reduced intrahepatic small bile
duct proliferation and reduced the levels of apoptosis in the
hepatocytes. Furthermore, the protein expression levels of collagen
I, collagen III, α-SMA and TIMP1 were decreased following Cpd 861
treatment. Taken together, these results have confirmed that Cpd
861 is a potentially useful anti-fibrotic agent.
On the other hand, BMP-7 is one member of the BMP
family that is expressed in a number of organized regions of the
early embryo, and was originally described in terms of its ability
to accelerate bone formation (26). Subsequently, a number of studies
have demonstrated that BMP-7 possesses anti-inflammatory and
anti-fibrotic properties in the fibrotic model (10), and is able to attenuate hepatic
fibrosis by negatively regulating the functions of TGF-β1 (11,12,27).
Studies have also indicated that the actions of BMP-7 are
transduced by Smad1, Smad5 and Smad8, which subsequently form a
complex with Smad4 to enter the nucleus to regulate gene
expression, whereas Smad2 and Smad3 regulate the activity of TGF-β1
(28–30). On the basis of the above-mentioned
evidence, in the present study a rat BDL model was used to
determine the mechanism(s) via which Cpd 861 regulates the fibrotic
process, and the results have revealed that the gene and protein
expression levels of components of the BMP-7/Smad signaling pathway
were elevated following treatment with Cpd 861. In addition, BMP-7
and p-Smad1/5/8 were observed in the cytoplasm and nuclei of the
hepatocytes. These results indicated that the BMP-7/Smad1/5/8
signaling pathway was activated on treatment with Cpd 861.
Nevertheless, the present study had certain
limitations. Further studies are required to evaluate the effects
of different dosages and durations of Cpd 861 treatment on the
BMP-7/Smad signaling pathway induced by BDL, and to clarify the
possible underlying molecular mechanisms.
In conclusion, we have demonstrated that an
attenuation of hepatic fibrosis and an amelioration of liver
function were detected in the Cpd 861-treated group, as indicated
by decreased protein levels of collagen I, collagen III, α-SMA and
TIMP1. Furthermore, elevated protein expression levels of BMP-7,
Smad1/5 and p-Smad1/5/8 were observed following treatment with Cpd
861 during hepatic fibrosis. Therefore, our collected data support
the concept that Cpd 861 attenuates hepatic fibrosis via the
upregulation of BMP-7/Smad signaling.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China [no. 81341090] and the WBE Liver
Fibrosis Foundation [no. CFHPC20120145]. We would also like to
thank Medjaden Bioscience Limited for assisting in the preparation
of this paper.
References
|
1
|
Lim YS and Kim WR: The global impact of
hepatic fibrosis and end-stage liver disease. Clin Liver Dis.
12:733–746. vii2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moreira RK: Hepatic stellate cells and
liver fibrosis. Arch Pathol Lab Med. 131:1728–1734. 2007.PubMed/NCBI
|
|
3
|
Brenner DA, Waterboer T, Choi SK,
Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A and
Rippe RA: New aspects of hepatic fibrosis. J Hepatol. 32(Suppl 1):
S32–S38. 2000. View Article : Google Scholar
|
|
4
|
Okuno M, Akita K, Moriwaki H, Kawada N,
Ikeda K, Kaneda K, Suzuki Y and Kojima S: Prevention of rat hepatic
fibrosis by the protease inhibitor, camostat mesilate, via reduced
generation of active TGF-β. Gastroenterology. 120:1784–1800. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ling H, Roux E, Hempel D, Tao J, Smith M,
Lonning S, Zuk A, Arbeeny C and Ledbetter S: Transforming growth
factor β neutralization ameliorates pre-existing hepatic fibrosis
and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS
One. 8:e544992013. View Article : Google Scholar
|
|
6
|
Yata Y, Gotwals P, Koteliansky V and
Rockey DC: Dose-dependent inhibition of hepatic fibrosis in mice by
a TGF-β soluble receptor: Implications for antifibrotic therapy.
Hepatology. 35:1022–1030. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reddi AH: Role of morphogenetic proteins
in skeletal tissue engineering and regeneration. Nat Biotechnol.
16:247–252. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huo L, Liu K, Pei J, Yang Y, Ye Y, Liu Y,
Sun J, Han H, Xu W and Gao Y: Fluoride promotes viability and
differentiation of osteoblast-like Saos-2 cells via BMP/Smads
signaling pathway. Biol Trace Elem Res. 155:142–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kretzschmar M and Massagué J: SMADs:
Mediators and regulators of TGF-β signaling. Curr Opin Genet Dev.
8:103–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang SL, Yang CQ, Qi XL, Yuan M, Chang YZ,
Yang L and Gao HJ: Inhibitory effect of bone morphogenetic
protein-7 on hepatic fibrosis in rats. Int J Clin Exp Pathol.
6:897–903. 2013.PubMed/NCBI
|
|
11
|
Chen BL, Peng J, Li QF, Yang M, Wang Y and
Chen W: Exogenous bone morphogenetic protein-7 reduces hepatic
fibrosis in Schistosoma japonicum-infected mice via transforming
growth factor-β/Smad signaling. World J Gastroenterol.
19:1405–1415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeisberg M, Hanai J, Sugimoto H, Mammoto
T, Charytan D, Strutz F and Kalluri R: BMP-7 counteracts
TGF-beta1-induced epithelial-to-mesenchymal transition and reverses
chronic renal injury. Nat Med. 9:964–968. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinoshita K, Iimuro Y, Otogawa K, Saika S,
Inagaki Y, Nakajima Y, Kawada N, Fujimoto J, Friedman SL and Ikeda
K: Adenovirus-mediated expression of BMP-7 suppresses the
development of liver fibrosis in rats. Gut. 56:706–714. 2007.
View Article : Google Scholar
|
|
14
|
Yin SS, Wang BE, Wang TL, Jia JD and Qian
LX: The effect of Cpd 861 on chronic hepatitis B related fibrosis
and early cirrhosis: A randomized, double blind, placebo controlled
clinical trial. Zhonghua Gan Zang Bing Za Zhi. 12:467–470. 2004.In
Chinese. PubMed/NCBI
|
|
15
|
Wang L, Wang BE, Wang J, Xiao PG and Tan
XH: Herbal compound 861 regulates mRNA expression of collagen
synthesis- and degradation-related genes in human hepatic stellate
cells. World J Gastroenterol. 14:1790–1794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Wang J, Wang BE, Xiao PG, Qiao YJ
and Tan XH: Effects of herbal compound 861 on human hepatic
stellate cell proliferation and activation. World J Gastroenterol.
10:2831–2835. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aghaei I, Shabani M, Doustar N, Nazeri M
and Dehpour A: Peroxisome proliferator-activated receptor-γ
activation attenuates motor and cognition impairments induced by
bile duct ligation in a rat model of hepatic cirrhosis. Pharmacol
Biochem Behav. 120:133–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Javadi-Paydar M, Ghiassy B, Ebadian S,
Rahimi N, Norouzi A and Dehpour AR: Nitric oxide mediates the
beneficial effect of chronic naltrexone on cholestasis-induced
memory impairment in male rats. Behav Pharmacol. 24:195–206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uchinami H, Seki E, Brenner DA and
D'Armiento J: Loss of MMP 13 attenuates murine hepatic injury and
fibrosis during cholestasis. Hepatology. 44:420–429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Shen H, Huang G, Hadi M, Choy P, Zhang M,
Minuk GY, Chen Y and Gong Y: Transforming growth factor-beta1
downregulation of Smad1 gene expression in rat hepatic stellate
cells. Am J Physiol Gastrointest Liver Physiol. 285:G539–G546.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang T, Chen SL, Lu XJ, Shen CY, Liu Y and
Chen YP: Bone morphogenetic protein 7 suppresses the progression of
hepatic fibrosis and regulates the expression of gremlin and
transforming growth factor β1. Mol Med Rep. 6:246–252.
2012.PubMed/NCBI
|
|
23
|
Ding HG, Tang SZ, Wang BE, Jia JD and Zhao
CH: Effects of herbal compound 861 on hepatic stellate cell
expressing endo-thelin-1 protein and mRNA. Chin J Hepatol.
11:3082003.In Chinese.
|
|
24
|
Yin C, Ma H, Wang A, Ma X, Jia J and Wang
B: Effect of compound 861 on tissue inhibitor of metalloproteinase
1 gene expression of HSC-T6 cells. Chin J Hepatol. 10:197–199.
2002.In Chinese.
|
|
25
|
You H, Wang B and Wang T: Proliferation
and apoptosis of hepatic stellate cells and effects of compound 861
on liver fibrosis. Chin J Hepatol. 8:78–80. 2000.In Chinese.
|
|
26
|
Cheifetz S, Li IW, McCulloch CA, Sampath K
and Sodek J: Influence of osteogenic protein-1 (OP-1;BMP-7) and
transforming growth factor-beta 1 on bone formation in vitro.
Connect Tissue Res. 35:71–78. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang LP, Dong JZ, Xiong LJ, Shi KQ, Zou
ZL, Zhang SN, Cao ST, Lin Z and Chen YP: BMP-7 attenuates liver
fibrosis via regulation of epidermal growth factor receptor. Int J
Clin Exp Pathol. 7:3537–3547. 2014.PubMed/NCBI
|
|
28
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wrana JL: Regulation of Smad activity.
Cell. 100:189–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang S and Hirschberg R: Bone
morphogenetic protein-7 signals opposing transforming growth factor
beta in mesangial cells. J Biol Chem. 279:23200–23206. 2004.
View Article : Google Scholar : PubMed/NCBI
|