Introduction
Breast cancer develops from the breast tissue. The
most common type of breast cancer is ductal carcinoma, which begins
in the lining of the milk ducts, while another type of breast
cancer is lobular carcinoma, which begins in the lobules of the
breast (1–3). Breast cancer accounts for >10% of
cancer among women worldwide, and it is the leading cause of
cancer-associated mortality in women aged 20–49 years (3,4).
Breast cancer incidence is increasing worldwide; in the USA,
~14,000 women under the age of 40 are diagnosed with breast cancer
annually and there are ~3,000 mortalities (5). Breast cancer risk factors are complex
and are likely to be associated with multiple factors, including
gender, age, genetics, family history of breast cancer, personal
history of breast cancer, race and ethnicity (6).
Angiogenesis is essential in breast cancer
progression and dissemination, and tumors have a limited ability to
grow without blood supply (7,8).
Therefore, inhibition of the formation of blood vasculature is an
effective way to stop or slow the growth of a cancer mass.
Accordingly, numerous angiogenesis inhibitors have been
investigated for use in the treatment of cancer. Chondromodulin I
(ChM-I), an endogenous anti-angiogenic factor expressed in
cartilage, has been reported to suppress tumorigenesis in
vivo, due to its anti-vascularization effect (9).
To further investigate the anti-vascularization
effect and antitumor activity of ChM-I, a recombinant ChM-I
expressing adenovirus vector was constructed and transfected into a
human breast cancer cell line. The stable expression of ChM-I in
the human breast cancer cell line was established.
Materials and methods
Patients and ethical approval
Human articular cartilage used in the present study
was obtained from the femoral condyles of patients undergoing knee
joint replacement surgery at Huashan Hospital, Fudan University
(Shanghai, China). All experimental procedures in the present study
were approved by the Ethics Committee of the Shanghai Fudan
University School of Medicine (Shanghai, China).
Cell culture
The MDA-MB-231 human breast cancer cell line and
293T cells were purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured at 37°C under 5% CO2 in Dulbecco's Modified
Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin (all: Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Construction of the human ChM-I cDNA
expression vector (pcDNA3-ChM-I)
The pcDNA3-ChM-I expression vector was established,
as described previously (10).
Briefly, total RNA was extracted from human articular cartilage
using the RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA), and
was reverse transcribed into cDNA for amplification by polymerase
chain reaction (PCR). The primers used to specifically amplify the
ChM-I cDNA are presented in Table
I and were obtained from Thermo Fisher Scientific, Inc. The
cycling conditions were as follows: 94°C for 3 min, followed by 30
cycles of 94°C for 1 min, 60°C for 1 min and 72°C for 2 min, and
then a final extension step at 72°C for 5 min. The PCR products
were separated by 1.5% agarose gel electrophoresis and the target
gene segment was retrieved and purified using the QIAquick Gel
Extraction kit (cat. no. 28704; Qiagen, Inc.). The pcDNA3.1 linear
plasmid (Invitrogen; Thermo Fisher Scientific, Inc.) underwent
double enzyme digestion with Hind III and Not I (both
from Thermo Fisher Scientific, Inc.), and the products of this
reaction were connected with the ChM-I cDNA overnight at 16°C to
produce the pcDNA3-ChM-I construct, which was then transformed into
XL1-Blue competent bacteria (New England BioLabs, Inc., Ipswich,
MA, USA). Positive bacterial colonies were selected for on Lysogeny
broth media containing ampicillin (Sigma-Aldrich, St. Louis, MO,
USA), and underwent plasmid extraction and purification using a
commercially available kit (cat. no. 12123; Qiagen, Inc.). The
extracted products were purified using the QIAquick PCR
Purification kit (cat. no. 28104; Qiagen, Inc.), and sequenced
(Invitrogen; Thermo Fisher Scientific, Inc.).
 | Table IPrimer sequences for polymerase chain
reaction. |
Table I
Primer sequences for polymerase chain
reaction.
| Gene | Primer sequence
(5′-3′) | Product length
(bp) |
|---|
| ChM-I | F:
ATCAGCAGGAAGGGGAAAGC | 308 |
| R:
TGGTCCCATCAGCATCAACC | |
| Cyclin D1 | F:
GGCGGAGGAGAACAAACAGA | 181 |
| R:
TGTGAGGCGGTAGTAGGACA | |
| Cyclin D3 | F:
ATGGAGCTGCTGTGTTG | 128 |
| R:
GAGAGGAGCCATCTAGACTA | |
| CDK6 | F:
TTCCCAGGCAGGCTTTTCAT | 245 |
| R:
GAAAGTTGGGCAGGCTGTAT | |
| β-actin | F:
TCCATCATGAAGTGTGACGT | 161 |
| R:
CTCAGGAGGAGCAATGATCT | |
Construction of the adenovirus ChM-I
vector
The recombinant adenoviral vector was constructed
according to a previously described method (11). Briefly, human ChM-I cDNA was
inserted into the Xho I and EcoRV restriction sites
of the pAdTrack-CMV shuttle vector (Invitrogen; Thermo Fisher
Scientific, Inc.), encoding green fluorescent protein (GFP). The
resultant DNA was linearized by digestion with Pme I (New
England BioLabs, Inc.), and subsequently cotransformed with an
adenoviral backbone plasmid (pAdEasy-1; Agilent Technologies, Inc.,
Santa Clara, CA, USA) into E coli BJ5183 (Invitrogen; Thermo
Fisher Scientific, Inc.). Recombinants were selected on media
containing kanamycin (Thermo Fisher Scientific, Inc.) and confirmed
by digestion with PacI.
Transfection of 293T cells
The linearized recombinant plasmid was transfected
into 293T cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). In 293T cells, recombination between the
homologous regions of the linearized transfer cosmid vector and the
adenovirus genome resulted in formation of the complete adenoviral
recombinant containing the ChM-I cDNA.
Measurement of virus titers
293T cells were used to measure the supernatant
virus titer. The transfected 293T cells were collected and genomic
DNA was extracted using the Genomic DNA Purification kit (cat. no.
K0512; Thermo Fisher Scientific, Inc.). The extracted DNA was
dissolved in 100 µl Tris-ethylenediaminetetraacetic acid
buffer (Thermo Fisher Scientific, Inc.) and underwent quantitative
(q)PCR using the Mx3000P® QPCR system (Agilent
Technologies, Inc.) to determine the number of vector copies in the
genomic DNA. The qPCR reaction mixture consisted of 1X Excite Real
Time Mastermix with SYBR Green (cat. no. 95088-050; BioGene,
Cambridge, UK), 40 nM each of ChM-I-specific sense and antisense
primers (Table I), 1.6 µl
genomic DNA (or distilled H2O for the control), and
H2O to a final volume of 20 µl. The thermal
cycler conditions included incubation at 95°C for 10 min, followed
by 50 cycles of 95°C for 30 sec and 59°C for 1 min. Integration of
the fluorescent SYBR green into the PCR product was monitored after
each annealing step. Amplification of one specific product was
confirmed by melting curve analysis, where a single melting peak
eliminated the possibility of primer-dimer association. For melting
curve analysis to be performed, the products were heated from 55 to
95°C after the 50 cycles.
Titers were calculated (IU/ml) according to the
following formula: IU/ml = (C × N × D × 1,000) / V, where
C=proviral copies per genome, N=number of cells at time of
transduction, D=dilution of vector preparation and V=volume of
diluted vector added in each well for transduction. To obtain an
accurate titer, average values were obtained from three of the
vector dilutions (12).
Transfection of the ChM-I recombinant
adenovirus into MDA-MB-231 human breast cancer cells
MDA-MB-231 human breast cancer cells
(5×104/ml) in the logarithmic phase were seeded into
each well of a 6-well plate and divided into three groups: i) The
ChM-I group (breast cancer cells transfected with ChM-I); ii) the
empty vector group (breast cancer cells transfected with empty
vector); and iii) the blank group (breast cancer cells).
After 12 h of culture and upon reaching 30–50%
confluence, the human breast cancer cells were transfected with the
ChM-I adenovirus vector. Briefly, based on the pre-established
experimental multiplicity of infection (MOI), 100 µl virus
or empty vector per 1 ml culture medium was added to each well. The
medium was replaced with the DMEM containing 10% FBS and 1%
penicillin/streptomycin after 12 h of culture. At 4 days following
transfection, the transfection efficiency was calculated based on
the number of GFP-positive cells under a fluorescent microscope
(Eclipse 80i; Nikon Corporation, Tokyo, Japan). When the
transfection efficiency had reached >80%, the cells were
collected to assess the expression of ChM-I by reverse
transcription (RT)-PCR. Briefly, total RNA was extracted from the
cells using TRIzol reagent and treated with DNase (both Invitrogen;
Thermo Fisher Scientific, Inc.), after which 1 mg RNA was
reverse-transcribed into cDNA using a RT-PCR kit (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The PCR cycling conditions were as follows: Amplification
at 94°C for 4 min, followed by 25 cycles at 60°C for 1 min, 72°C
for 3 min and 94°C for 1 min. The PCR products were resolved in a
1.5% agarose gel. The forward and reverse primers for ChM-I are
presented in Table I.
Cell proliferation in vitro
Cell Counting kit-8 (CCK-8; Dojindo China Co., Ltd.,
Beijing, China) was used to test the cell proliferation in the
ChM-I, empty vector and blank groups. In total, 103
cells in 100 µl medium were seeded into each well of the
96-well plate. On days 1, 3, 5 and 7, 10 µl CCK-8 solution
was added to each well prior to examination. After 3 h of culture,
the absorbance was measured spectrophotometrically at 450 nm
(DU® Series 700 UV/Vis Scanning Spectrophotometer
(Beckman Coulter, Inc., Brea, CA, USA). These experiments were
conducted at least in triplicate.
Colony formation assays
Cells in the ChM-I, empty vector and blank cell
groups were cultured on 35 mm culture plates as described (9). The cells were detached and suspended
in culture medium containing 0.68% malting agar (Sigma-Aldrich).
The cell suspension was then plated on culture medium containing
0.4% agarose that had been allowed to harden beforehand. The
culture medium was changed every 2–3 days. The numbers of colonies
were measured under a phase contrast microscope (Olympus CX31;
Olympus Soft Imaging Solutions GmbH, Münster, Germany) on days 7,
14 and 21 of culture. The experiment was conducted in
triplicate.
Analysis of the mRNA expression levels of
cell-cycle associated genes in target cells
RT-qPCR was used to investigate the mRNA expression
levels of genes associated with the cell-cycle. Briefly, total RNA
was extracted from the human breast cancer cells using TRIzol
reagent, according to the manufacturer's protocol. RNA was reverse
transcribed into cDNA using the Reverse Transcription kit (Qiagen,
Inc.). PCR was performed in a qPCR detection system (Bio-Rad
Laboratories, Hercules, CA, USA) and conducted with iQ SYBR Green
Supermix (Bio-Rad). The primer sequences are presented in Table I. The cycling conditions were as
follows: One cycle at 50°C for 2 min; one cycle at 95°C for 10 min;
40 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec;
and one cycle at 72°C for 10 min. Amplification reactions were
performed in duplicate and the amount of cDNA in the reactions was
normalized to β-actin. The specificity of the amplification was
confirmed by 2% agarose gel electrophoresis and by analysis of the
melting curves. An amplification reaction control with no reverse
transcriptase enzyme was performed in order to assess the
interference of potential genomic DNA in the RNA solution. Relative
mRNA expression levels were calculated using the 2−ΔΔCq
method (13).
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed using the SPSS 17.0 software (SPSS Inc.,
Chicago, IL, USA). One-way analysis of variance followed by
Fisher's Least Significant Difference test were used to assess
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Transfection of the ChM-I recombinant
adenovirus in 293T cells
The ChM-I recombinant adenovirus was transfected
into 293T cells. Fluorescence microscopy was used to GFP
fluorescence following transfection, and the transfection rate was
>95% (Fig. 1). RT-qPCR was used
to determine the number of vector copies associated with genomic
DNA extracted from transduced 293T cells. Vector copy numbers in
293T cells are normalized to human RNaseP gene copies and presented
as proviral copies per genome equivalent. The average value
obtained from 3 of the vector dilutions was 5.19×109
IU/ml (Table II).
 | Table IIVirus titer calculation. |
Table II
Virus titer calculation.
| Sample | V | C | N | D | IU | Mean |
|---|
| 1 | 10 | 261.75 | 2×105 | 1 | 5.23E+09 | 5.19E±09 |
| 2 | 1 | 20.05 | 2×105 | 1 | 4.10E+09 | |
| 3 | 0.1 | 3.12 |
2×105 | 1 | 6.24E+09 | |
Transfection of the ChM-I recombinant
adenovirus in human breast cancer cells
The ChM-I recombinant adenovirus was successfully
transfected and expressed constitutively in human breast cancer
cells. The expression of GFP was observed in the experimental group
under fluorescence microscope examination. Following recombinant
adenovirus transfection for 7 days in human breast cancer cells,
the transfection rate was >90% (Fig. 2). The result of RT-qPCR showed that
expression of ChM-I was observed in the ChM-I transfected cell
group, while no expression was identified in the in control groups
(Fig. 3).
Ad-ChM-I inhibits the growth of human
breast cancer cells in vitro
Compared with the two other groups, there was a
significantly decrease in the cell growth of human breast cancer
cell lines transfected with Ad-ChM-I cultures, indicating that
ChM-I suppresses the proliferation of breast cancer cells (Fig. 4A).
Colony formation assay
A colony formation assay was conducted. Ad-ChM-I
infection markedly suppressed the number of colonies of human
breast cancer cells, a result that was consistent with the in
vitro CCK-8 results (Fig.
4B).
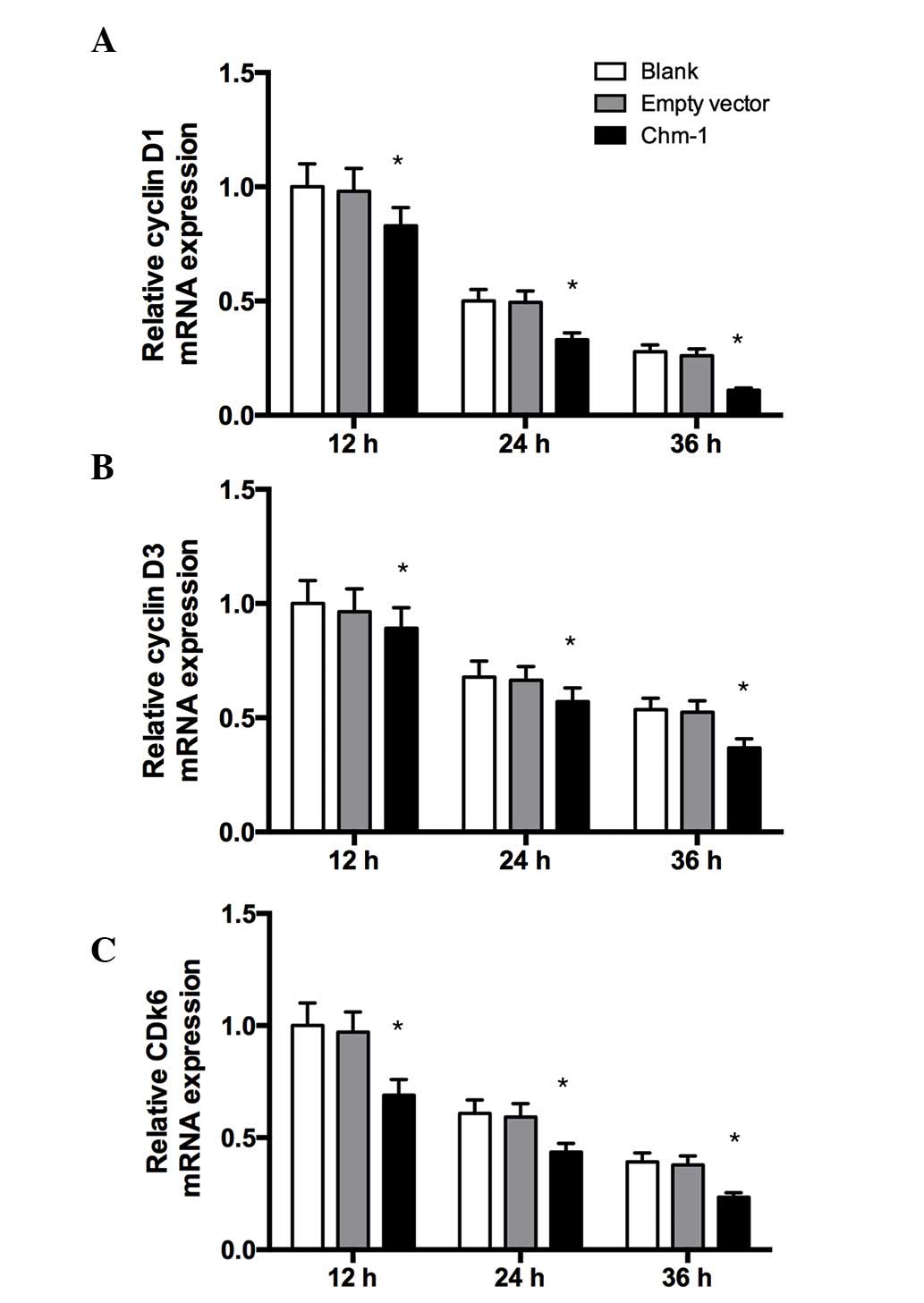
ChM-I suppresses the expression of cell
cycle-related genes in human breast cancer cells
To investigate the mechanism of ChM-I-induced
suppression of tumor cell growth, the expression levels of cell
cycle-related genes in human breast cancer cells were examined
in vitro by RT-qPCR analysis. As shown in Fig. 5, Ad-ChM-I inhibited the levels of
certain cell cycle-related genes after infection, the levels of
cyclin D1, cyclin D3, and cyclin-dependent kinase (CDK)6 were
significantly decreased by Ad-ChM-I.
Discussion
Angiogenesis is essential in tumor development and
subsequent growth, invasion and metastasis (14). Tumors require a blood supply to
grow and spread, and blood vessels are required for tumors to grow
beyond a few millimeters in size. Tumors can secrete chemical
signals that stimulate angiogenesis to form blood vessels to
increase supply. In addition, tumors can also stimulate nearby
normal cells to produce angiogenesis signaling molecules (15,16).
Among the various angiogenic factors that are
involved in tumor development, vascular endothelial growth factor
(VEGF) is the most prominent (17,18).
Thereby VEGF has been considered as a therapeutic target, and
angiogenesis inhibitors have been designed to prevent the formation
of novel blood vessels, to stop or slow the growth or spread of
tumors (19,20). Accordingly, a variety of drugs
blocking VEGF signal have been developed in recent years (21,22).
ChM-I is a 25-kDa glycoprotein, which contains two
distinctive structural domains: The N-terminal third of the
molecule is a hydrophilic domain that contains O-linked and
N-linked oligosaccharide chains, and the C-terminal two-thirds is a
hydrophobic domain that contains all of the cysteine residues, and
is considered as an effective anti-vascualarization factor
(23). Hakuno et al
(24) reported that ChM-I is a
crucial factor for maintaining normal cardiac valvular function by
preventing angiogenesis that may lead to valvular heart diseases.
Klinger et al (25) showed
that CmH-I stabilizes the chondrocyte phenotype and inhibits
endochondral ossification of porcine cartilage repair tissue. Mera
et al (9) reported that
ChM-I directly suppresses the proliferation of tumor cells in an
anchorage-independent manner. In addition, Hiraki et al
(26) reported that ChM-I was
identified as an angiogenesis inhibitor, and found that ChM-I is
specifically expressed in the avascular zone of cartilage in
developing bone, but not present in calcifying cartilage. Purified
ChM-I inhibited DNA synthesis and proliferation of vascular
endothelial cells as well as tube morphogenesis in vitro
(26).
The central importance of tumor neovascularization
has been emphasized by experimental and clinical trials in various
tumor types (27,28), and ChM-I has been shown to exhibit
anti-vascularization action, thus, the present study aimed to
transfect ChM-I into human breast cancer cells in order to
investigate the effects of ChM-I.
In the present study, an adenoviral vector
expressing ChM-I was constructed. Use of adenoviral vectors offers
multiple advantages for gene replacement therapy, as they combine
efficient delivery, an ability to transduce proliferating and
non-proliferating cells, a capacity to integrate into the host
chromatin to provide stable long-term expression of the transgene,
an absence of any viral genes in the vector and an absence of
interference from preexisting viral immunity (29). In the present study, ChM-I was
successfully transfected into breast cancer cells and stably
expressed. The effect of ChM-I on the proliferation on breast
cancer cells was analyzed using CCK-8 and colony forming assays. It
was demonstrated that ChM-I had a significant inhibitory effect on
breast cancer cell proliferation. It is has previously been shown
that cyclin D1, cyclin D3 and CDK6 are able to promote cell
division, therefore, the expression of these genes was analyzed
following ChM-I transfection. Ad-ChM-I significantly decreased the
mRNA expression levels of cyclin D1, cyclin D3 and CDK6. These
results suggested that ChM-I had a significant inhibitory effect on
breast cancer cell division and proliferation.
In conclusion, recombinant ChM-I was successfully
constructed and transfected into human breast cancer cells, wherein
it was shown to be stably expressed. The in vitro results
suggested that ChM-I was able to inhibit the growth of human breast
cancer cells. Further in vivo studies are required to
explore the role of ChM-I in human breast cancer cells.
Acknowledgments
This study was supported by the National Natural
Science Fund of China (grant no. 81072188).
References
|
1
|
Brinton LA, Smith L, Gierach GL, Pfeiffer
RM, Nyante SJ, Sherman ME, Park Y, Hollenbeck AR and Dallal CM:
Breast cancer risk in older women: Results from the NIH-AARP diet
and health study. Cancer Causes Control. 25:843–857. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Institute: Breast cancer.
What You Need To Know About. National Institutes of Health;
Bethesda, MD: 2012
|
|
3
|
Nyante SJ, Dallal CM, Gierach GL, Park Y,
Hollenbeck AR and Brinton LA: Risk factors for specific
histopathological types of postmenopausal breast cancer in the
NIH-AARP diet and health study. Am J Epidemiol. 178:359–371. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasco AJ: Breast cancer and the
environment. Horm Res. 60(Suppl 3): S502003. View Article : Google Scholar
|
|
5
|
Ruddy KJ, Greaney ML, Sprunck-Harrild K,
Meyer ME, Emmons KM and Partridge AH: Young women with breast
cancer: A focus group study of unmet needs. J Adolesc Young Adult
Oncol. 2:153–160. 2013. View Article : Google Scholar :
|
|
6
|
John EM, Hopper JL, Beck JC, Knight JA,
Neuhausen SL, Senie RT, Ziogas A, Andrulis IL, Anton-Culver H, Boyd
N, et al: The breast cancer family registry: An infrastructure for
cooperative multinational, interdisciplinary and translational
studies of the genetic epidemiology of breast cancer. Breast Cancer
Res. 6:R375–R389. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fantozzi A and Christofori G: Mouse models
of breast cancer metastasis. Breast Cancer Res. 8:2122006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zetter BR: Angiogenesis and tumor
metastasis. Annu Rev Med. 49:407–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mera H, Kawashima H, Yoshizawa T,
Ishibashi O, Ali MM, Hayami T, Kitahara H, Yamagiwa H, Kondo N,
Ogose A, et al: Chondromodulin-1 directly suppresses growth of
human cancer cells. BMC Cancer. 9:1662009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xing S, Wang Z, Xi H, Zhou L, Wang D, Sang
L, Wang X, Qi M and Zhai L: Establishment of rat bone mesenchymal
stem cell lines stably expressing Chondromodulin I. Int J Clin Exp
Med. 5:34–43. 2012.PubMed/NCBI
|
|
11
|
Lin L, Fu X, Zhang X, Chen LX, Zhang JY,
Yu CL, Ma KT and Zhou CY: Rat adipose-derived stromal cells
expressing BMP4 induce ectopic bone formation in vitro and in vivo.
Acta Pharmacol Sin. 27:1608–1615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Zeng X, Liu Y, Liang C, Zhang H,
Liu C, Du W and Zhang Z: Construction and characterization of a
PDCD5 recombinant lentivirus vector and its expression in tumor
cells. Oncol Rep. 28:91–98. 2012.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Zheng K, Li HY, Su XL, Wang XY, Tian T, Li
F and Ren GS: Chemokine receptor CXCR7 regulates the invasion,
angiogenesis and tumor growth of human hepatocellular carcinoma
cells. J Exp Clin Cancer Res. 29:312010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishida N, Yano H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar
|
|
16
|
Wels J, Kaplan RN, Rafii S and Lyden D:
Migratory neighbors and distant invaders: Tumor-associated niche
cells. Genes Dev. 22:559–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999.PubMed/NCBI
|
|
19
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9(Suppl 1):
S2–S10. 2004. View Article : Google Scholar
|
|
20
|
Ferrara N: VEGF as a therapeutic target in
cancer. Oncology. 69(Suppl 3): S11–S16. 2005. View Article : Google Scholar
|
|
21
|
Shibuya M: VEGF-VEGFR signals in health
and disease. Biomol Ther (Seoul). 22:1–9. 2014. View Article : Google Scholar
|
|
22
|
Ma J and Waxman DJ: Combination of
antiangiogenesis with chemotherapy for more effective cancer
treatment. Mol Cancer Ther. 7:3670–3684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miura S, Kondo J, Kawakami T, Shukunami C,
Aimoto S, Tanaka H and Hiraki Y: Synthetic disulfide-bridged cyclic
peptides mimic the anti-angiogenic actions of chondromodulin-I.
Cancer Sci. 103:1311–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hakuno D and Fukuda K: Role of
anti-angiogenic factor chondromodulin-I for maintaining cardiac
valvular function. Clin Calcium. 17:361–372. 2007.In Japanese.
PubMed/NCBI
|
|
25
|
Klinger P, Surmann-Schmitt C, Brem M,
Swoboda B, Distler JH, Carl HD, von der Mark K, Hennig FF and Gelse
K: Chondromodulin 1 stabilizes the chondrocyte phenotype and
inhibits endochondral ossification of porcine cartilage repair
tissue. Arthritis Rheum. 63:2721–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hiraki Y, Inoue H, Iyama K, Kamizono A,
Ochiai M, Shukunami C, Iijima S, Suzuki F and Kondo J:
Identification of chondromodulin I as a novel endothelial cell
growth inhibitor. Purification and its localization in the
avascular zone of epiphyseal cartilage. J Biol Chem.
272:32419–32426. 1997. View Article : Google Scholar
|
|
27
|
Fox SB, Generali DG and Harris AL: Breast
tumour angiogenesis. Breast Cancer Res. 9:2162007. View Article : Google Scholar
|
|
28
|
Miller KD: Recent translational research:
Antiangiogenic therapy for breast cancer - where do we stand?
Breast Cancer Res. 6:128–132. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muruve DA: The innate immune response to
adenovirus vectors. Human Gene Ther. 15:1157–1166. 2004. View Article : Google Scholar
|