Introduction
Glioma is the most common type of malignancy of the
central nervous system in adults worldwide (1). Glioma has a very poor prognosis and
the natural history of the disease is particularly short, typically
<1 year (2). High-grade glioma
presents a challenge with regard to research. Despite the current
treatment regimen of surgery and radiotherapy with concomitant
temozolomide administration, median survival is ~1 year (3,4). The
poor response to therapy may be due to the intratumoral
heterogeneity of these malignancies at the cellular and molecular
levels (5–7). Radiotherapy has been important in
prolonging the survival of patients with glioma since the 1970s
(8). However, although
radiotherapy is widely adopted for glioma treatment, the
radioresistance of glioma cells limits its success (9).
Ras-related C3 botulinum toxin substrate 1 (Rac1) is
among the most extensively characterized members of the Rho family
of small GTPases, which are adhesion- and growth-factor activated
molecular switches that are important in tumor development and
progression (10,11). In addition, Rac1 has been
identified to contribute to radioresistance in head and neck
squamous cell carcinomas (12).
WASP-family verprolin-homologous protein-2 (WAVE2) is a member of
the family of Wiskott–Aldrich Syndrome protein (WASp)/WAVE
proteins, which is activated at the plasma membrane by Rac1 and
uses the actin-related protein 2/3 (Arp2/3) complex to generate a
dendritic array of branched filaments responsible for the extension
of lamellipodia (13,14). WAVE2 is strongly associated with
migration of a range of tumor cells, and is implicated in tumor
cell invasion and metastasis via regulating the Arp2/3 complex
(15). In addition, activation of
Rac results in dephosphorylation of cofilin (16), and the Arp2/3 complex serves as a
key upstream factor for the recruitment of modulators of
lamellipodia formation, such as capping protein or cofilin
(17). A previous study
demonstrated that blocking the Rac1-WAVE2-Arp2/3 signaling pathway
in carcinoma cells results in marked suppression of cell migration
and invasion (18).
Cofilin-1 (CFL-1) is a non-muscle isoform of the
actin depolymerizing factor/cofilin protein family, which
accelerates actin dynamics in vitro and in vivo
(19,20). The actin-associated protein, cofilin binds and severs
actin filaments (21). In
addition, dysfunction of CFL-1 is essential for cell viability and
migration (22,23). A previous study revealed that CFL-1
was significantly upregulated in radioresistant astrocytomas
(24) and it is potentially
involved in decreasing radiosensitivity in U251 cells (25). Thus, the present study proposed
that the Rac1-WAVE-Arp2/3 signaling pathway may be involved in the
radioresistant phenotype, via CFL-1, and may present as a novel
therapeutic target for glioma.
In the present study, U251 cells, a human glioma
cell line, were selected as an in vitro model to investigate
whether the Rac1-WAVE-Arp2/3 signaling pathway is involved in
radioresistance, and to elucidate its regulatory role in CLF-1
expression.
Materials and methods
Cell culture
U251 human glioma cells were obtained from Nanjing
KeyGen Biotech Co. Ltd. (Nanjing, China). Radioresistant U251
(RR-U251) human glioma cells were established according to a
previously described method (25).
U251 and RR-U251 cells were cultured in Gibco Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with Gibco 10% fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (both Thermo Fisher Scientific, Inc.) in a 37°C
humidified incubator with 5% CO2.
Transfection
The short hairpin (sh)RNA-WAVE2 plasmid was
synthesized by Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
U251 cells (1×105/ml) were seeded into 6-well plates 24
h prior to transfection and 1 ml DMEM (with serum and antibiotics)
was added. Upon reaching ~50% confluence the U251 cells in the
6-well plates were transfected using Lipofectamine™2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The medium was replaced with fresh
DMEM containing 10% FBS 6 h after transfection. Following
transfection (24 h), cell fluorescence was observed under a
fluorescence microscope (Axiovert 40 CFL; Zeiss AG, Oberkochen,
Germany) and the transfection efficiency was determined, according
to the number of fluorescent cells.
Cell viability assay
Cell viability was determined using a
3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) assay
(Sigma-Aldrich, St. Louis, MO, USA). Briefly, the normal U251 and
RR-U251 cells (1×105/ml) were seeded into separate
96-well plates. After 24 h, 50 µmol/l NSC 23766 (Topscience
Biotech Co., Ltd., Shanghai, China) or 200 µmol/lCK-666
(Sigma-Aldrich) dissolved in 150 µl dimethyl sulfoxide
(DMSO; Biosharp, Hefei, China) was added to each well for 0.5–4 h.
The cells treated with NSC 23766, shRNA-WAVE2, and CK-666, and the
untreated cells, were irradiated using a cobalt-60 source at 0.5
Gy/min for 10 min, followed by washing with phosphate-buffered
saline (PBS), trypsin digestion (Nanjing Sunshine Biotechnology
Co., Ltd., Nanjing, China) to separate the cells from the wells and
centrifugation at 37°C for 6 min at 300 × g. The cells were then
incubated in fresh DMEM (containing antibiotics) for an additional
48 h at 37°C. MTT solution (20 µl) was added to each well
and the cells were incubated at 37°C for 4 h. Aliquots of 150
µl DMSO were added into each well to dissolve the formazan
following removal of the old medium from the plates. The optical
density (OD) of the samples was measured at a wavelength of 570 nm
using a microplate reader (Multiskan Ascent; Thermo Fisher
Scientific, Inc.). Cell viability (%) was calculated using the
following equation: Cell viability (%) = 100×
(ODtreatment/ODcontrol).
Cell migration assay
Cell migration was examined using a wound-healing
assay. Normal U251 and RR-U251 cells, treated with NSC 23766,
shRNA-WAVE2 and CK-666, and the untreated cells were irradiated,
which was followed by a washout. Cells were subsequently seeded
into 6-well plates and allowed to reach confluency. The glioma
cells were scratched using a 200-µl tip pipette to create
the wound line. Fresh DMEM was added to each well after the plates
were washed twice with PBS. Sequential images were obtained using
the Axiovert 40 CFL microscope by capturing photomicrographs at 0
and 24 h at the same site, and the scratch area was measured using
a ruler. The rate of migration was further analyzed using the
following equation: Migration ratio = (Width 0 h − Width 24 h) /
Width 0 h ×100%.
Cell invasion assessment
Cell invasion was measured using a Matrigel-coated
Transwell chamber kit (Corning Incorporated, Corning, NY, USA). The
normal U251 and RR-U251 cells, treated with NSC 23766, shRNA-WAVE2
and CK-666, and the untreated cells were irradiated, which was
followed by a washout. The monolayer cells were then suspended in
serum-free DMEM at a density of 1×105/ml. Aliquots (100
µl) of suspension was seeded into the top chambers. DMEM
supplemented with 15% FBS was added to the lower chamber. Cells
were then incubated in a 5% CO2 atmosphere at 37°C.
Following incubation for 24 h, the top chamber was removed and the
cells remaining on the upper surface of the membrane were removed
using a medical cotton swab. The invasive cells on the lower
surface were fixed with formaldehyde (Dynamic Chemical Industry
Co., Ltd., Nanjing, China) and stained with crystal violet
(Biosharp) for 10 min. The chambers were photographed in five
randomly selected fields (magnification, ×40) per well under a
Axiovert 40 CFL microscope. All experiments were performed three
times.
Western blot analysis
U251 and RR-U251 cells were washed with ice-cold PBS
three times and then frozen at −80°C for ≥24 h before lysing the
cells using a lysis buffer (Thermo Fisher Scientific, Runcorn, UK).
Following centrifugation for 15 min at 1,000 × g, the supernatant
was collected. All samples were diluted in loading buffer (Nanjing
Sunshine Biotechnology Co., Ltd.) and boiled for 3 min. Thirty
micrograms of each protein sample were resolved by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis before being
blotted onto a nitrocellulose membrane (Nanjing Sunshine
Biotechnology Co., Ltd.). Following blocking with 5% non-fat milk,
the membranes were incubated overnight at 4°C with rabbit
anti-CFL-1 polyclonal antibody (1:1,000; cat. no. ab29038; Abcam,
Cambridge, MA, USA) and mouse anti-GAPDH monoclonal antibody
(1:1,000; cat. no. KC-5G4; Kangchen Biotech Co., Ltd., Zhejiang,
China), followed by incubation with horseradish
peroxidase-conjugated goat anti-rabbit (1:10,000; cat. no. ZB-2301;
Nanjing Shengxing Biotechnology Co., Ltd., Nanjing, China) and goat
anti-mouse (1:10,000; cat. no. SN133; Nanjing Sunshine
Biotechnology Co., Ltd.) secondary antibodies for 1 h at room
temperature. After washing three times using TBST, the protein
bands were detected by enhanced chemiluminescence (ECL; Pierce;
Thermo Fisher Scientific, Inc., Shanghai, China). The intensity of
protein bands was quantified using Quantity One 4.62 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All statistical analyses were conducted using SPSS
19.0 software (IBM SPSS, Armonk, NY, USA). Statistical analyses
were performed by means of the Student's t-test and the
Mann-Whitney U test. Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Confirmation of transfection
efficiency
Transfection of shRNA-WAVE2 duplexes led to a stable
exogenous gene expression with ~80–90% efficiency in U251 cells, as
shown by the green fluorescent protein-labeled reporter method
(Fig. 1).
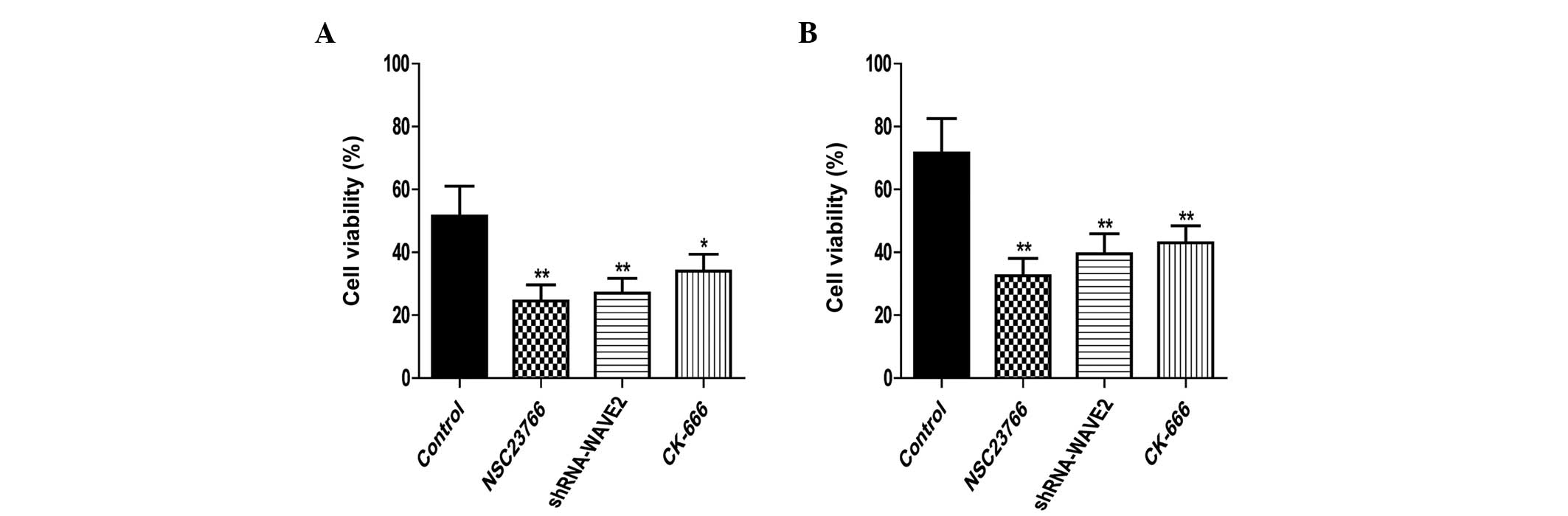
Effects of inhibiting the
Rac1-WAVE2-Arp2/3 signaling pathway on U251 cell proliferation
The proliferation rates of U251 cells were
determined by the MTT assay. The proliferation of normal U251 and
RR-U251 cells was significantly downregulated by treatment with NSC
23766 (P=0.005 and 0.002, respectively) and CK-666 (P=0.022 and
0.007, respectively), and by transfection with shRNA-WAVE2 (P=0.009
and 0.006, respectively; Fig.
2).
Inhibition of Rac1-WAVE2-Arp2/3 reduces
U251 cell migration ability
The migratory ability of U251 cells was determined
using a wound healing assay. Following radiation therapy, the
migratory abilities of normal U251 and RR-U251 cells were markedly
decreased upon treatment with Rac1 and Arp2/3 inhibitors, NSC 23766
and CK-666, respectively, or transfection with shRNA-WAVE2, as
compared with control cells (Fig.
3).
Inhibition of Rac1-WAVE-Arp2/3 impairs
U251 cell invasion ability
The role of Rac1-WAVE-Arp2/3 in cell
invasion was assessed using the Transwell assay. As presented in
Fig. 4, compared with control
cells, the invasion potential of U251 cells was inhibited by NSC
23766 and CK-666, and by transfection with shRNA-WAVE2 for
Rac1-WAVE2-Arp2/3 in normal U251 and RR-U251 cells. These results
indicate that inhibition of Rac1-WAVE-Arp2/3 impairs U251 cell
invasion.
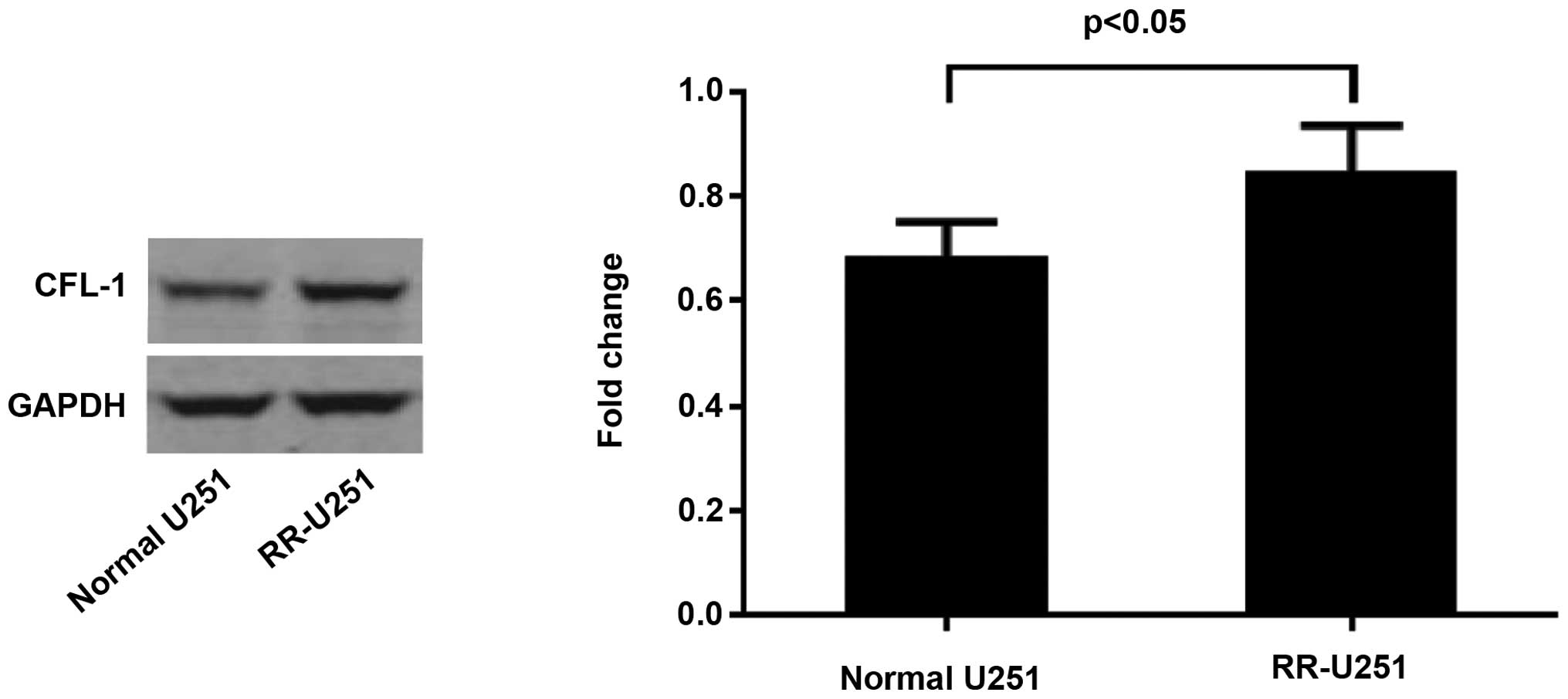
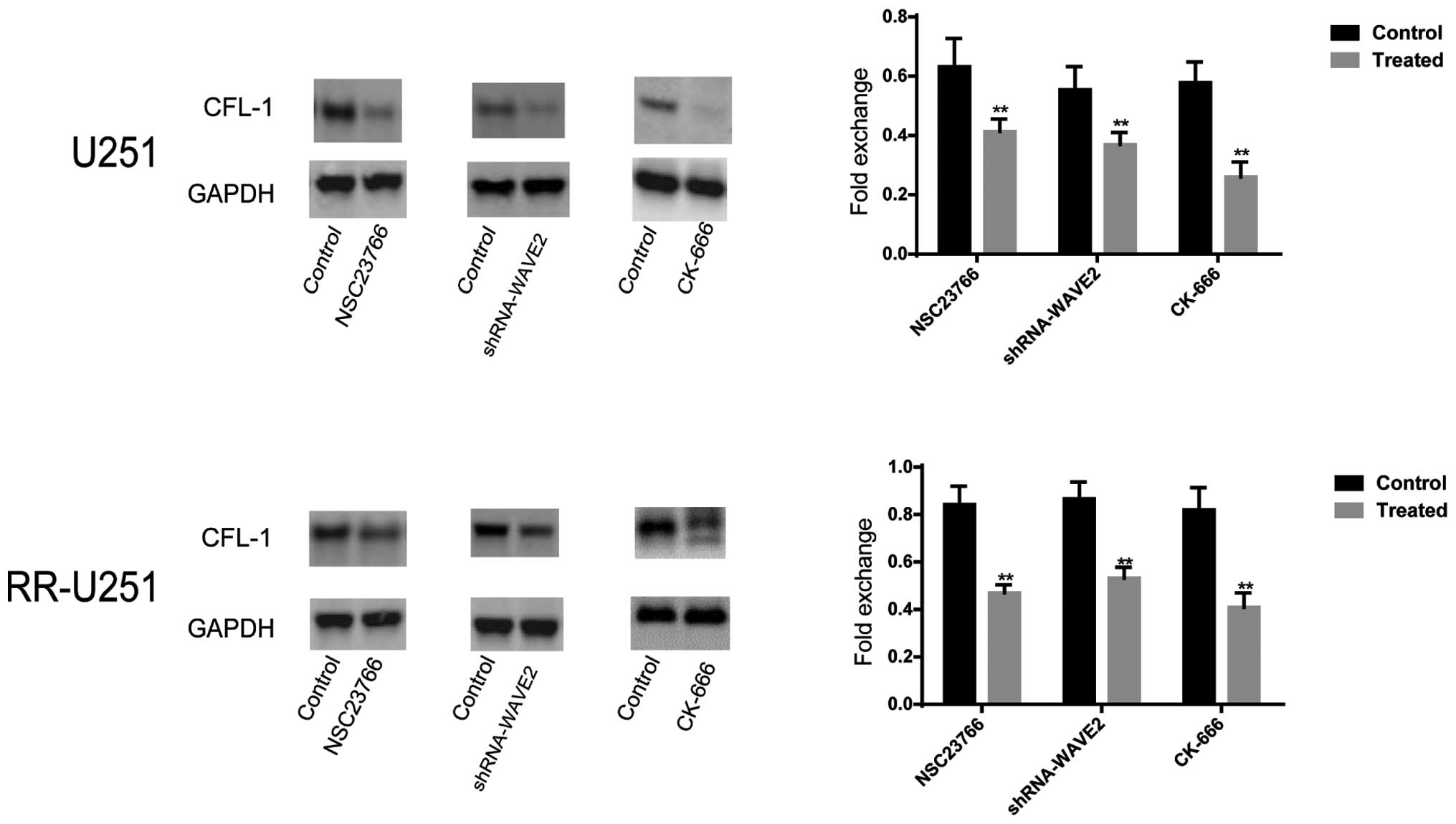
Protein expression levels of CFL-1 in
U251 and RR-U251 cells
The protein expression of CFL-1 was assessed by
western blot and densitometric analyses. As compared with the
normal U251 cells, the protein expression level of CFL-1 was
significantly elevated in the RR-U251 cells (P<0.05; Fig. 5). Conversely, the protein
expression level of CFL-1 was significantly downregulated in normal
U251 and RR-U251 cells treated with NSC 23766 (P=0.004 and
P<0.001, respectively) and CK-666 (P<0.001 for both), or
transfected with shRNA-WAVE2 (P=0.003 and 0.002, respectively), as
compared with the control cells (Fig.
6).
Discussion
In the present study, cell viability, cell migration
and cell invasion abilities were observed to be reduced
consistently with the decreased expression of CFL-1 protein when
the Rac1-WAVE2-Arp2/3 signaling pathway was inhibited. These
findings indicate that inhibition of the Rac1-WAVE2-Arp2/3
signaling pathway may promote radiosensitivity in U251 human glioma
cells, and this regulatory role may involve the downregulation of
CFL-1 protein expression, which has been found to contribute to
radiosensitivity in human glioma by suppressing tumorigenic
properties, including cell viability, cell migration and invasion
ability (25).
A previous study reported that CFL-1 potentially
contributed to radioresistant astrocytomas and U251 glioma cells
(24,25). In addition, it has been shown that
Rac1 contributes to radioresistance of head and neck squamous cell
carcinomas (12). Rac signaling
recruits and activates WAVE, which nucleates branched actin
networks via the Arp2/3 complex in tumor development (26). Conversely, Rac is linked to
dephosphorylation of cofilin (16). These findings suggest that the
Rac1-WAVE-Arp2/3 signaling pathway may be correlated with
radiosensitivity and has a marked association with CFL-1 in
glioma.
Almost half of all cancer patients receive
radiotherapy, which is currently a major treatment option; however,
recent reports have suggested that radiotherapy increases the
migration and invasion of various cancer cell types, including lung
cancer, hepatocellular carcinoma and glioma (27–29).
A major factor controlling the metastatic nature of cancer cells is
their motility. Alterations in molecular pathways controlling
regulation may lead to tumor cell migration and invasion (15). The identification of the signaling
pathways of tumor cell metastasis may provide novel diagnostic and
therapeutic markers of treatment-resistant cancer (30). Rac1 activation induces lamellipodia
formation via WAVE, which uses the Arp2/3 complex to promote the
formation of densely branched filaments (31). Evidence has indicated that Arp2/3
and cofilin act synergistically to generate novel actin filaments
(32). The present study proposed
that the Rac1-WAVE-Arp2/3 signaling pathway is involved in
radioresistance in U251 glioma cells exhibiting a high CFL-1
protein expression level.
Metastatic progression of malignant tumors resistant
to conventional therapeutic approaches is a challenge in clinical
oncology. Despite previous investigation and clinical research, no
effective treatment has been established to prevent or combat the
metastatic spread of malignant tumors (33). Tumor invasion and metastasis are
important factors influencing the poor prognosis and severity of
cancer, hence elucidating the mechanism of invasion and metastasis
is considered to be a crucial task.
The mechanisms of metastasis include cell
detachment, migration, invasion into the surrounding tissue,
extravasations, and transplantation followed by formation of tumor
emboli (34). It has been reported
that inhibition of the Rac1-dependent signaling pathway is an
important mechanism underlying cancer metastasis, particularly in
cancer cell invasion (35). Cancer
cell migration and invasion are closely associated with
lamellipodia formation and migration, and the extension of
lamellipodia is driven by WASp family proteins, which activate the
Arp2/3 complex to catalyze the formation of a branched actin
filament array at the leading edge of lamellipodia (36). In addition, cofilin localization is
dependent on the mechanism by which cell peripheral actin networks
are nucleated and maintained, and its targeting is highly sensitive
to Arp2/3 complex activity and cannot be solely mediated by
interaction with actin filaments (37).
Cell migration and invasion is significantly
increased in radioresistant glioma cells (21), and dysregulation of certain targets
may regulate the signaling pathways of tumor metastasis (38). The identification of CFL-1 as an
important downstream effector protein of the Rac1-WAVE2-Arp2/3
signaling pathway, which mediates tumor cell migration and invasion
in vitro, highlights the essential role of the
Rac1-WAVE2-Arp2/3 signaling pathway in CFL-1 promoting
radioresistance in U251 human glioma cells. Although a direct
interaction between CFL-1 and the Rac1-WAVE2-Arp2/3 signaling
pathway was not demonstrated in the present study, the results
suggested that they may act in concert.
In conclusion, the results of the present study
demonstrated that the Rac1-WAVE2-Arp2/3 signaling pathway promotes
radioresistance via CFL-1 in U251 human glioma cells in
vitro; however, further studies are required to examine the
exact molecular mechanism underlying this effect. Additional glioma
cell lines should be investigated to confirm the role of CFL-1 and
the Rac1-WAVE2-Arp2/3 signaling pathway in glioma. In addition,
further studies using rodent models are required to determine the
mechanism by which CFL-1 and the Rac1-WAVE2-Arp2/3 signaling
pathway contribute to the radioresistance of glioma. The results of
the present study may provide a basis for the use of gene therapy
in the treatment of patients with glioma.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
NSFC81172390) and the Health Bureau of Nanjing (grant no.
ZKX10021).
References
|
1
|
Ford E, Catt S, Chalmers A and Fallowfield
L: Systematic review of supportive care needs in patients with
primary malignant brain tumors. Neuro Oncol. 14:392–404. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pace A, Metro G and Fabi A: Supportive
care in neurooncology. Curr Opin Oncol. 22:621–626. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Catt S, Chalmers A and Fallowfield L:
Psychosocial and supportive-care needs in high-grade glioma. Lancet
Oncol. 9:884–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shapiro JR, Yung WK and Shapiro WR:
Isolation, karyotype and clonal growth of heterogeneous
subpopulations of human malignant gliomas. Cancer Res.
41:2349–2359. 1981.PubMed/NCBI
|
|
6
|
Bonavia R, Inda MM, Cavenee WK and Furnari
FB: Heterogeneity maintenance in glioblastoma: A social network.
Cancer Res. 71:4055–4060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sottoriva A, Spiteri I, Piccirillo SG,
Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C and Tavaré
S: Intratumor heterogeneity in human glioblastoma reflects cancer
evolutionary dynamics. Proc Natl Acad Sci USA. 110:4009–4014. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walker MD, Strike TA and Sheline GE: An
analysis of dose-effect relationship in the radiotherapy of
malignant gliomas. Int J Radiat Oncol Biol Phys. 5:1725–1731. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang L, Li B, Tang S, Guo H, Li W, Huang
X, Yan W and Zou F: Mitochondrial KATP channels control glioma
radioresistance by regulating ROS-Induced ERK activation. Mol
Neurobiol. 52:626–637. 2015. View Article : Google Scholar
|
|
10
|
Michaelson D, Abidi W, Guardavaccaro D,
Zhou M, Ahearn I, Pagano M and Philips MR: Rac1 accumulates in the
nucleus during the G2 phase of the cell cycle and promotes cell
division. J Cell Biol. 181:485–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prudnikova TY, Rawat SJ and Chernoff J:
Molecular pathways: Targeting the kinase effectors of RHO-family
GTPases. Clin Cancer Res. 21:24–29. 2015. View Article : Google Scholar :
|
|
12
|
Skvortsov S, Dudás J, Eichberger P,
Witsch-Baumgartner M, Loeffler-Ragg J, Pritz C, Schartinger VH,
Maier H, Hall J, Debbage P, et al: Rac1 as a potential therapeutic
target for chemo-radioresistant head and neck squamous cell
carcinomas (HNSCC). Br J Cancer. 110:2677–2687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Machesky LM and Insall RH: Signaling to
actin dynamics. J Cell Biol. 146:267–272. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pollard TD and Borisy GG: Cellular
motility driven by assembly and disassembly of actin filaments.
Cell. 112:453–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lane J, Martin T, Weeks HP and Jiang WG:
Structure and role of WASP and WAVE in Rho GTPase signalling in
cancer. Cancer Genomics Proteomics. 11:155–165. 2014.PubMed/NCBI
|
|
16
|
Ma X, Espana-Serrano L, Kim WJ, Thayele
PH, Nie Z and Daaka Y: βArrestin1 regulates the guanine nucleotide
exchange factor RasGRF2 expression and the small GTPase
Rac-mediated formation of membrane protrusion and cell motility. J
Biol Chem. 289:13638–13650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koestler SA, Steffen A, Nemethova M,
Winterhoff M, Luo N, Holleboom JM, Krupp J, Jacob S, Vinzenz M,
Schur F, et al: Arp2/3 complex is essential for actin network
treadmilling as well as for targeting of capping protein and
cofilin. Mol Biol Cell. 24:2861–2875. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ko HS, Kim JS, Cho SM, Lee HJ, Ahn KS, Kim
SH and Lee EO: Urokinase-type plasminogen activator expression and
Rac1/WAVE-2/Arp2/3 pathway are blocked by pterostilbene to suppress
cell migration and invasion in MDA-MB-231 cells. Bioorg Med Chem
Lett. 24:1176–1179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bamburg JR: Proteins of the ADF/cofilin
family: Essential regulators of actin dynamics. Annu Rev Cell Dev
Biol. 15:185–230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carlier MF, Ressad F and Pantaloni D:
Control of actin dynamics in cell motility. Role of ADF/cofilin. J
Biol Chem. 274:33827–33830. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maciver SK and Hussey PJ: The ADF/cofilin
family: Actin-remodeling proteins. Genome Biol. 3:reviews30072002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moriyama K and Yahara I: The
actin-severing activity of cofilin is exerted by the interplay of
three distinct sites on cofilin and essential for cell viability.
Biochem J. 365:147–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gurniak CB, Perlas E and Witke W: The
actin depolymerizing factor n-cofilin is essential for neural tube
morphogenesis and neural crest cell migration. Dev Biol.
278:231–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan H, Yang K, Xiao H, Zou YJ, Zhang WB
and Liu HY: Over-expression of cofilin-1 and phosphoglycerate
kinase 1 in astrocytomas involved in pathogenesis of
radioresistance. Cns Neurosci Ther. 18:729–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du HQ, Chen L, Wang Y, Wang LJ, Yan H, Liu
HY and Xiao H: Increasing radiosensitivity with the downregulation
of cofilin-1 in U251 human glioma cells. Mol Med Rep. 11:3354–3360.
2015.
|
|
26
|
Dang I, Gorelik R, Sousa-Blin C, Derivery
E, Guérin C, Linkner J, Nemethova M, Dumortier JG, Giger FA,
Chipysheva TA, et al: Inhibitory signalling to the Arp2/3 complex
steers cell migration. Nature. 503:281–284. 2013.PubMed/NCBI
|
|
27
|
Ho JN, Kang GY, Lee SS, Kim J, Bae IH,
Hwang SG and Um HD: Bcl-XL and STAT3 mediate malignant actions of
gamma-irradiation in lung cancer cells. Cancer Sci. 101:1417–1423.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS,
Lee SH, Park IC, Rhee CH and Hong SI: Ionizing radiation enhances
matrix metalloproteinase-2 secretion and invasion of glioma cells
through Src/epidermal growth factor receptor-mediated p38/Akt and
phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res.
66:8511–8519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang W, Eddy R and Condeelis J: The
cofilin pathway in breast cancer invasion and metastasis. Nat Rev
Cancer. 7:429–440. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le Clainche C, Schlaepfer D, Ferrari A,
Klingauf M, Grohmanova K, Veligodskiy A, Didry D, Le D, Egile C,
Carlier MF and Kroschewski R: IQGAP1 stimulates actin assembly
through the N-WASP-Arp2/3 pathway. J Biol Chem. 282:426–435. 2007.
View Article : Google Scholar
|
|
32
|
DesMarais V, Macaluso F, Condeelis J and
Bailly M: Synergistic interaction between the Arp2/3 complex and
cofilin drives stimulated lamellipod extension. J Cell Sci.
117:3499–3510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arnold CR, Abdelmoez A, Thurner G, Debbage
P, Lukas P, Skvortsov S and Skvortsova II: Rac1 as a
multifunctional therapeutic target to prevent and combat cancer
metastasis. Oncoscience. 1:513–521. 2014. View Article : Google Scholar
|
|
34
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Lu S, Jiang J, Jia X, Dong X and
Bu P: Hsa-microRNA-101 suppresses migration and invasion by
targeting Rac1 in thyroid cancer cells. Oncol Lett. 8:1815–1821.
2014.PubMed/NCBI
|
|
36
|
Lorenz M, Yamaguchi H, Wang Y, Singer RH
and Condeelis J: Imaging sites of N-wasp activity in lamellipodia
and invadopodia of carcinoma cells. Curr Biol. 14:697–703. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bernstein BW and Bamburg JR: ADF/cofilin:
A functional node in cell biology. Trends Cell Biol. 20:187–195.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sreekumar R, Sayan BS, Mirnezami AH and
Sayan AE: MicroRNA control of invasion and metastasis pathways.
Front Genet. 2:582011. View Article : Google Scholar
|