Introduction
Breast cancer is a common malignancy that affects
the health of women worldwide (1).
Currently, breast cancer treatment typically requires surgery and
adjuvant chemotherapy or radiotherapy. Among various treatment
strategies, chemotherapy has remained one of the most effective
tools for the treatment of breast cancer (2–4).
However, the phenomenon of multidrug resistance (MDR) severely
limits the efficacy of chemotherapy. MDR is the predominant cause
of the failure of chemotherapeutic treatment, therefore, it is
important to investigate methods that may reverse MDR during
chemotherapy of breast cancer (5,6).
Overexpression of ATP-binding cassette (ABC) family proteins and
elevation of the apoptotic threshold contribute to drug resistance
(7–9). P-glycoprotein (P-gp; also termed ABC
subfamily B member 1, ABCB1), multidrug resistance proteins (MRPs;
also termed ABCCs), and breast cancer resistance protein (BCRP;
also termed ABCG2) are commonly overexpressed in chemoresistant
cells (10).
The human ABCB1 gene, also termed multidrug
resistance 1 (MDR1), encodes P-gp. Overexpression of this gene is
considered to be one of the major obstacles to successful cancer
chemotherapy (11). The MDR1
phenotype is commonly observed to produce resistance to
chemotherapy in breast cancer (12). The overexpression of P-gp has also
been observed in the tumor tissue of 40–50% of patients with cancer
(13). Inhibition of P-gp-mediated
drug efflux may increase the sensitivity of tumor cells to
chemotherapeutics, and enhance the success of chemotherapy for
cases of multidrug-resistant cancer (14,15).
Thus, inhibiting the function of P-gp and MRPs, or enhancing the
efficacy of apoptosis induced by chemotherapeutics, has become
important during the investigation of novel breast cancer
treatments (16). Although several
agents have been demonstrated to effectively reverse P-gp-mediated
MDR, no drugs have been successfully developed for clinical use.
The observation that numerous plant-derived dietary compounds
modulate P-gp transport has led to interest in the possible use of
natural compounds, which exhibit fewer side effects than
traditional chemotherapy, for cancer treatment (17–19).
The feasibility of using traditional Chinese
medicine to combat MDR has received increasing attention and
extensive research (20). Psoralen
is the main active ingredient extracted from the natural products
of Psoralea corylifolia, and is widely used as an
anti-neoplastic agent in the treatment of leukemia and other types
of cancer. Previous studies have demonstrated psoralen to be a
potent inhibitor of cutaneous T-cell lymphoma (21–23),
and cytotoxic to MEC-1 mucoepidermoid carcinoma cells in
vitro (24). Furthermore,
intraperitoneal administration of psoralen can inhibit the growth
of ascitic tumors in mice (25).
However, the effects and mechanisms of psoralen on MDR remain
unclear. Therefore, in the present study, the effect of psoralen on
MDR in breast cancer cells was investigated. An adriamycin
(ADR)-resistant human breast cancer cell line (MCF-7/ADR) was used
to determine whether psoralen may reverse MDR by modulating the
function of P-gp.
Materials and methods
Reagents
Psoralen was obtained from Baoji Herbest Bio-Tech
Co., Ltd. (Baoji, China). Cell culture reagents were purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Adriamycin (ADR) was obtained from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China). Mouse monoclonal P-gp (ab3083) and β-actin
(ab49900) antibodies were purchased from Abcam (Cambridge, MA,
USA). Peroxidase-conjugated Affinipure goat anti-mouse IgG
(SA00001-1) was obtained from ProteinTech Group, Inc. (Chicago, IL,
USA). A RevertAid First Strand cDNA Synthesis kit (#K1621) and
Power SYBR Green PCR Master Mix (#4367659) were purchased from
Thermo Fisher Scientific, Inc. TRIzol,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylterazolium bromide (MTT)
and Rhodamine 123 (Rh 123) were obtained from Sigma-Aldrich (St.
Louis, MO, USA).
Cell culture
MCF-7 human breast cancer cells and ADR-resistant
(MCF-7/ADR) cells were obtained from the Laboratory of Type Culture
Collection, Binzhou University of Medicine (Binzhou, China). The
MCF-7 cell line was cultured in RPMI-1640. The MCF-7/ADR cell line
was cultured in the same medium containing 1 μg/ml ADR to
maintain the MDR phenotype. All media were supplemented with 10%
Gibco fetal bovine serum, penicillin (100 U/ml) and streptomycin
(100 μg/ml) (all from Thermo Fisher Scientific, Inc.), and
all cells were incubated at 37°C in a 5% CO2 humidified
atmosphere.
Cell viability assay
Inhibition of cell proliferation by psoralen was
determined using the MTT assay. The MCF-7/ADR cells were seeded at
1×104 cells/well in 96-well flat-bottomed culture plates
with 100 μl RPMI-1640 for 8 h. RPMI-1640 medium alone was
used as a blank control. The medium was then removed and replaced
by fresh medium containing a range of psoralen concentrations (2,
4, 6, 8, 12, 16 and 20 μg/ml) and cells were incubated for
48 h. Following addition of 20 μl MTT solution (5 mg/ml),
incubation was continued for 4 h at 37°C. The supernatant was then
removed and 150 μl dimethyl sulfoxide was added to each well
and incubated for 10 min, with agitation to dissolve the purple
formazan crystals. The absorbance at 490 nm was measured using an
automatic micro-plate reader (EL×800; BioTek Instruments, Inc.,
Winooski, VT, USA). Values are presented as the mean ± standard
deviation (SD) from three independent experiments. The IC10 value
was defined as the concentration resulting in 90% cell survival.
The IC10 value was calculated using the SPSS software version 17.0
(SPSS, Inc., Chicago, IL, USA). The IC10 concentration was used as
the experimental concentration in the proceeding experiments.
MDR reversal activity
The effect of psoralen (4, 8 and 12 μg/ml) on
MDR in MCF-7 and MCF-7/ADR cells treated with ADR was measured
using MTT assay. The concentration of drug that inhibited 50% of
cells (IC50) was calculated, and was subsequently used to
calculated the MDR reversal fold. The reversal fold value was
calculated by dividing the IC50 value of the MDR cells (MCF-7/ADR)
by the value of the treated cells (MCF-7/ADR + psoralen).
ADR and Rh 123 efflux assays
The intercellular ADR and Rh 123 content in
MCF-7/ADR cells treated with psoralen were analyzed by flow
cytometry as previously described (26). The cells treated with the IC10 of
psoralen were cultured at 37°C for 3 h. ADR and Rh 123 were added
to the cells at a final concentration of 5 μg/ml. The cells
were incubated for a further 3 h and 0.5 h, respectively,
harvested, washed three times with cold phosphate-buffered saline
(PBS), and the fluorescence intensity was measured by flow
cytometric analysis on a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The mRNA expression of MDR1 was measured by RT-qPCR.
MCF-7/ADR cells (1×106 cells/ml) were seeded in 6-well
culture plates and incubated for 8 h to allow attachment. The media
was refreshed and psoralen was added to the experimental group at
the IC10 concentration (8 μg/ml), and incubation was
continued for 48 h. Cells were harvested following treatment,
washed twice with cold PBS and collected by scraping. According to
the manufacturer's protocols, total RNA was isolated from 6-well
plates using TRIzol reagent, and then subjected to RT-qPCR using
the RevertAid First Strand cDNA Synthesis kit and Power SYBR Green
PCR Master Mix according to the manufacturer's protocol. The RT
reactions were performed at 42°C for 60 min and 70°C for 60 min.
The cDNA was dissolved in 20 μl
diethylpyrocarbonate-H2O and used in the proceeding PCR
reactions. In addition, the mRNA levels of β-actin were measured as
a reference and used to normalize the mRNA levels of the drug
resistance genes. The primer sequences were designed and supplied
from Sangon Biotech Co., Ltd. (Shanghai, China) as follows: MDR1, F
5′-CCCATCATTGCAATAGCAGG-3′ and R 5′-GTTCAAACTTCTGCTCCTAG-3′;
β-actin, F 5′-TGTCACCAACTGGGACGATA-3′ and R
5′-GGGGTGTTGAAGGTCTCAAA-3′. The cDNA (1 μl) was amplified by
PCR on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), at 95°C for 1 min and 45
sec, followed by 35 cycles of 95°C for 30 sec and 60°C for 30 sec,
with a final extension at 72°C for 7 min. The data were analyzed by
2−ΔΔCq (27).
Western blot analysis
The MCF-7/ADR cells treated with psoralen (8
μg/ml), and untreated MCF-7 and MCF-7/ADR cells were
incubated for 48 h. The cell lysates were prepared in
radioimmunoprecipitation assay buffer. Protein concentrations were
determined by bicinchoninic acid assay using a GeneQuant 1300
spectrophotometer (GE Healthcare Life Sciences, Milwaukee, WI, USA)
to measure absorbance at 562 nm. Proteins were separated by 8%
SDS-PAGE (80 V for 20 min and 100 V for 70 min) and transferred
onto polyvinylidene fluoride membranes (Bio-Rad Laboratories,
Inc.). Following blocking with 5% non-fat dry milk, the membranes
were incubated with the mouse P-gp (1:400) and β-actin (1:5,000)
antibodies overnight at 4°C. The membranes were washed with
Tris-buffered saline with 0.1% Tween 20 (TBS-T) and incubated for 2
h with peroxidase-conjugated anti-mouse secondary antibodies
(1:5,000), then washed again with TBS-T. The blots were detected
using Clarity Western ECL Blotting Substrate (Bio-Rad Laboratories,
Inc.). The relative photographic density was quantified using
BandScan software, version 4.0 (Glyko Biomedical, Ltd., Hayward,
CA, USA). All experiments were performed in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Significant
differences between the groups were evaluated with t-tests. The
values are expressed as the mean ± SD. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibitory effects of psoralen on cell
proliferation of MCF-7/ADR cells
Prior to the experiments of the current study, no
cytotoxic effects of psoralen on MCF-7/ADR cells had been
established. Therefore, the cytotoxic effects of psoralen were
evaluated prior to use. MTT assays were performed to evaluate the
antiproliferative effects of psoralen on MCF-7/ADR cells. The cells
were treated with the different concentrations of psoralen (2, 4,
6, 8, 12, 16 and 20 μg/ml) for 48 h. As presented in
Fig. 1, the concentration
resulting in a 10% growth inhibition (IC10) of psoralen was 8
μg/ml in MCF-7/ADR cells.
Effect of psoralen on MCF-7/ADR cell
resistance to ADR
Effects of psoralen on the MDR were evaluated by an
MTT assay. The results demonstrated that psoralen can reduce MDR.
The sensitivity of the MCF-7/ADR cells to ADR were 2.0-, 17.5- and
44-fold when the cells were treated with 4, 8 and 12 μg/ml
psoralen, respectively. These results indicated that psoralen
significantly reduces MDR and increases the cytotoxicity of ADR in
MCF-7/ADR cells in a dose-dependent manner.
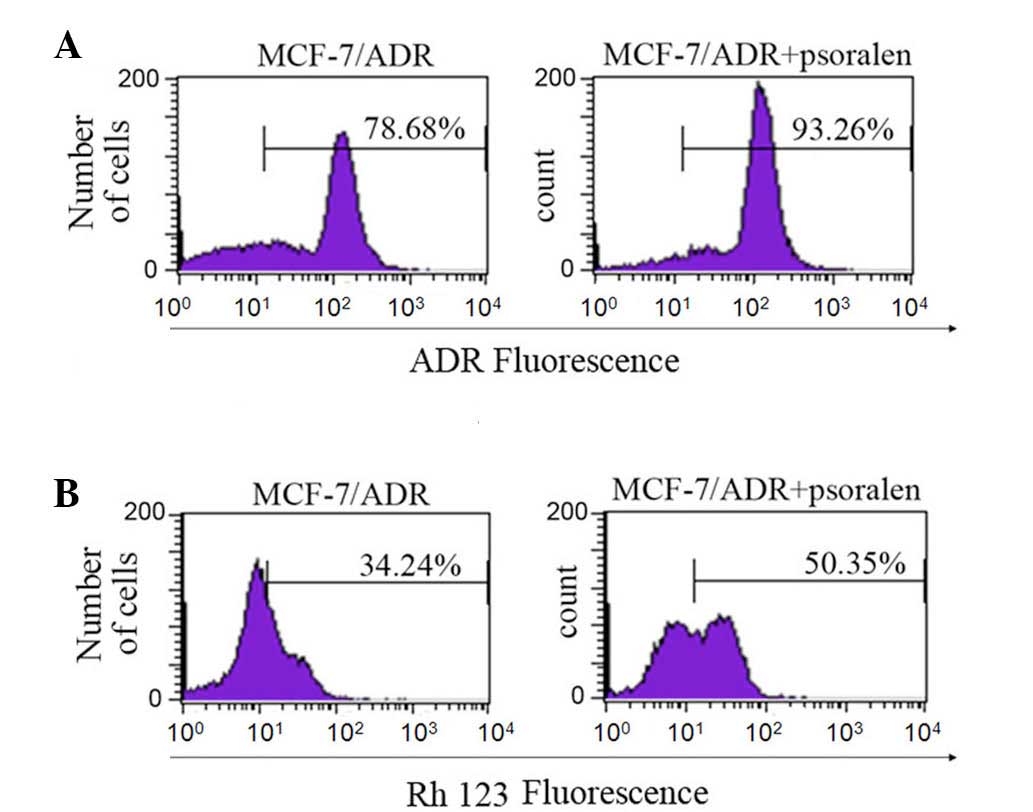
Psoralen inhibits the transport function
of P-gp
The ability of psoralen to inhibit P-gp-mediated
transport was analyzed using the P-gp substrates, ADR and Rh 123
(28). Flow cytometric analysis
was performed to determine the effect of psoralen on the
accumulation and efflux of ADR and Rh 123 (Fig. 2). Compared with cells without
psoralen, the fluorescence index of ADR was increased by 1.2-fold
in cells treated with 8 μg/ml psoralen (P=0.027). The same
doses of psoralen increased the fluorescence index of Rh 123 by
1.6-fold (P=0.022). The results indicate that psoralen increases
the accumulation of the ADR and Rh 123 anticancer drugs in
MCF-7/ADR cells, which may be associated with an effect on P-gp
transport.
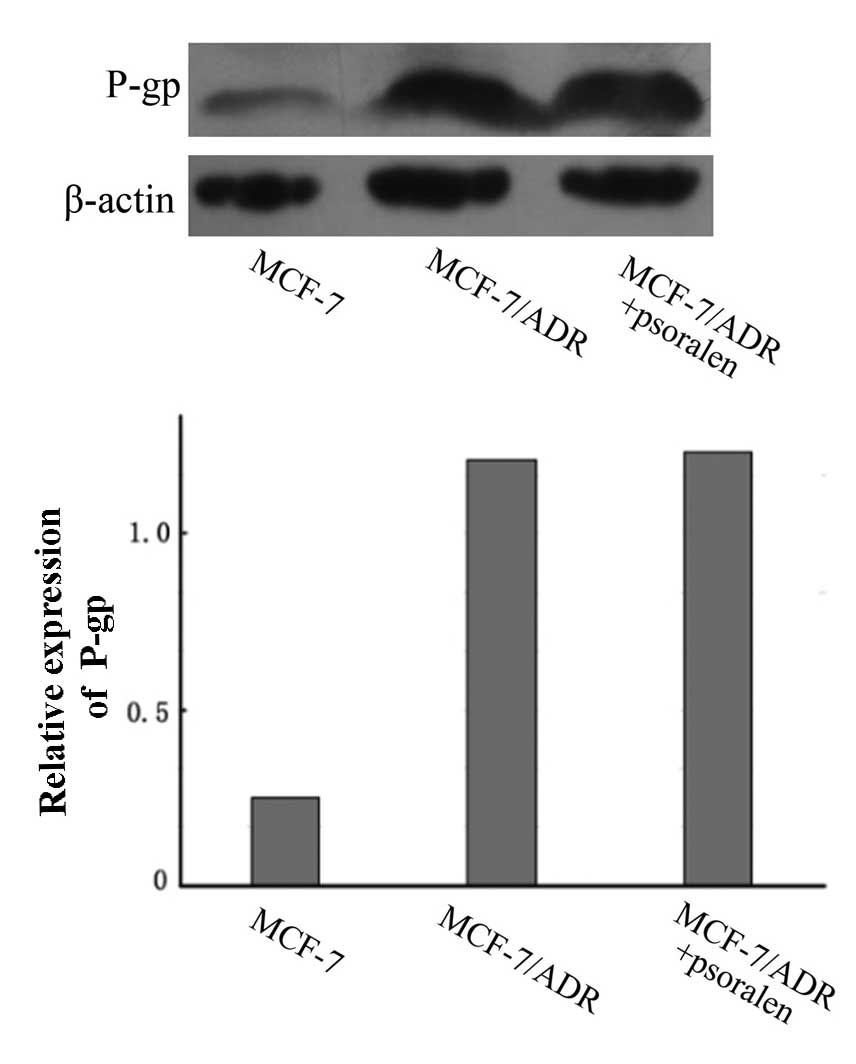
Psoralen has no marked effect on the
expression of P-gp
RT-qPCR and western blotting were performed to
measure the effect of psoralen on the expression of P-gp at the
mRNA and protein levels, respectively. As demonstrated in Fig. 3, the western blot analysis
indicated that the P-gp protein expression levels were markedly
increased in MCF-7/ADR cells compared with the parental MCF-7
cells. However, compared with untreated cells, no change in the
P-gp protein expression levels in MCF-7/ADR cells were observed
following treatment with psoralen (P>0.05).
As determined by RT-qPCR, no significant difference
was observed in the MDR1 mRNA expression levels between untreated
and psoralen-treated MCF-7/ADR cells (P>0.05; Fig. 4).
In summary, the MDR1 mRNA levels and P-gp protein
levels in MCF-7/ADR cells were not changed by psoralen treatment.
The above results demonstrate that the effect of psoralen on MDR
may be associated with inhibition of the P-gp transporter, rather
than reducing the expression levels of P-gp mRNA or protein.
Discussion
Numerous types of cancer cells eventually develop
MDR following treatment with chemotherapeutic drugs (29). MDR is an important issue, as
overcoming MDR may enhance chemotherapy results and improve the
outcome for patients with cancer (30). One of the mechanisms of MDR is
overexpression of P-gp (31),
which increases drug efflux and eventually results in the reduced
efficacy of chemotherapeutic drugs (32). Extensive research efforts have
attempted to reduce MDR using inhibitors of the drug-efflux pump
and various other compounds to improve the therapeutic efficiency
of chemotherapy (33,34). Several P-gp inhibitors have been
investigated for their potential to reduce MDR. However, none have
currently been approved for clinical use (35).
A large body of literature now exists indicating
that the herbal medicines or natural compounds may be feasible for
use as potent chemopreventive drugs (36,37).
The goal of the present study was to assess whether psoralen, an
active ingredient extracted from Psoralea corylifolia, may
reverse MDR by modulating the function of P-gp. MTT assays
demonstrated that psoralen is a cytotoxic agent, which is
consistent with previous results in A549/D16 human lung cancer
cells (38), with an IC10 at 8
μg/ml. However, no cell toxicity was detected when psoralen
was used at 8 μg/ml as a single agent. This indicates that
the concentration of psoralen used in these experiments is
clinically achievable.
To assess the potential of psoralen to reduce MDR,
its capacity to sensitize MCF-7/ADR cells was measured. The results
of the present study demonstrated that 8 μg/ml psoralen
significantly reduced the resistance of MCF-7/ADR cells to ADR,
whereas it had no effect on the parental MCF-7 cells. The current
study demonstrated the potential of psoralen as a P-gp-mediated MDR
reversal agent, and this result was further confirmed by the
psoralen-induced reduction to ADR resistance in P-gp-overexpressing
MCF-7/ADR cells.
P-gp-mediated MDR can be reduced by downregulating
P-gp expression or inhibiting P-gp efflux activity. In the
experiments of the current study, the P-gp transport function was
analyzed by measuring the efflux of P-gp substrates using flow
cytometry. This analysis provides a measure of P-gp-mediated
transport activity by comparing the intracellular concentrations of
ADR and Rh 123 in P-gp overexpressing MCF-7/ADR cells, in the
presence and absence of psoralen. These results demonstrated that
the intracellular accumulation of ADR and Rh 123 were significantly
increased in MCF-7/ADR cells treated with psoralen, compared with
untreated cells, however, psoralen had no significant effect on the
parental MCF-7 cells. These results are in agreement with the
demonstrated effect of psoralen on MDR, indicating that psoralen
alters P-gp function and transport to increase the intracellular
accumulation of drugs. However, the results of the present study
indicated that psoralen did not alter P-gp expression at the mRNA
or protein level, suggesting that psoralen does not reduce MDR via
inhibition of P-gp expression. Due to the effect of psoralen on
MDR, it may be a promising chemosensitizer for clinical use in
combination with other anticancer drugs.
P-gp is a transmembrane drug efflux protein with
trans-membrane and nucleotide-binding domains. The transport
activity of P-gp requires energy from ATP hydrolysis, thus, the
ATPase activity of P-gp reflects its transport activity. Substrate
recognition and binding occur in the transmembrane domains of P-gp,
and are an essential requirement for transportation. Detailed
understanding of the mechanisms by which psoralen inhibits P-gp
transport function may be important for overcoming MDR. Thus,
further research should be undertaken to elucidate these
mechanisms, particularly the effect on P-gp ATPase activity.
In conclusion, the current study demonstrated that
psoralen significantly reduces P-gp-mediated MDR in human breast
cancer MCF-7/ADR cells at pharmacologically achievable
concentrations, and affects MDR reversal by inhibiting P-gp
function. Based on the results of the present study, psoralen has
the potential to be coadministered with chemotherapeutic agents to
improve the efficacy of chemotherapy and to reduce P-gp-mediated
MDR.
Acknowledgments
The present work was supported by the National
Natural Science Foundation of China (nos. 30972932 and
81173601).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Xu H, Dai X, Zhu Z, Liu B and Lu X:
Enhanced in vitro and in vivo therapeutic efficacy of codrug-loaded
nanoparticles against liver cancer. Int J Nanomedicine.
7:5183–5190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao X, Luo J, Gong T, Zhang ZR, Sun X and
Fu Y: Coencapsulated doxorubicin and bromotetrandrine lipid
nanoemulsions in reversing multidrug resistance in breast cancer in
vitro and in vivo. Mol Pharm. 12:274–286. 2015. View Article : Google Scholar
|
|
4
|
Shuhendler AJ, Cheung RY, Manias J, Connor
A, Rauth AM and Wu XY: A novel doxorubicin-mitomycin C
co-encapsulated nanoparticle formulation exhibits anti-cancer
synergy in multidrug resistant human breast cancer cells. Breast
Cancer Res Treat. 119:255–69. 2010. View Article : Google Scholar
|
|
5
|
Duan Z, Zhang J, Ye S, Shen J, Choy E,
Cote G, Harmon D, Mankin H, Hua Y, Zhang Y, et al: A-770041
reverses paclitaxel and doxorubicin resistance in osteosarcoma
cells. BMC Cancer. 14:6812014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Susa M, Iyer AK, Ryu K, Choy E, Hornicek
FJ, Mankin H, Milane L, Amiji MM and Duan Z: Inhibition of ABCB1
(MDR1) expression by an siRNA nanoparticulate delivery system to
overcome drug resistance in osteosarcoma. PLoS One. 5:e107642010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi E, Iyer AK, Hornicek FJ, Amiji
MM and Duan Z: Lipid-functionalized dextran nanosystems to overcome
multidrug resistance in cancer: A pilot study. Clin Orthop Relat
Res. 471:915–925. 2013. View Article : Google Scholar :
|
|
8
|
Brambilla D, Zamboni S, Federici C, Lugini
L, Lozupone F, De Milito A, Cecchetti S, Cianfriglia M and Fais S:
P-glycoprotein binds to ezrin at amino acid residues 149–242 in the
FERM domain and plays a key role in the multidrug resistance of
human osteosarcoma. Int J Cancer. 130:2824–2834. 2012. View Article : Google Scholar
|
|
9
|
Pakos EE and Ioannidis JP: The association
of P-glycoprotein with response to chemotherapy and clinical
outcome in patients with osteosarcoma. A meta-analysis. Cancer.
98:581–589. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneiderman RS, Shmueli E, Kirson ED and
Palti Y: TTFields alone and in combination with chemotherapeutic
agents effectively reduce the viability of MDR cell sub-lines that
over-express ABC transporters. BMC Cancer. 10:2292010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barthomeuf C, Grassi J, Demeule M,
Fournier C, Boivin D and Béliveau R: Inhibition of P-glycoprotein
transport function and reversion of MDR1 multidrug resistance by
cnidiadin. Cancer Chemother Pharmacol. 56:173–181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leonard GD, Fojo T and Bates SE: The role
of ABC transporters in clinical practice. Oncologist. 8:411–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Feng D, Han L, Fan J, Zhang X,
Wang X, Ye L, Shi X and Feng M: Combination of apolipoprotein
A1-modi liposome-doxorubicin with autophagy inhibitors overcame
drug resistance in vitro. J Pharm Sci. 103:3994–4004. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH, Choi AR, Kim YK and Yoon S:
Co-treatment with the anti-malarial drugs mefloquine and primaquine
highly sensitizes drug-resistant cancer cells by increasing P-gp
inhibition. Biochem Biophys Res Commun. 441:655–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Georgantzopoulou A, Skoczynska E, Van den
Berg JH, Brand W, Legay S, Klein SG, Rietjens IM and Murk AJ: P-gp
efflux pump inhibition potential of common environmental
contaminants determined in vitro. Environ Toxicol Chem. 33:804–813.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin J, Wang FP, Wei H and Liu G: Reversal
of multidrug resistance of cancer through inhibition of
P-glycoprotein by 5-bromotetrandrine. Cancer Chemother Pharmacol.
55:179–188. 2005. View Article : Google Scholar
|
|
17
|
Jodoin J, Demeule M and Beliveau R:
Inhibition of the multidrug resistance P-glycoprotein activity by
green tea polyphenols. Biochim Biophys Acta. 1542:149–159. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang EJ, Casciano CN, Clement RP and
Johnson WW: Inhibition of P-glycoprotein transport function by
grapefruit juice psoralen. Pharm Res. 18:432–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Pietro A, Conseil G, Pérez-Victoria JM,
Dayan G, Baubichon-Cortay H, Trompier D, Steinfels E, Jault JM, de
Wet H, Maitrejean M, et al: Modulation by flavonoids of cell
multidrug resistance mediated by P-glycoprotein and related ABC
transporters. Cell Mol Life Sci. 59:307–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chai S, To KK and Lin G: Circumvention of
multi-drug resistance of cancer cells by Chinese herbal medicines.
Chin Med. 5:262010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edelson R, Berger C, Gasparro F, Jegasothy
B, Heald P, Wintroub B, Vonderheid E, Knobler R, Wolff K, Plewig G,
et al: Treatment of cutaneous T-cell lymphoma by extracorporeal
photochemotherapy. Preliminary results. N Engl J Med. 316:297–303.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McGinnis KS, Shapiro M, Vittorio CC, Rook
AH and Junkins-Hopkins JM: Psoralen plus long-wave UV-A (PUVA) and
bexarotene therapy: An effective and synergistic combined adjunct
to therapy for patients with advanced cutaneous T-cell lymphoma.
Arch Dermatol. 139:771–775. 2013.
|
|
23
|
Querfeld C, Rosen ST, Kuzel TM, Kirby KA,
Roenigk HH Jr, Prinz BM and Guitart J: Long-term follow-up of
patients with early-stage cutaneous T-cell lymphoma who achieved
complete remission with psoralen plus UV-A monotherapy. Arch
Dermatol. 141:305–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu JZ, Situ ZQ, Chen JY, Liu B and Wang W:
Chemosensitivity of salivary gland and oral cancer cell lines. Chin
Med J (Engl). 105:1026–1028. 1992.
|
|
25
|
Latha PG, Evans DA, Panikkar KR and
Jayavardhanan KK: Immunomodulatory and antitumour proper ties of
Psoralea corylifolia seeds. Fitoterapia. 71:223–231. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiang QF, Zhang DM, Wang JN, Zhang HW,
Zheng ZY, Yu DC, Li YJ, Xu J, Chen YJ and Shang CZ: Cabozantinib
reverses multidrug resistance of human hepatoma HepG2/adr cells by
modulating the function of P-glycoprotein. Liver Int. 35:1010–1023.
2015. View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Minderman H, O'Loughlin KL, Pendyala L and
Baer MR: VX-710 (biricodar) increases drug retention and enhances
chemosensitivity in resistant cells overexpressing P-glycoprotein,
multidrug resistance protein, and breast cancer resistance protein.
Clin Cancer Res. 10:18261834. View Article : Google Scholar
|
|
29
|
Eicher C, Dewerth A, Kirchner B, Warmann
SW, Fuchs J and Armeanu-Ebinger S: Development of a drug resistance
model for hepatoblastoma. Int J Oncol. 38:447–454. 2011.
|
|
30
|
Oliveira Rodrigues F, Dos Santos RE, de
Oliveira AL, de Lima Rozenowicz R, de Melo MB and Scheffer DK:
Prognostic assessment of polymorphisms of the MDR-1 and GSTP1 genes
in patients with stage II and III breast cancer submitted to
neoadjuvant chemotherapy. Breast J. 18:185–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tiwari AK, Sodani K, Dai CL, Ashby CRJ and
Chen ZS: Revisiting the ABCs of multidrug resistance in cancer
chemotherapy. Curr Pharm Biotechnol. 12:570–594. 2011. View Article : Google Scholar
|
|
32
|
Ernst R, Kueppers P, Stindt J, Kuchler K
and Schmitt L: Multidrug efflux pumps: Substrate selection in
ATP-binding cassette multidrug efflux pumps - first come, first
served? FEBS J. 277:540–549. 2010. View Article : Google Scholar
|
|
33
|
Liscovitch M and Lavie Y: Cancer multidrug
resistance: A review of recent drug discovery research. IDrugs.
5:349–355. 2002.
|
|
34
|
Lavie Y, Cao H, Volner A, Lucci A, Han TY,
Geffen V, Giuliano AE and Cabot MC: Agents that reverse multidrug
resistance, tamoxifen, verapamil, and cyclosporin A, block
glycosphingolipid metabolism by inhibiting ceramide glycosylation
in human cancer cells. J Biol Chem. 272:1682–1687. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ward AB, Szewczyk P, Grimard V, Lee CW,
Martinez L, Doshi R, Caya A, Villaluz M, Pardon E, Cregger C, et
al: Structures of P-glycoprotein reveal its conformational
flexibility and an epitope on the nucleotide-binding domain. Proc
Natl Acad Sci USA. 110:13386–13391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen WT, Yang TS, Chen HC, Chen HH, Chiang
HC, Lin TC, Yeh CH, Ke TW, Chen JS, Hsiao KH and Kuo ML:
Effectiveness of a novel herbal agent MB-6 as a potential adjunct
to 5-fluoracil-based chemotherapy in colorectal cancer. Nutr Res.
34:585–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Montazeri AS, Raei M, Ghanbari A, Dadgari
A, Montazeri AS and Hamidzadeh A: Effect of herbal therapy to
intensity chemotherapy-induced nausea and vomiting in cancer
patients. Iran Red Crescent Med J. 15:101–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsieh MJ, Chen MK, Yu YY, Sheu GT and
Chiou HL: Psoralen reverses docetaxel-induced multidrug resistance
in A549/D16 human lung cancer cells lines. Phytomedicine.
21:970–977. 2014. View Article : Google Scholar : PubMed/NCBI
|