Introduction
Spinal cord injury (SCI) is a devastating clinical
condition for which there is currently no fully restorative
treatment. Following SCI, the resulting scar tissue and myelin
debris can produce a hostile environment in which neurite outgrowth
and axonal regeneration is hampered by the limited intrinsic
regenerative capacity of injured neurons, due to the lack of
neurotrophic support and the presence of growth inhibitory
molecules in myelin (1,2). Specific myelin components, such as
myelin-associated glycoprotein (MAG) and the C-terminal of Nogo-A
(Nogo-66), are major inhibitory molecules of spinal cord repair
after SCI (3,4).
Nogo protein is a central myelin-derived proteins
expressed in the white matter of central neural system (CNS), and
has been confirmed to block axon regeneration following injury
(5). Three isoforms (Nogo-A, -B,
and -C) are generated through alternative splicing and differential
promoter usage from a single Nogo gene. The inhibitory action of
the Nogo protein is dependent on the Nogo-66 domain, which is
present in all three isoforms. Nogo-66 is a 66-amino-acid sequence
between N- and C-terminals. Nogo-A exerts its inhibitory effects by
binding to the Nogo receptor via the Nogo-66 domain. Studies have
shown that the Nogo-66 high-affinity receptor (NgR) is a common
receptor of Nogo, MAG and oligodendrocyte myelin glycoprotein
(OMgp) (6–14). The NgR is a member of a family of
three CNS-enriched glycosyl phosphatidylinositol-linked proteins
(6–8). NgR functions as the ligand binding
component of a tripartite receptor system consisting of Lingo-1 and
tumour necrosis factor (TNF) receptor family members, p75NTR or
TROY (9). Inhibition of NgR with
function-blocking antibodies or short hairpin RNA interference
(shRNA)-mediated knockdown have been reported to demonstrate that
NgR is essential for Nogo66, MAG and OMgp inhibitory effects
(10–12). NgR mRNA is detected in numerous
types of neurons in the CNS and distribution of NgR protein is
consistent with its mRNA (13,14).
The expression of myelin inhibitors or receptor-related proteins
can be knocked down by RNA interference to reduce neuronal
apoptosis and promote axonal regeneration after injury (15,16).
Growth Associated Protein 43 (GAP-43), a plasticity
and growth protein, is expressed at high levels in neuronal growth
cones during development and axonal regeneration (17,18).
GAP-43 is a crucial component of the axon and presynaptic terminal,
and its nonsense mutation causes defects in motor axon outgrowth
and pathfinding (19).
A novel therapeutic strategy is required for neuron
regeneration and SCI repair. The aim of the present study was to
establish an efficient and highly specific shRNA expression and
delivery system to knock down the Nogo gene and to detect effects
of the Nogo shRNA system on SCI repair in a rat model.
Materials and methods
Animals
Sprague Dawley rats (n=40; age, 2–3 weeks) the Basic
Medicine Animal Laboratory of Jilin University were housed at a
room temperature of 22±2°C with a 12-h dark:light cycle and free
access to food and water. The present study was approved by the
Jilin University Animal Ethics Committee (Changchun, China).
Preparation of Nogo-A shRNA
The Nogo-66 gene has 66 amino acids between two
transmembrane regions of Nogo proteins. The expression of Nogo-66
has a strong inhibitory effect on CNS regeneration (20). Two specific shRNA sequences were
designed to target the cDNA sequence of Nogo-66 (1024–1089 amino
acid fragment) and the primers were as follows: Sense, 5′-GGG CGT
GAT CCA GGC TATCTT-3′ and antisense, 5′-GAT AGC CTG GAT CAC
GCCCTT-3′; sense 5′-GGC CAC CCA TTC AGG GCATTT-3′ and antisense,
5′-ATG CCC TGA ATG GGT GGCCTT-3′. Non-effective scrambled shRNA was
used as a negative control, and sequences were as follows: Sense,
5′-GGC GGT AGT CAC GGT CATCTT-3′ and antisense, 5′-GAT GTG ACC GTG
ACT ACC GCCTT-3′. The pShuttle-U6 (Department of Immunology, Jilin
University School of Medicine, Jilin, China) was used as a template
for polymerase chain reaction (PCR). The upstream primers targeting
the U6 promoter and downstream primers with a Nogo-A gene reverse
complementary target sequence were synthesized by Sangon
Bioengineering, Inc. (Shanghai, China). The sequences were as
follows: Upstream, 5′-CTCGAGCCCCAGTGGAAAGACGCG-3′ for U6F and
downstream, 5′-GGA TCC AAA AAA GAT AGC CTG GAT CAC GCC CTT CAA GCT
TCA AGG GCG TGA TCC AGG CTA TCG GTG TTT CGT CCT TTC CACAA-3′;
5′-GGA TCC AAA AAA ATG CCC TGA ATC CCT GGC CTT CAA GCT TCA AGG CCA
CCC ATT CAG GGC ATG GTG TTT CGT CCT TTC CACAA-3′; and 5′-GGA TCC
AAA AAA GAT GTG ACC GTG ACT ACC GCC TCA AGC TTC GGC GGT AGT CAC GGT
CAT CTT GGT GTT TCG TCC TTT CCA CAA-3′ for primers of hairpins 1, 2
and 3, respectively. The PCR systems were composed of 1 µl
pShuttle-U6 (100 ng/µl), 2.5 µl U6F (10
pmol/µl), 2.5 µl hairpins (10 pmol/µl), 3
µl dNTP (2.5 mmol/l), 5 µl 10X Buffer, 3 µl
MgCl2, 0.5 µl Taq DNA polymerase, 0.1 µl
Pfu DNA polymerase, 2 µl DMSO and double distilled
H2O up to 50 µl. The PCR cycling conditions were
as follows: 94°C for 5 min; 94°C 30 sec, 55°C for 30 sec, 72°C for
1 min, 30 cycles and 72°C for final extension. The PCR products
that were amplified using the Bio-Rad T100 thermocycler (Bio-Rad
Laboratories, Shanghai, China) were named U6shNogo1, U6shNogo2,
U6shNogo3 and U6shNogo3 containing. Scrambled shRNA was used as the
negative control. The U6shNogos were ligated into the pUCm-T vector
(Bio Basic Inc., Markham, ON, Canada), and the ligation products
were transfected into E. coli DH5α competent cells. The
plasmids were extracted and purified using an Aurum Plasmid Mini
kit (cat no. 732-6400, Bio-Rad Laboratories). U6shNogos were cut
from the T-vector with EcoRI and SalI, and then cloned into the
AdMaxTM adenovirus shuttle plasmids pDC316 (Microbix Biosystems
Inc., Toronto, ON, Canada), to construct the pDCU6shNogo1,
pDCU6shNogo2 and pDCU6shNogo3 (with the scrambled sequence as the
negative control) plasmids. The fragment of interest was cut by
EcoRI and SalI restriction enzymes for identification. AdMax
Systems (Microbix Biosystems Inc., Toronto, ON, Canada) were used
as the adenovirus packaging system to package the recombinant
pDCU6shNogos, and the resulting recombinant virus (rAdUNogos) was a
replication deficient adenovirus due to missing E1/E3 regions.
Interference of the endogenous Nogo gene
by adenovirus-mediated Nogo shRNA transfection via local injection
in SCI model rats
Sprague Dawley rats (n=40) underwent spinal cord
hemisection for modeling of SCI according to the methods of
previous studies (21–24). After 7 days, transfection of the
recombinant adenovirus carrying pDCU6shNogos into SCI rats was
performed by direct local injection of 5 µl rAdUNogos
containing 10 µg Nogo shRNA into the spinal cord. The rats
were divided randomly into four separate groups as follows: Group
A, 10 µg shRNA Nogo1 (rAdUshNogo1); group B, 10 µg
shRNA Nogo2 (rAdUshNogo2); group C, 10 µg shRNA Nogo3
(rAdUshNogo3 containing the chaotic sequence) served as the
negative control; and group D, model control, saline containing 10
µg empty adenoviral vector.
At 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 days after
injection, the animals in each group were narcotized with sodium
pentobarbital (30–40 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) and
sacrificed by cervical dislocation.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the spinal cord at the
location of the injury 3 days subsequent to injection. Nogo mRNA
was detected by RT-qPCR. Briefly, total RNA was transcribed into
cDNAs. PCR amplification was performed using cDNA as the template.
Nogo-A primers were as follows: Sense, 5′-TCA AAG GTG ACT GAG
GCAGC-3′ and antisense, 5′-ACT GGG CTG CAC TAC AGAAG-3′. Primers
for the internal reference, GAPDH were as follows: Sense, 5′-GGG
CCA AAA GGG TCA TCATC-3′ and antisense, 5′-AAC CTG GTC CTC AGT
GTAGC-3′. The PCR system of 30 µl was composed 2 µl
of the cDNA template (100 ng/µl), 3 µl 10X buffer,
0.5 µl dNTP (10 mmol/l), 0.5 µl Taq DNA polymerase, 3
µl MgCl2 (25 mmol/l), 1 µl primers (10
pmol/l) for each, double distilled H2O, 19 µl.
The PCR thermocycling conditions were as follows: 94°C for 1 min;
94°C 30 sec, 55°C for 45 sec, 72°C for 1 min, 30 cycles and 72°C
for 3 min for the final extension. The PCR products were separated
on a 1% agarose gel.
Western blotting
The Nogo-A protein expression levels of each group
were detected by western blotting 3 days after injection. GAP-43
protein expression levels in the shRNA Nogo1 group and the model
control 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 days after injection were
also determined by western blotting. The procedures were performed
according to the manufacturer's protocol (Thermo Scientific Pierce
Fast Western Blot kit, ECL substrate; cat no. 35050; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) as described previously
(25). Briefly, the spinal cord
tissues were homogenated and lysed with radioimmunoprecipitation
assay buffer. Proteins were separated on an 10% SDS-PAGE gel and
electroblotted onto a polyvinylidene fluoride membrane.
Consecutively, the membrane was blocked in 5% skimmed milk (Inner
Mongolia Yili Industrial Group Co., Ltd., Hohhot, China) in PBS-T
at room temperature for 2 h, incubated in either anti-Nogo-A
(diluted 1:2,000; cat no. sc-25660) or anti-GAP-43 rabbit
polyclonal antibodies (diluted 1:500; cat no. sc-10786) all
purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA)
overnight at 4°C, incubated in horseradish peroxidase-conjugated
anti-rabbit IgG (diluted 1:5,000; cat no. 31460; Thermo Fisher
Scientific, Inc.) at room temperature for 1 h, and visualized using
enhanced chemiluminescence reagent. β-actin (diluted 1:5,000; cat
no. sc-130656) was used as the housekeeping internal reference.
Images were analyzed using Image-Pro Plus (version 6.0; Media
Cybernetics Inc., Rockville, MD, USA).
In addition, the functional recovery of rats with
SCI was assessed through the Basso, Beattie and Bresnahan (BBB)
scoring system (26–30), 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10
days after injection.
Statistical analysis
Data were analyzed using SPSS statistical software
(version 17.0; SPSS, Chicago, IL, USA). Student's t-test and
analysis of variance, with Duncan's multiple range test post-hoc
analysis, were performed for comparison. P<0.05 was considered
to indicate a statistically significant difference.
Results
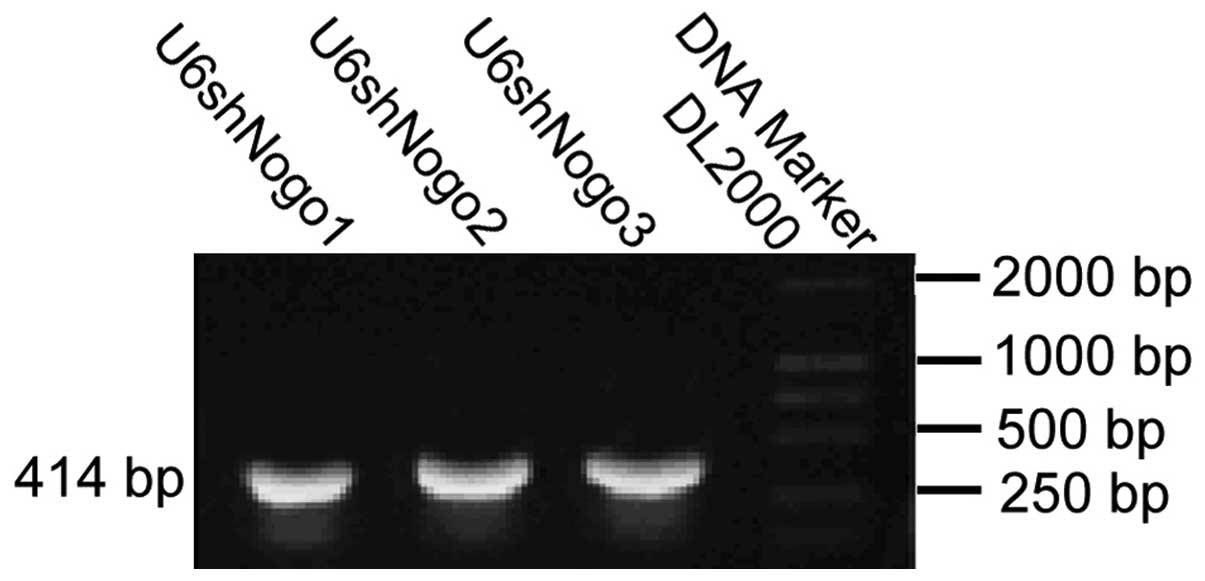
Construction of small hairpins with the
U6 promoter
The upstream primer and the downstream primer with a
reverse primer complementary target sequence of the U6 promoter
were synthesized. The pShuttle-U6 was used as a template, U6F as
upstream primers, hairpins 1, 2 and 3 as the downstream primer. A
414 bp fragment with a U6 promoter and short hairpin loop was
obtained (Fig. 1). U6shNogos were
cut from the T vector with EcoRI and SalI, and then
cloned into pDC316, to construct the pDCU6shNogo1, pDCU6shNogo2 and
pDCU6shNogo3 plasmids. The 414 bp fragment was released by
EcoRI and SalI restriction digestion, thus it was
demonstrated that U6shNogos were properly inserted into the pDC316
plasmid.
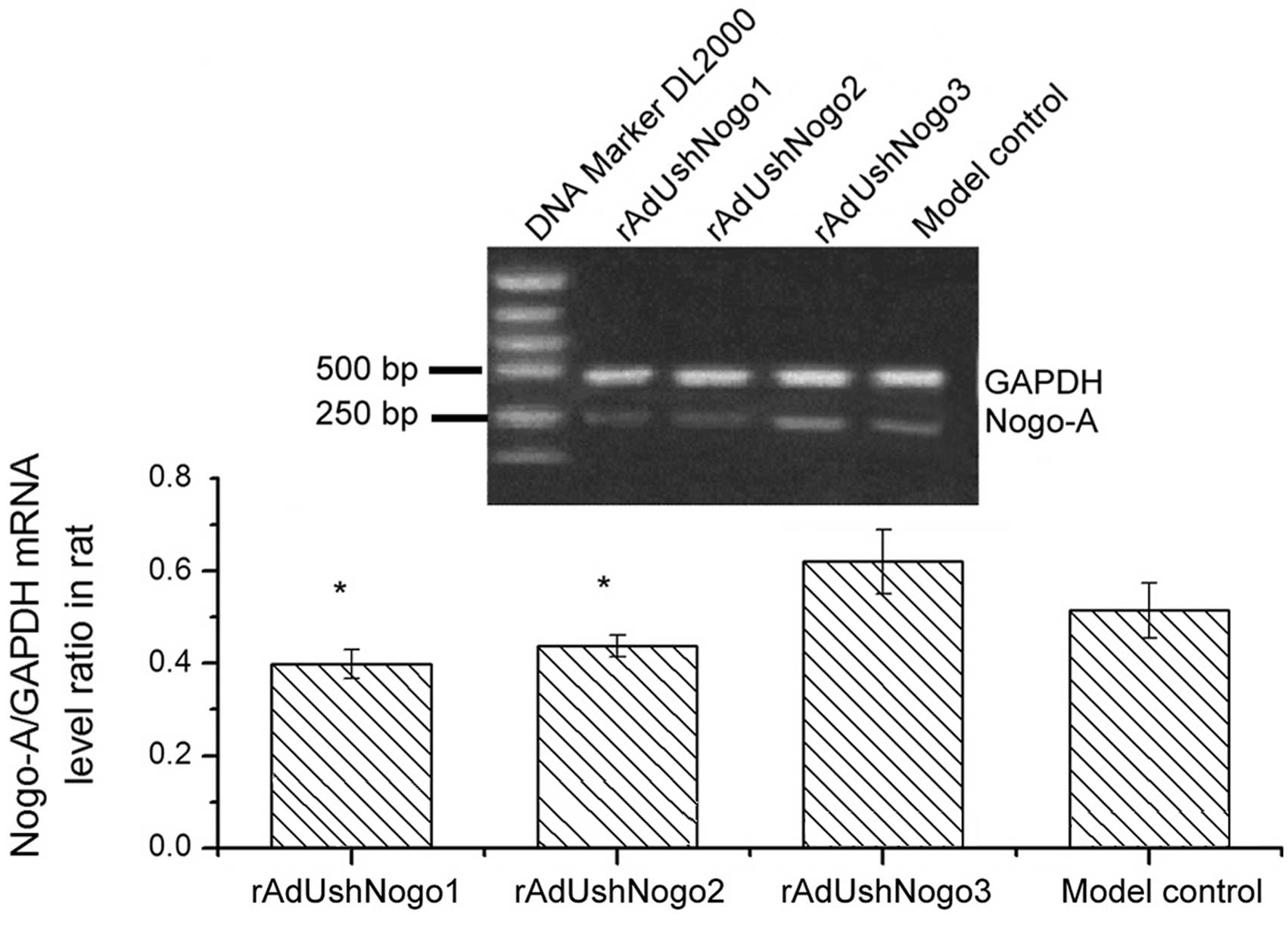
Nogo-A mRNA levels in the rats with SCI
following injection with rAdUshNogos
RT-PCR results showed that, compared with the
rAdUshNogo3 group, Nogo mRNA relative expression levels of
rAdUshNogo1 and rAdUshNogo2 groups were significantly reduced by
35.5% (P<0.05) and 31.5% (P<0.05), respectively. Compared
with the model control, the expression level of Nogo mRNA in the
oligodendrocytes of the rAdUshNogo3 group was not identified to be
significantly different (P>0.05). Thus, the shRNAs were
successfully transcribed in animals, and participated in the
transcriptional regulation of the Nogo-A gene. shRNA interference
in vivo effectively inhibited the expression of the Nogo-A
gene, thus providing favorable conditions for regeneration of axons
(Fig. 2).
Nogo-A protein blots of rats with SCI
after injection with rAdUshNogos
Compared with the rAdUshNogo3 group (negative
control), the relative expression of Nogo protein in
oligodendrocytes of rAdUshNogo1 group decreased by 29.5%
(P<0.05), and rAdUshNogo2 group decreased by 28.2% (P<0.05)
(Fig. 3). The shRNAs inhibited
Nogo-A expression at the protein level.
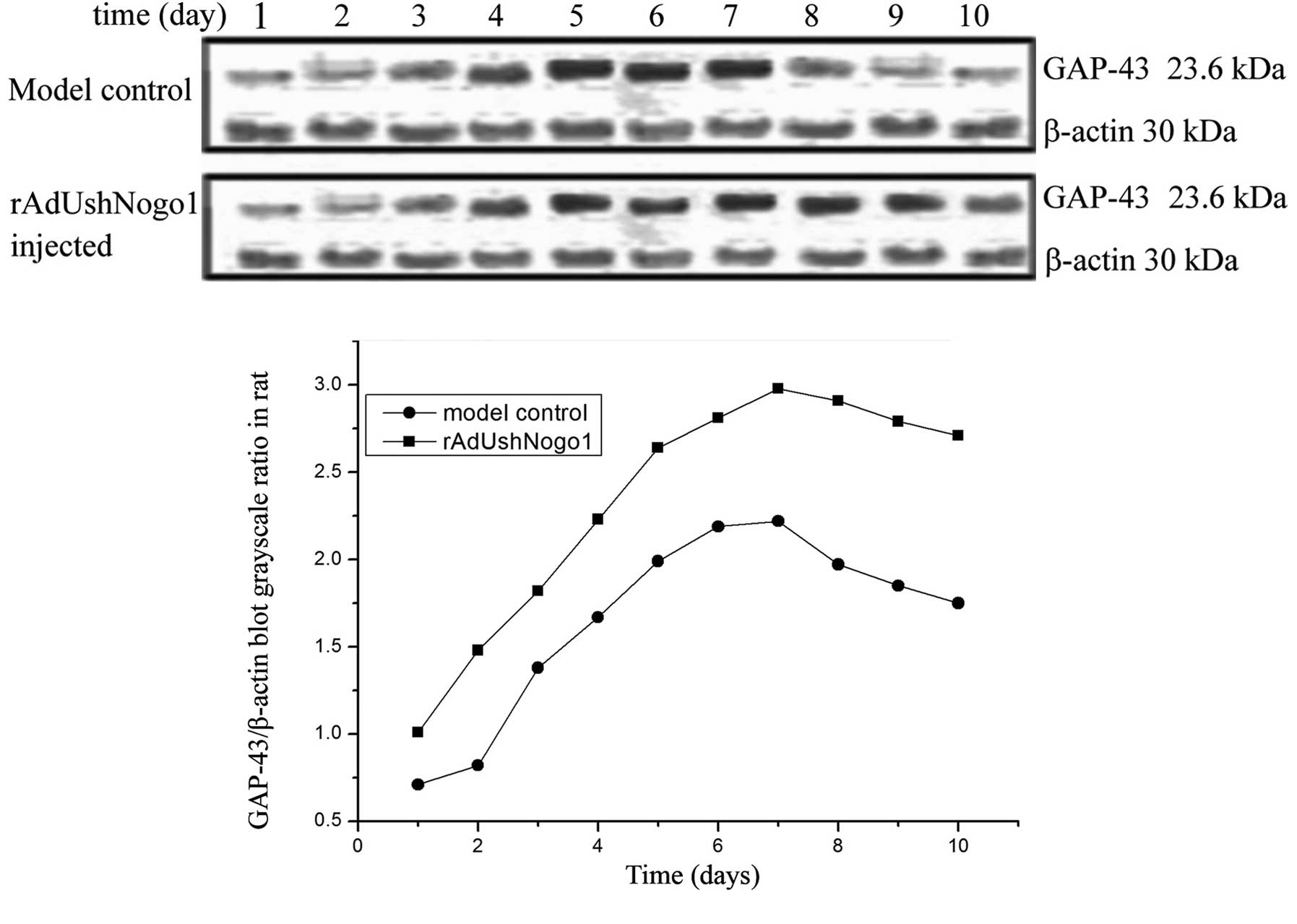
GAP-43 protein blots from rats with SCI
following injection with rAdUshNogo1
The GAP-43 expression levels in the model control
and rAdUshNogo1 group increased. The GAP-43 expression level of the
rAdUshNogo1 group was significantly increased, and reached the
maximum at 7 days. After 2 days, the expression levels of GAP-43 in
the rAdUshNogo1 group were markedly higher compared with the model
control. After 7 days, the GAP-43 expression levels of the
rAdUshNogo1 group and the model control reduced. However, the
expression of GAP-43 in the rAdUshNogo1 group was not significantly
reduced (Fig. 4).
Functional recovery of rats with SCI
following injection with rAdUshNogo1
The BBB scores (0–21 points) of the rats were
determined using a double-blind method as reported in the
literature (31). A lower score
indicated more serious injury. Prior to spinal cord hemisection
surgery, all rats achieved 21 points in the BBB tests. One day
following hemisection, the BBB scores were ~1 for all rats. The BBB
scores were significantly increased in the rAdUshNogo1 group on
days 6, 8, 10 (P<0.05; Fig. 5)
compared with the model control.
Discussion
The spinal cord has a limited capacity for
regeneration and this is largely attributable to the presence of
cellular substrates that are unsuitable for growth. In vitro
and in vivo evidence supports the ability of Nogo-A to
inhibit neurite outgrowth (15,32–34).
In the present study, an animal model of SCI was
generated, and virus-mediated small hairpin RNAs were injected into
the spinal cord region by local injection. Nogo expression in the
SCI region was detected by RT-PCR analysis. Compared with the
rAdUshNogo3 group (negative control), Nogo mRNA relative expression
levels in the SCI region decreased by 45.0% (P<0.05) following
injection with rAdUshNogo1 and 40.0% following injection with
rAdUshNogo2 (P<0.05). This indicated that the adenoviral vector
that was constructed in vivo successfully transcribed short
hairpin RNAs, which participated in Nogo-transcriptional
regulation. rAdUshNogo1 and rAdUshNogo2 effectively suppressed the
expression of the Nogo-A gene in vivo, and rAdUshNogo3 did
not inhibit Nogo-A gene expression, indicating that the designed
small hairpins were specific.
GAP-43 is a specific phosphoprotein of neural
tissue. It is located in neurons, regenerated Schwann cells and
glial cells, and is considered as the molecular marker of axon
growth and plasticity (35,36).
High expression of GAP-43 is considered to be typical in the repair
of nerves, and is closely associated with neuronal growth within
the spinal cord (37,38). In the process of neuronal growth,
GAP-43 may affect axon growth by altering G protein activity of the
growth cones, which guide axonal extension and growth. The
interaction of G protein with its receptor generates inhibitory
signals, resulting in the growth arrest of growth cones (39). When GAP-43 and G-protein bind, the
disinhibition signal allows the axons to continue growth (39).
In the present study, GAP-43 expression in the
spinal cord was increasing following SCI, indicating that GAP-43
participated in the growth and repair of the spinal cord. shRNAs
against Nogo-A were injected into the rats with SCI. The
experimental animals were divided into the rAdUshNogo1 group and
the model control. The rAdUshNogo1 group underwent injection with
rAdUshNogo1 for different time points of 1 to 10 days. The relative
GAP-43 expression was detected by western blotting, with β-actin
was used as a control. The results showed that, GAP-43 expression
increased with time and reached a peak at 7 days. The GAP-43
expression of the rAdUshNogo1 group and the model control gradually
increased, but the GAP-43 expression of the rAdUshNogo1 group was
markedly higher than that of the model control. The BBB scores of
the rAdUshNogo1 group were significantly higher than those of the
model control after 6 days, indicating that the virus-mediated
small hairpin was effectively transcribed in vivo and
inhibited the expression of Nogo gene, which promoted axonal
regeneration.
In conclusion, the adenoviral vector-mediated Nogo
shRNA interference can effectively inhibit the expression of Nogo-A
in oligodendrocytes and in rats with SCI, and upregulate the
production of GAP-43 in rats with SCI. Knockdown by Nogo shRNAs has
the potential to become an effective method for the treatment of
SCI.
References
|
1
|
Chytrova G, Ying Z and Gomez-Pinilla F:
Exercise normalizes levels of MAG and Nogo-A growth inhibitors
after brain trauma. Eur J Neurosci. 27:1–11. 2008. View Article : Google Scholar
|
|
2
|
Filbin MT: Myelin-associated inhibitors of
axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci.
4:703–713. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaudhry N and Filbin MT:
Myelin-associated inhibitory signaling and strategies to overcome
inhibition. J Cereb Blood Flow Metab. 27:1096–1107. 2007.
View Article : Google Scholar
|
|
4
|
Lenzlinger PM, Shimizu S, Marklund N,
Thompson HJ, Schwab ME, Saatman KE, Hoover RC, Bareyre FM, Motta M,
Luginbuhl A, et al: Delayed inhibition of Nogo-A does not alter
injury-induced axonal sprouting but enhances recovery of cognitive
function following experimental traumatic brain injury in rats.
Neuroscience. 134:1047–1056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen MS, Huber AB, van der Haar ME, Frank
M, Schnell L, Spillmann AA, Christ F and Schwab ME: Nogo-A is
amyelin-associated neurite outgrowth inhibitor and an antigen for
monoclonal antibody IN-1. Nature. 403:434–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fournier AE, GrandPre T and Strittmatter
SM: Identification of a receptor mediating Nogo-66 inhibition of
axonal regeneration. Nature. 409:341–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
GrandPré T, Nakamura F, Vartanian T and
Strittmatter SM: Identification of the Nogo inhibitor of axon
regeneration as a Reticulon protein. Nature. 403:439–444. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prinjha R, Moore SE, Vinson M, Blake S,
Morrow R, Christie G, Michalovich D, Simmons DL and Walsh FS:
Inhibitor of neurite outgrowth in humans. Nature. 403:383–384.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu BP, Cafferty WB, Budel SO and
Strittmatter SM: Extracellular regulators of axonal growth in the
adult central nervous system. Philos Trans R Soc Lond B Biol Sci.
361:1593–1610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Domeniconi M, Cao Z, Spencer T,
Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y,
et al: Myelin-associated glycoprotein interacts with the nogo66
receptor to inhibit neurite outgrowth. Neuron. 35:283–290. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Otori Y, Wei JY and Barnstable CJ:
Neurotoxic effects of low doses of glutamate on purified rat
retinal ganglion cells. Invest Ophthalmol Vis Sci. 39:972–981.
1998.PubMed/NCBI
|
|
12
|
Ahmed Z, Dent RG, Suggate EL, Barrett LB,
Seabright RJ, Berry M and Logan A: Disinhibition of
neurotrophin-induced dorsal root ganglion cell neurite outgrowth on
CNS myelin by siRNA-mediated knockdown of NgR, p75NTR and Rho-A.
Mol Cell Neurosci. 28:509–523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Acevedo L, Yu J, Erdjument-Bromage H, Miao
RQ, Kim JE, Fulton D, Tempst P, Strittmatter SM and Sessa WC: A new
role for Nogo as a regulator of vascular remodeling. Nat Med.
10:382–388. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marklund N, Fulp CT, Shimizu S, Puri R,
McMillan A, Strittmatter SM and McIntosh TK: Selective temporal and
regional alterations of Nogo-A and small proline-rich repeat
protein 1A (SPRR1A) but not Nogo-66 receptor (NgR) occur following
traumatic brain injury in the rat. Exp Neurol. 197:70–83. 2006.
View Article : Google Scholar
|
|
15
|
Buchli AD and Schwab ME: Inhibition of
Nogo: A key strategy to increase regeneration, plasticity and
functional recovery of the lesioned central nervous system. Ann
Med. 37:556–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fouad K, Klusman I and Schwab ME:
Regenerating corticospinal fibers in the Marmoset (Callitrix
jacchus) after spinal cord lesion and treatment with the
anti-Nogo-A antibody IN-1. Eur J Neurosci. 20:2479–2482. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christman CW, Salvant JB Jr, Walker SA and
Povlishock JT: Characterization of a prolonged regenerative attempt
by diffusely injured axons following traumatic brain injury in the
adult cat: A light and electron microscopic immunocytochemical
study. Acta Neuropathol. 94:329–337. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hulsebosch CE, DeWitt DS, Jenkins LW and
Prough DS: Traumatic brain injury in rats results in increased
expression of GAP-43 that corelates with behavioral recovery.
Neurosci Lett. 255:83–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emery DL, Royo NC, Fischer I, Saatman KE
and McIntosh TK: Plasticity following injury to the adult central
nervous system: Is recapitulation of a developmental state worth
promoting? J Neurotrauma. 20:1271–1292. 2003. View Article : Google Scholar
|
|
20
|
Koda M, Hashimoto M, Murakami M, Yoshinaga
K, Ikeda O, Yamazaki M, Koshizuka S, Kamada T, Moriya H, Shirasawa
H, et al: Adenovirus vector-mediated in vivo gene transfer of
brain-derived neurotrophic factor (BDNF) promotes rubrospinal
axonal regeneration and functional recovery after complete
transection of the adult rat spinal cord. J Neurotrauma.
21:329–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao YY, Yuan Y, Chen Y, Jiang L, Liao RJ,
Wang L, Zhang XN, Ohtsu H, Hu WW and Chen Z: Histamine promotes
locomotion recovery after spinal cord hemisection via inhibiting
astrocytic scar formation. CNS Neurosci Ther. 21:454–462. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Shao Y, Zhao C, Cai J and Sun S:
N-methyl-D-aspartate receptor antagonist MK-801 prevents apoptosis
in rats that have undergone fetal spinal cord transplantation
following spinal hemisection. Exp Ther Med. 8:1731–1736.
2014.PubMed/NCBI
|
|
23
|
Latini L, Bisicchia E, Sasso V, Chiurchiù
V, Cavallucci V, Molinari M, Maccarrone M and Viscomi MT:
Cannabinoid CB2 receptor (CB2R) stimulation delays rubrospinal
mitochondrial-dependent degeneration and improves functional
recovery after spinal cord hemisection by ERK1/2 inactivation. Cell
Death Dis. 5:e14042014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grosso MJ, Matheus V, Clark M, van Rooijen
N, Iannotti CA and Steinmetz MP: Effects of an immunomodulatory
therapy and chondroitinase after spinal cord hemisection injury.
Neurosurgery. 75:461–471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Han Y, Ye W, Liu F, Zhuang K and
Wu G: Alpha tocopherol treatment reduces the expression of Nogo-A
and NgR in rat brain after traumatic brain injury. J Surg Res.
182:e69–e77. 2013. View Article : Google Scholar
|
|
26
|
Yan M, Yang M, Shao W, Mao XG, Yuan B,
Chen YF, Ye ZX, Liang W and Luo ZJ: High-dose ascorbic acid
administration improves functional recovery in rats with spinal
cord contusion injury. Spinal Cord. 52:803–808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaneko A, Matsushita A and Sankai Y: A 3D
nanofibrous hydrogel and collagen sponge scaffold promotes
locomotor functional recovery, spinal repair, and neuronal
regeneration after complete transection of the spinal cord in adult
rats. Biomed Mater. 10:0150082015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xian-Hui D, Xiao-Ping H and Wei-Juan G:
Neuroprotective effects of the Buyang Huanwu decoction on
functional recovery in rats following spinal cord injury. J Spinal
Cord Med. Oct 20–2014.Epub ahead of print. PubMed/NCBI
|
|
29
|
Yamaya S, Ozawa H, Kanno H, Kishimoto KN,
Sekiguchi A, Tateda S, Yahata K, Ito K, Shimokawa H and Itoi E:
Low-energy extracorporeal shock wave therapy promotes vascular
endothelial growth factor expression and improves locomotor
recovery after spinal cord injury. J Neurosurg. 121:1514–1525.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Liu Z, Chen H, Duan Z, Zhang L,
Chen L and Li B: Synergic effects of EPI-NCSCs and OECs on the
donor cells migration, the expression of neurotrophic factors, and
locomotor recovery of contused spinal cord of rats. J Mol Neurosci.
55:760–769. 2015. View Article : Google Scholar
|
|
31
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spencer T, Domeniconi M, Cao Z and Filbin
MT: New roles for old proteins in adult CNS axonal regeneration.
Curr Opin Neurobiol. 13:133–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schwab ME: Nogo and axon regeneration.
Curr Opin Neurobiol. 14:118–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Filbin MT: Recapitulate development to
promote axonal regeneration: Good or bad approach? Philos Trans R
Soc Lond B Biol Sci. 361:1565–1574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang XY and Zhang JT: Effects of
ginsenoside Rg1 on synaptic plasticity of freely moving rats and
its mechanism of action. Acta Pharmacol Sin. 22:657–662.
2001.PubMed/NCBI
|
|
36
|
Hassiotis M, Ashwell KW, Marotte LR,
Lensing-Höhn S and Mai JK: GAP-43 Immunoreactivity in the brain of
the developing and adult wallaby (Macropus eugenii). Anat Embryol
(Berl). 206:97–118. 2002. View Article : Google Scholar
|
|
37
|
Tolner EA, van Vliet EA, Holtmaat AJ,
Aronica E, Witter MP, da Silva FH and Gorter JA: GAP-43 mRNA and
protein expression in the hippocampal and parahippocampal region
during the course of epileptogenesis in rats. Eur J Neurosci.
17:2369–2380. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carrasco J, Penkowa M, Giralt M, Camats J,
Molinero A, Campbell IL, Palmiter RD and Hidalgo J: Role of
metallothionein-III following central nervous system damage.
Neurobiol Dis. 13:22–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McIlvain VA, Robertson DR, Maimone MM and
McCasland JS: Abnormal thalamocortical pathfinding and terminal
arbors lead to enlarged barrels in neonatal GAP-43 heterozygous
mice. J Comp Neurol. 462:252–264. 2003. View Article : Google Scholar : PubMed/NCBI
|