Introduction
Tuberculosis (TB) results in high rates of mortality
and morbidity globally. According to global TB reports in 2014, an
estimated 9.6 million individuals developed TB and 1.5 million
mortalities were caused by TB (1).
Despite progress being made on TB, childhood TB remains an epidemic
in numerous developing countries. There were an estimated 53,000 TB
cases among children (<15 years of age) and 74,000 TB-associated
mortalities in 2012, which account for the 6 and 8% of the global
totals, respectively. It has been reported that there was no marked
decrease in the prevalence of childhood TB from 1979 to 2000 in
China, and patients with bacteriologically-negative TB remain a
large proportion of TB patients (2,3).
There remains a long way to go for China to achieve their 2015
targets of eliminating TB. From 2016, the aim is to end the global
TB epidemic by implementing the End TB Strategy. Adopted by the
World Health Assembly in May 2014, the strategy serves to reduce
the number of TB mortalities by 90% by 2030 (compared with 2015
levels), decrease the number of new cases by 80% and ensure that no
family is financially burdened due to TB (1). To achieve this goal, novel and
effective methods for the early development of childhood TB are
required.
MicroRNA (miRNA) is a class of highly conserved,
single-stranded RNA molecules (length, 18–25 nt) that regulate
expression levels of their target mRNAs (4,5).
Emerging research has demonstrated that serum miRNA is stable to
repeated freezing and thawing as well as heat, acidic and alkaline
conditions and other extremes. It may have potential as a useful
biomarker for disease diagnosis, the effects of therapeutics and
prognosis (6–10). Previous studies have demonstrated
that miRNA is associated with numerous diseases, including cancer
and heart, immune and infectious diseases (11–14).
Altered levels of miRNAs following Mycobacterium
tuberculosis (MTB) infection have been reported, however, these
studies focused on adults rather than children (15–18).
Therefore, the present study aimed to identify and validate the
altered levels of circulating miRNAs in childhood TB. Furthermore,
the present study aimed to determine the diagnostic value of single
and combined miRNAs in childhood TB.
Materials and methods
Ethics statement
The present study was reviewed and approved by the
ethics committee of Chongqing Medical University (Chongqing,
China). Written informed consent was obtained from participants'
parents at the time of enrollment in the present study.
Diagnostic process
Children with the following clinical manifestations
were enrolled as suspected TB cases: i) Cough, fever and weight
loss lasting >2 weeks; ii) pneumonia not responding to
antibiotics; iii) and other clinical findings, including
hydrothorax, tuberculin test (+), interferon-γ release assay (+),
anti-tuberculosis antibody (+), PCR (+) and chest radiography
suggestive of TB. Preliminary screening was conducted according to
previously described guidelines (19). Cases that failed to meet the
criteria, had pulmonary infection other than TB or had other
preliminary diagnoses were excluded. The patients' sputum and
stomach lavage fluid were collected to isolate MTB using
Lowenstein-Jensen medium and an acid-fast bacillus test. Cases with
successful isolation of MTB were regarded as culture-positive
tuberculosis, while cases with no successful isolation of MTB were
regarded as culture-negative tuberculosis. Healthy children were
selected from a child care center at the Children's Hospital of
Chongqing Medical University (Chongqing, China) between February
2012 and July 2013. Written informed consent was obtained from the
patient's family. These children did not recently suffer from any
infectious diseases and did not have any immunodeficiency
diseases.
Sample collection and handing
A total of 129 children were recruited from the
Children's Hospital of Chongqing Medical University (Chongqing,
China). Following preliminary screening, 28 patients and 24 healthy
children were included in the present study. Three active pulmonary
TB cases (confirmed by MTB culture) and three healthy samples were
used for microarray detection. The remaining 25 patients and 21
healthy children had samples taken for use in reverse
transcription-quantitative polymerase chain reaction validation.
There was no significant difference in age or gender between the
two groups. The demographic and clinical characteristics of
patients are summarized in Table
I. Fresh blood samples were collected from the participants in
2.0 ml anticoagulant tubes. Within 4 h of collection, red blood
cell lysis buffers produced in our laboratory were used to collect
peripheral white blood cells for total RNA extraction. A total of 3
ml 1X red blood cell lysis buffer per ml of blood was added and
mixed sufficiently. Centrifugation was then performed at 6,000 x g
at room temperature for 8–10 min and the supernatant was discarded.
Subsequently, 1 ml 1X red blood cell lysis buffer per tube was
added, mixed and centrifuged at 6,000 × g at room temperature for 5
min. The supernatant was discarded and the sediment was white blood
cells.
 | Table IClinical characteristics of childhood
TB. |
Table I
Clinical characteristics of childhood
TB.
| Characteristic | Culture-positive
TB | Culture-negative
TB |
|---|
| Total | 14 | 14 |
| Age (years, mean ±
SD) | 8.4±5.7 | 6.7±4.9 |
| Gender
(male/female) | 11/3 | 5/9 |
| History of close TB
contact | 7 | 4 |
| TST positive | 4 | 10 |
| Cough for >2
weeks | 8 | 5 |
| Fever for >2
weeks | 8 | 10 |
| Night sweats for
>2 weeks | 0 | 0 |
| Weight loss/failure
to thrive | 0 | 2 |
| Radiographic features
of TB | 14 | 14 |
| Culture-positive
TB | 14 | 0 |
| HIV-positive | 0 | 0 |
| HBV-positive | 0 | 0 |
RNA extraction
Total RNA was isolated from peripheral white blood
cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocols. Each
sample was dissolved in 50 µl RNAase-free water. RNA
quantity and purity was measured using a NanoDrop spectrophotometer
(NANODROP 1000; Thermo Fisher Scientific, Inc.). In solution, the
high quantity RNA had an A260/A280 ratio of 1.8–2.1. The total RNA
concentration was 100–400 ng/µl.
Analysis of miRNA microarray data
miRNA microarray assays were performed using the
Agilent Human miRNA microarray platform (Agilent Technologies,
Inc., Santa Clara, CA, USA). Labeling and hybridization were
performed according to the protocols in the Agilent miRNA
microarray system. The microarray was scanned by Agilent miRNA
Scanner and the microarray data was normalized using R-language
programming (http://www.r-project.org). The
normalized data was analyzed according to log 2-transformed
expression level and the expression levels were tested by Student's
t-test and the Wilcoxon signed rank sum test. The miRNAs that
indicated significantly different expression in the two tests and
had at least a 2-fold changes were filtered. An miRNA may target
different genes and a gene may be targeted by different miRNAs. In
order to understand the complicated associations between miRNAs and
target genes, Targetscan (http://genes.mit.edu/targetscan/index.html) and Pictar
(http://pictar.bio.nyu.edu) databases were used to
predict the target genes. The miRNA-gene network was built to
observe closely associated miRNAs following TB infection using
Cytoscape (version 3.0.1; http://www.cytoscape.org/).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (500 ng) was isolated and miRNA was
obtained by poly (A) tail method. miRNAs in the center of the
network were selected for validation using a SYBR green RT-qPCR
assay (All-In-One miRNA qRT-PCR Detection kit, GeneCopoeia, Inc.,
Rockville, MD, USA). The miRNA primers were synthesized by
GeneCopoeia, Inc. and the expression level was normalized to U6.
The miRNA was polyadenylated by poly(A) polymerase and reverse
transcribed to cDNA under conditions of 37°C for 60 min followed by
85°C for 5 min. The RT reaction was conducted in a reaction volume
of 10 µl, which contained 2.5 U/µl PolyA polymerase
(0.4 µl), 0.4 µl RTase mix, 2 µl 5X Reaction
Buffer, 500 ng total RNA and variable volume of RNase Free
dH2O. Synthesized cDNA was diluted 5 times for Real-Time
PCR reaction. The total volume of the reaction mixture was 20
µl, including 10 µl of 2X All-In-One qPCR mix, 2
µl All-In-One qPCR Primer (2 µM), 2 µl
Universal Adaptor PCR Primer (2 µM), 2 µl cDNA and 4
µl dH2O. The PCR was conducted with incubation at
95°C for 10 min, and 40 cycles of 95°C for 10 sec, 55°C for 20 sec
and 72°C for 10 sec using a CFX96 Real-Time system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Each sample was run in
triplicate. For the RT-qPCR data, the relative expression level of
each miRNA was calculated according to the different cycle
quantification (Cq) values between the target miRNAs and miR-U6 by
using the 2−ΔΔCq method (20).
Statistical analysis
Statistical analysis was performed with SPSS
software (version 21; IBM SPSS, Armonk, NY, USA). The data are
presented as the mean ± standard deviation. An unpaired t-test was
used for statistical analysis and P<0.05 was considered to
indicate a statistically significant difference. Receiver operating
characteristic (ROC) curve was generated to evaluate the diagnostic
value of miRNAs. The area under the curve (AUC) and 95% confidence
intervals (CI) were calculated to determine the specificity and
sensitivity. To increase the diagnostic accuracy, a combination of
circulating miRNAs was analyzed. The ROC curve and multivariate
logistic regression in the ROC curves were calculated with MedCalc
11.4.3.0 statistical software (MedCalc Software bvba, Ostend,
Belgium).
Results
Identification of circulating miRNAs from
children with TB
Following preliminary screening, a total of 29
miRNAs were altered between the TB group and the control. Of these,
15 miRNAs were upregulated and 14 were downregulated when compared
with the healthy control group (Table
II). Cluster analysis based on differentially expressed miRNAs
indicated marked distinctions between the two groups (Fig. 1).
 | Table IIFC of miRs between children with
tuberculosis and healthy controls. |
Table II
FC of miRs between children with
tuberculosis and healthy controls.
| ID | logFC |
|---|
| miR-142-5p | 1.165432929 |
| miR-29b | 1.289097829 |
|
miR-21* | 1.67673294 |
| miR-542-5p | 1.812354699 |
| miR-32 | 1.86960397 |
| miR-142-3p | 1.887657013 |
| miR-95 | 2.410210413 |
| miR-144 | 2.099441438 |
|
miR-17* | 2.128855333 |
| miR-141 | 2.278684837 |
| miR-33a | 2.372570115 |
| miR-136 | 2.571642478 |
| miR-324-5p | 2.845130237 |
| miR-193a-3p | 3.94172976 |
| miR-503 | 4.518278633 |
| miR-31 | −5.138973159 |
| miR-564 | −3.577222134 |
| miR-1 | −2.229848349 |
|
miR-181a-2* | −2.048150209 |
| miR-874 | −1.99302363 |
| miR-1305 | −1.894597861 |
| miR-10a | −1.824343578 |
| miR-1275 | −1.756667644 |
| miR-125b | −1.675341028 |
| miR-342-5p | −1.637504768 |
| miR-150 | −1.370167992 |
| miR-155 | −1.282465779 |
| miR-342-3p | −1.131121391 |
| miR-146a | −1.00258041 |
Construction of the miRNA-gene
network
To investigate whether the unique miRNA profile and
their target genes are associated with the progression of TB
infection, and to identify miRNAs that are closely associated with
childhood TB, an miRNA-gene network was constructed. miRNAs and
their target genes have a complex association as miRNAs may target
genes directly or indirectly. The miRNAs and genes closest to the
center are more likely to have altered expression levels. The
network demonstrated miR-1, miR-155, miR-31, miR-146a, miR-10a,
miR-125b, miR-150, miR-29b, miR-141, miR-17*, miR-32, miR-33a,
miR-503 and miR-144 were in the center of the network (Fig. 2).
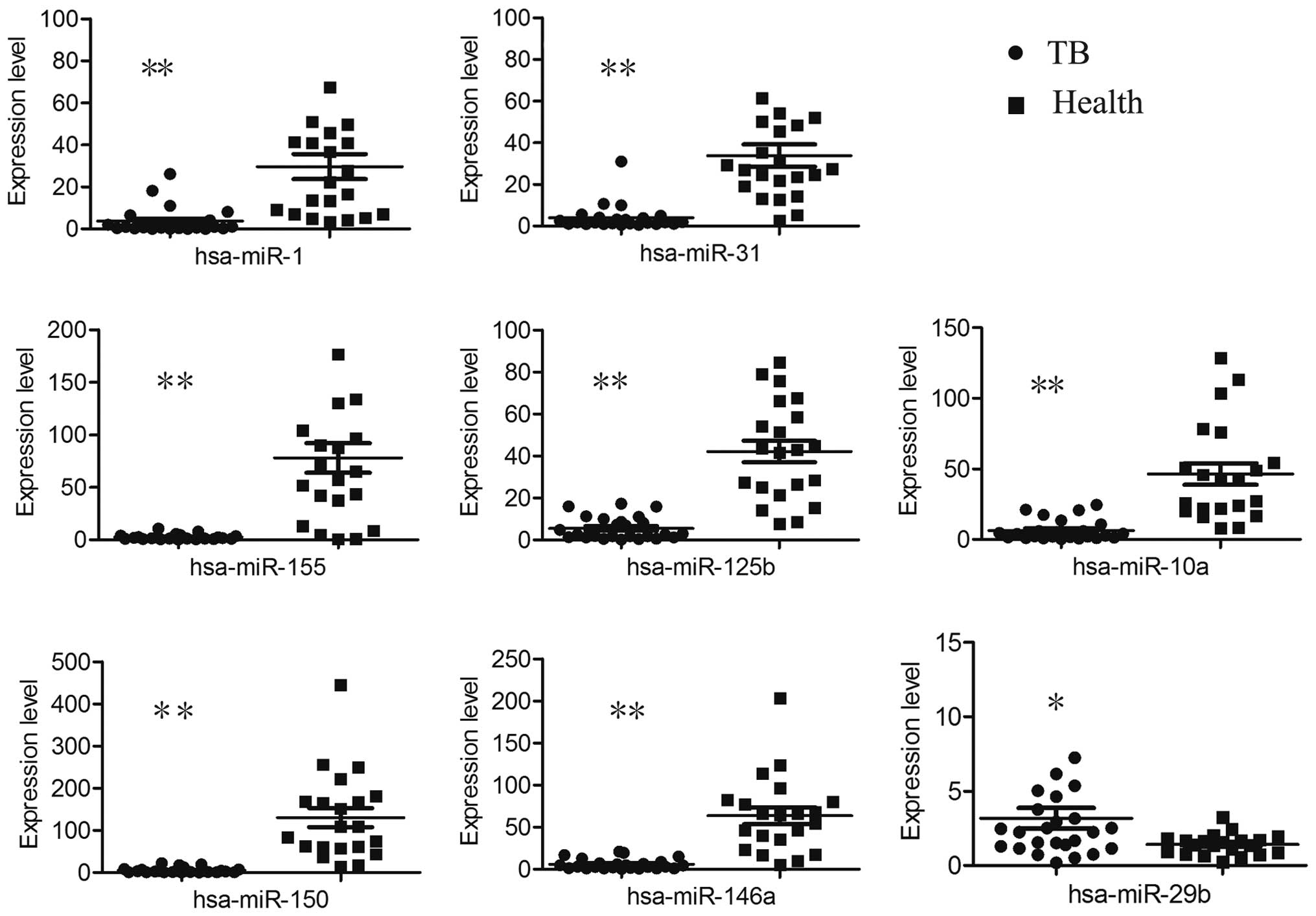
Validation of the altered miRNAs in
independent samples
Fourteen candidate miRNAs were selected for further
verification in 25 children with TB and 21 healthy controls. When
compared with healthy controls, miR-1, miR-155, miR-31, miR-146a,
miR-10a, miR-125b and miR-150 were downregulated while miR-29 was
upregulated in the TB cases (Fig.
3).
Circulating miRNA to diagnose childhood
TB
ROC was performed to evaluate the potential of
microRNA as a biomarker to diagnose childhood TB. The data
demonstrated the diagnostic value of single miRNA was as follows:
miR-1
50>miR-146a>miR-125b>miR-31>miR-10a>miR-1>miR-155>miR-29.
The data demonstrated miR-1, miR-10a, miR-125b miR-146a, miR-150,
miR-155 and miR-31 exhibited a reliable discrimination yielding AUC
values of 0.926 (95% CI, 0.809–0.982), 0.950 (95% CI, 0.843–0.993),
0.962 (95% CI, 0.860–0.996), 0.964 (95% CI, 0.862–0.997), 0.989
(95% CI, 0.902–1.0), 0.918 (95% CI, 0.799–0.978) and 0.952 (95% CI,
0.844–0.994) respectively. Using an optical cutoff (maximum Youden
index; sensitivity +, specificity −1), the sensitivity of the seven
miRNAs was 76, 76, 100, 100, 100, 96 and 95.8% respectively and the
specificity was 100, 100, 81, 81, 90.5, 85.7 and 90.5%
respectively. The miR-29b had a lower discrimination yielding an
AUC value of 0.697 (95% CI, 0.544–9.824), with optical cutoff, the
sensitivity and specificity were 56 and 90.5% respectively.
Logistic regression analysis with the ROC curves was used to
identify combined miRNAs, which may aid in the diagnosis of
childhood TB, the result indicated marked separation between TB and
healthy groups with an AUC value of 0.996 (95% CI, 0.914–1.0), the
sensitivity was 95.8% and the specificity was 100% (Fig. 4).
Discussion
Childhood TB cases are currently diagnosed by
history of close contact with a patient with TB, clinical
manifestations, chest radiography, tuberculin test, interferon-γ
release assay, anti-tuberculosis antibody or PCR. However, children
typically lack clinical symptoms, with >50% children
asymptomatic at early stages of the disease (21,22).
The gold standard to diagnose TB is microbiological isolation of
MTB; however, the load of MTB is often low in tested fluids,
including sputum, stomach lavage fluid and bronchoalveolar lavage
fluid. In addition, the culture of clinical specimens requires long
incubation times (4–8 weeks), by which point it is too late to
influence clinical strategies of treatment and the success rate of
the treatment is low, <30–40% (17,23,24).
Identification of a novel biomarker to allow early diagnosis of
childhood tuberculosis is required.
There are numerous studies that describe the miRNA
profile following TB infection and miR-155, miR-155, miR-200C,
miR-193a-3p, miR-595, miR-432, miR-9, miR-582-5p, miR-144 and
miR-29b have been validated in other studies (15,18,25–28).
The microarray data from the present study is largely inconsistent
with previous studies (17,27,29,30).
For example, data from the present study indicated that miR-155 was
downregulated in the TB group, however, Wu et al (30) demonstrated that miR-155 was
upregulated in the purified protein derivative challenged
peripheral blood mononuclear cells of patients with active TB.
Consistent with previous studies, miR-141, miR-32 and miR-29b were
overexpressed in the TB group of the present study. The expression
level of miR-144 has varied in previous studies, Wang et al
(27) suggested miR-144 is
upregulated in TB patients, while Wu et al (30) observed downregulation. In the
present study, no significant difference was observed in the miRNA
between TB children and healthy children. Furthermore, microarray
data from the present study has suggested a difference in
expression in a number of miRNAs that have not yet been reported,
including miR-31, miR-342-5p, miR-193a-3p, miR-10 and miR-33a. This
may be a result of different experimental protocols, experimental
conditions and samples.
The miRNA-gene network was constructed to observe
key miRNAs. The further validation data demonstrated eight of the
miRNAs in the center of the network had significantly different
expression levels. Of these miRNAs, miR-1, miR-155, miR-31,
miR-146a, miR-10a, miR-125b, miR-150, miR-141, miR-144, miR-17*,
miR-32 and miR-503 were downregulated while miR-29 was upregulated
in the TB group. The diagnostic values of these miRNAs were
analyzed using ROC curves. The data demonstrated each of the
miRNAs, excluding miR-29b, had a reliable diagnostic value and they
all exhibited moderate specificity and sensitivity. Furthermore,
the ROC curve data indicated the diagnostic value of single miRNA
was as follows: miR-150>miR-146a>miR-125b>miR-31>
miR-10a>miR-1>miR-155>miR-29.
However, a combination of the eight miRNAs
demonstrated increased diagnostic value with an AUC of 0.996 (95%
CI, 0.914–1.0), the sensitivity and specificity were 95.8 and 100%,
respectively. It indicated combined identification of miR-1,
miR-155, miR-31, miR-146a, miR-10a, miR-125b, miR-150 and miR-29
may be a novel early diagnostic biomarker.
In conclusion, the present study is the first, to
the best of our knowledge, to analyze the circulating miRNA profile
in childhood TB. Results from the current study suggest multiple
miRNAs may be suitable to serve as potential biomarkers for the
diagnosis of childhood TB.
Acknowledgments
The authors would like to thank all the patients and
healthy children who participated in the present study at the
Children's Hospital of Chongqing. The present study was supported
by NSFC (grant no. 81071406) and the Medical Scientific Research of
Chongqing (grant no. 20142046).
Abbreviations:
|
miRNA
|
microRNA
|
|
TB
|
tuberculosis
|
|
MTB
|
Mycobacterium tuberculosis
|
|
ROC
|
receiver operational curve
|
|
AUC
|
area under the curve
|
|
CI
|
confidence interval
|
References
|
1
|
World Health Organization: Global
Tuberculosis Report 2015. 20th edition. World Health Organization;
2015
|
|
2
|
Li L: The national epidemiological
sampling survey analysis of childhood tuberculosis from 1979 to
2000. Chinese Medical Journal. 80:16782004.In Chinese.
|
|
3
|
World Health Organisation: Global
tuberculosis report 2013. 2013
|
|
4
|
Krishnarao A: MicroRNAs – From Basic
Science to Disease Biology. Cambridge University Press; Cambridge,
UK: 2010
|
|
5
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chim SS, Shing TK, Hung EC, Leung TY, Lau
TK, Chiu RW and Lo YM: Detection and characterization of placental
microRNAs in maternal plasma. Clin Chem. 54:482–490. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alevizos I and Illei GG: MicroRNAs as
biomarkers in rheumatic diseases. Nat Rev Rheumatol. 6:391–398.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilad S, Meiri E, Yogev Y, Benjamin S,
Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed
N, et al: Serum microRNAs are promising novel biomarkers. PloS One.
3:e31482008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Hu Z, Wang W, Ba Y, Ma L, Zhang C,
Wang C, Ren Z, Zhao Y, Wu S, et al: Identification of ten serum
microRNAs from a genome-wide serum microRNA expression profile as
novel noninvasive biomarkers for nonsmall cell lung cancer
diagnosis. Int J Cancer. 130:1620–1628. 2012. View Article : Google Scholar
|
|
10
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: A new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connell RM, Kahn D, Gibson WS, Round JL,
Scholz RL, Chaudhuri AA, Kahn ME, Rao DS and Baltimore D:
MicroRNA-155 promotes autoimmune inflammation by enhancing
inflammatory T cell development. Immunity. 33:607–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ura S, Honda M, Yamashita T, Ueda T,
Takatori H, Nishino R, Sunakozaka H, Sakai Y, Horimoto K and Kaneko
S: Differential microRNA expression between hepatitis B and
hepatitis C leading disease progression to hepatocellular
carcinoma. Hepatology. 49:1098–1112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gidlöf O and Erlinge D: MicroRNAs in the
failing heart-novel therapeutic targets? Scand Cardiovasc J.
48:328–334. 2014. View Article : Google Scholar
|
|
14
|
Ji J, Shi J, Budhu A, Yu Z, Forgues M,
Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al: MicroRNA
expression, survival and response to interferon in liver cancer. N
Engl J Med. 361:1437–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu Y, Yi Z, Wu X, Li J and Xu F:
Circulating microRNAs in patients with active pulmonary
tuberculosis. J Clin Microbiol. 49:4246–4251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spinelli SV, Diaz A, D'Attilio L,
Marchesini MM, Bogue C, Bay ML and Bottasso OA: Altered microRNA
expression levels in mononuclear cells of patients with pulmonary
and pleural tuberculosis and their relation with components of the
immune response. Mol Immunol. 53:265–269. 2013. View Article : Google Scholar
|
|
17
|
Zhang X, Guo J, Fan S, Li Y, Wei L, Yang
X, Jiang T, Chen Z, Wang C, Liu J, et al: Screening and
identification of six serum microRNAs as novel potential
combination biomarkers for pulmonary tuberculosis diagnosis. PloS
One. 8:e810762013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abd-El-Fattah AA, Sadik NA, Shaker OG and
Aboulftouh ML: Differential microRNAs expression in serum of
patients with lung cancer, pulmonary tuberculosis and pneumonia.
Cell Biochem Biophys. 67:875–884. 2013. View Article : Google Scholar
|
|
19
|
Subspecialty Group of Respiratory
Diseases; Society of Pediatrics, Chinese Medical Association:
Editorial Board, Chinese Journal of Pediatrics: Diagnostic
standards and therapeutic recommendations for pulmonary
tuberculosis in children. Zhonghua Er Ke Za Zhi. 44:249–251.
2006.In Chinese.
|
|
20
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anderson ST, Kaforou M, Brent AJ, Wright
VJ, Banwell CM, Chagaluka G, Crampin AC, Dockrell HM, French N,
Hamilton MS, et al: Diagnosis of childhood tuberculosis and host
RNA expression in Africa. N Engl J Med. 370:1712–1723. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eamranond P and Jaramillo E: Tuberculosis
in children: Reassessing the need for improved diagnosis in global
control strategies. Int J Tuberc Lung Dis. 5:594–603.
2001.PubMed/NCBI
|
|
23
|
Zar HJ, Connell TG and Nicol M: Diagnosis
of pulmonary tuberculosis in children: New advances. Expert Rev
Anti Infect Ther. 8:277–288. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wallis RS, Pai M, Menzies D, Doherty TM,
Walzl G, Perkins MD and Zumla A: Biomarkers and diagnostics for
tuberculosis: Progress, needs and translation into practice.
Lancet. 375:1920–1937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maertzdorf J, Weiner J III, Mollenkopf HJ;
TBornotTB Network; Bauer T, Prasse A, Müller-Quernheim J and
Kaufmann SH: Common patterns and disease-related signatures in
tuberculosis and sarcoidosis. Proc Natl Acad Sci USA.
109:7853–7858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi Y, Cui L, Ge Y, Shi Z, Zhao K, Guo X,
Yang D, Yu H, Cui L, Shan Y, et al: Altered serum microRNAs as
biomarkers for the early diagnosis of pulmonary tuberculosis
infection. BMC Infect Dis. 12:384–393. 2012. View Article : Google Scholar
|
|
27
|
Wang C, Yang S, Sun G, Tang X, Lu S,
Neyrolles O and Gao Q: Comparative miRNA expression profiles in
individuals with latent and active tuberculosis. PloS One.
6:e258322011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yi Z, Fu Y, Ji R, Li R and Guan Z: Altered
microRNA signatures in sputum of patients with active pulmonary
tuberculosis. PloS One. 7:e431842012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Wang X, Jiang J, Cao Z, Yang B and
Cheng X: Modulation of T cell cytokine production by
miR-144* with elevated expression in patients with
pulmonary tuberculosis. Mol Immunol. 48:1084–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu J, Lu C, Diao N, Zhang S, Wang S, Wang
F, Gao Y, Chen J, Shao L, Lu J, et al: Analysis of microRNA
expression profiling identifies miR-155 and miR-155* as
potential diagnostic markers for active tuberculosis: A preliminary
study. Hum Immunol. 73:31–37. 2012. View Article : Google Scholar
|