Introduction
Vitiligo is a common type of dermatosis,
characterized by depigmentation of the skin and mucosae due to the
loss of melanocytes, most likely as a result of autoimmune effects
(1). It is predominantly caused by
the destruction of melanocytes in the skin, mucous membranes and
the retina, which results in white patches of skin on different
parts of the body (1,2). The hair growing in these
vitiligo-affected areas usually turns white. At present, leucoderma
treatment is predominantly focused on drug therapy, surgical
treatment and physical therapy (3). The transplantation of cultured
autologous pure melanocytes contributes significantly in leucoderma
therapy. Melanocytes are considered to be more vulnerable to the
damaging effects of oxidative stress, compared with keratinocytes
and fibroblasts (4–6). Oxidative stress is also one of the
inducing factors causing vitiligo (7). Therefore, the repair of injured
melanocytes and the renewal of melanocytes are key for the
treatment of vitiligo.
Traditional Chinese medicine has been used for
various disease therapies in China for thousands of years (8). Cuscutae semen is the dry root of
Cuscuta australis and Cuscuta chinensis, which has
been used for treating various kidney conditions in China (9,10).
It has also long been used for drinking (11). According to the Chinese
Pharmacopoeia (2005, 2010) (12,13),
Cuscutae semen has favorable effects on vitiligo treatment, and it
is Component of the Chinese herbal compound prescription, Chi Tu
Ting, which is used extensively in the treatment of leucoderma
(14). Bioactive compounds,
including alkaloids, anthraquinones, hyperoside, flavonoids,
glycosides, sterols, tannic acid and saccharides are secondary
metabolites found in Cuscutae semen (15,16).
However, there has been little screening of the specific compounds
closely associated with the effect of Cuscutae semen on vitiligo.
In our previous study, six compounds obtained from Cuscutae semen,
including quercetin, astragalin,
quercetin-3-O-β-D-galactoside-7-O-β-glucoside, β-carotene, lutein
and hyperoside [2-(3,4-dihydro
xyphenyl)-3-(B-D-gala-ctopyranosyloxy)-5,7-dihydroxy] and
hyperoside (Fig. 1) exhibited
significant effects in melanogenesis.
In the present study, the protective ability of
hyperoside against hydrogen peroxide
(H2O2)-induced damage in melanocytes was
investigated, and the possible mechanisms involved was examined.
The results of these investigations aimed to provide novel
direction and understanding for the treatment of vitiligo.
Materials and methods
Melanocyte culture
Human primary epidermal melanoyctes (cat. no.
PCS-200-012) were purchased from American Type Culture Collection
(ATCC; Rockville, MD, USA) and cultured in dermal cell basal medium
supplemented with a melanocyte growth kit and
antimicrobials/antimycotics (0.5 ml gentamicin-amphotericin B and
0.5 ml penicillin-streptomycin-amphotericin B) (ATCC). All cultures
were incubated in a humidified incubator with 5% CO2 at
37°C.
Hyperoside
Hyperoside, with a purity of 98.78%, was obtained as
a canary yellow needle-shaped crystal (Nanjing Zelang Medical
Technological Co., Ltd., Nanjing, China). The hyperoside was
dissolved in an appropriate volume of dimethylsulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) and diluted to the desired
concentrations prior to use, with the final concentration of DMSO
maintained <0.5%.
Cell viability
A standard tetrazolium bromide (MTT) assay was used
to assess cell viability. Briefly, the melanocytes
(5×103 cells/well) were seeded into 96-well plates. The
cells were treated with hyperoside (0, 2, 10 and 50 µg/ml)
for 2 h at 37°C, following which the melanocytes were exposed to
H2O2 (200 µM) for 24 h at 37°C.
Subsequently, 50 ml MTT (Sigma-Aldrich, St. Louis, MO, USA)
solution (2 mg/ml) in phosphate-buffered saline (PBS) was added to
each well and incubated for an additional 4 h at 37°C. The medium
was then removed, and the cells were incubated with 200 µl
DMSO in the dark for 30 min to dissolve the violet crystals. The
absorbance was read at 570 nm on an automatic microplate reader
(550; Bio-Rad Laboratories, Inc., Hercules, CA, USA), with DMSO as
a blank control. All assays were performed in multiples of give and
repeated at least three times.
Cell apoptosis assessment
Following treatment with hyperoside (0, 2, 10 and 50
µg/ml) for 2 h, the H2O2 (200
µM)-treated melanocytes were stained with Annexin V-propium
iodide (Beyotime Institute of Biotechnology, Shanghai, China), and
apoptosis rates were analyzed using a flow cytometer (FACS-Calibur;
BD Biosciences, Hercules, CA, USA).
Mitochondria membrane potential
(MMP)
Rhodamine-123 (Rho-123) dye (Sigma-Aldrich) was used
to detect the changes in MMP in the cells. The cells
(5×104 cells/well) were cultured in a 24-well plate.
Following hyperoside (0, 2, 10 and 50 µg/ml) pretreatment
for 2 h, and H2O2 exposure for 24 h, the
cells were washed with PBS, incubated with Rho-123 (10 mg/ml) at
37°C for 20 min and subsequently subjected to flow cytometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The reverse
transcription reactions were performed using M-MuLV reverse
transcriptase (Promega Corporation, Madison, WI, USA), following
the same protocol as Baek et al (17). Samples were pretreated with DNase
(Promega Corporation) for 1 h at 37°C in order to avoid
contamination. The qPCR reaction was performed on an ABI 7500
(Applied Biosystems; Thermo Fisher Scientific, Inc.) thermal cycler
using 5 µl cDNA template, 1 µl primer pairs (10
µM) and 10 µl of a standard SYBR Green PCR kit
(Thermo Fisher Scientific, Inc.) to a final reaction volume of 20
µl. The following cycling parameters were used: 95°C for 10
min, followed by 40 cycles of 95°C for 15 sec and 60°C for 45 sec
and a final extension step at 72°C for 5 min. The relative mRNA
expression levels of target genes were compared with GAPDH, which
were calculated using the 2−ΔΔCq method (18). The primers used for each gene
(synthesized by Generay Biotech Co., Ltd., Shanghai, China) were as
follows: B cell lymphoma-2 (Bcl-2), forward
5′-AGACCGAAGTCCGCAGAACC-3′ and reverse 5′-GAGACCACACTGCCCTGTTG-3′
(product 113 bp); Bcl-2-associated X protein (Bax), forward
5′-GCGACTGATGTCCCTGTCTC-3′ and reverse 5′-GGCCTCAGCCCATCTTCTTC-3′
(product 132 bp); caspase 3, forward 5′-AACTGGACTGTGGCATTGAG-3′ and
reverse 5′-ACAAAGCGACTGGATGAACC-3′ (product 161 bp); and GAPDH,
forward 5′-ATCACTGCCACCCAGAAG-3′ and reverse
5′-TCCACGACGGACACATTG-3′ (product 191 bp). The experiment was
repeated three times.
Western blot analysis
The treated and untreated melanocytes were harvested
and washed twice with PBS, followed by lysis in ice-cold
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) with freshly added 0.01% protease inhibitor cocktail
(Sigma-Aldrich) and incubation on ice for 30 min. The cell lysate
was centrifuged at 12,000 × g for 10 min at 4°C. The supernatant
(20–30 µg protein) was run on a 10% SDS-PAGE gel and
transferred electrophoretically onto a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
then blocked with 5% skim milk, followed by incubation with primary
antibodies at 4°C. Antibodies against rabbit polyclonal Bcl-2 (cat.
no. Sc-492; 1:400), and rabbit polyclonal Bax (cat. no. Sc-493;
1:400) were purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA), rabbit polyclonal caspase 3 (cat. no. ab44976; 1:500) was
purchased from Abcam (Cambridge, UK). Antibodies against rabbit
monoclonal phosphorylated (p)-AKT (cat. no. 4060; 1:1,000), rabbit
polyclonal AKT (cat. no. 9272; 1:1,000), rabbit monoclonal p-p38
(cat. no. 4511; 1:1,000), rabbit monoclonal p38 (cat. no. 8690;
1:1,000) and rabbit monoclonal GAPDH (cat. no. 5471; 1:1,500) were
purchased from Cell Signaling Technology (Danvers, MA, USA). The
blots were then incubated for 1 h at room temperature with goat
anti-mouse secondary antibody (cat no. A0216; 1:1,000; Beyotime
Institute of Biotechnology) or polyclonal goat anti-rabbit
secondary antibody (cat no. A0208; 1:1,000; Beyotime Institute of
Biotechnology) and visualized using enhanced chemiluminescence (EMD
Millipore).
Statistical analysis
The GraphPad Prism 5.0 software system (GraphPad
Software, Inc., San Diego, CA, USA) was used for statistical
analysis. Data are expressed as the mean ± standard deviation.
Student's t-test was used to compared the differences between two
groups, and one-way analysis of variance was used for comparing
more than two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Hyperoside stimulates melanocyte
proliferation
For the purpose of evaluating the proliferation
promoting ability of hyperoside on melanocytes, cell viability was
detected following treatment with different concentrations of
hyperoside. As shown in Fig. 2,
hyperoside significantly increased the proliferation of the
melanocytes in a time- and dose-dependent manner, compared with the
control group. However, treatment with high concentrations of
hyperoside (100 and 200 µg/ml) had no significant effects on
cell viability, compared with the 50 µg/ml hyperoside
treatment group. As a result, doses of 5, 10 and 50 µg/ml
were selected for treatment in the subsequent investigations.
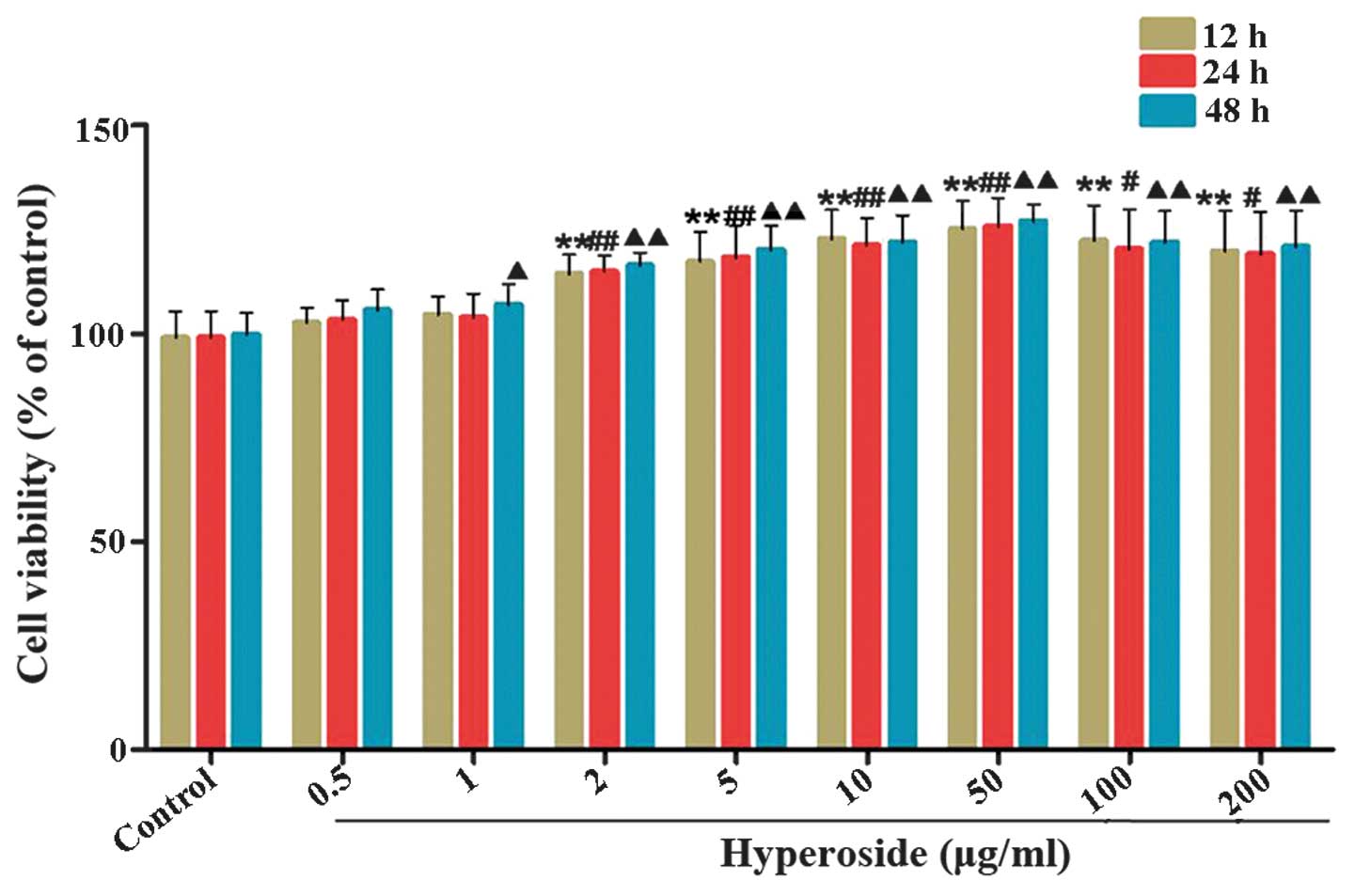 | Figure 2Effects of hyperoside on the
proliferation of melanocytes. (A) Following exposure of melanocytes
to various concentrations of hyperoside (0, 0.5, 1, 2, 5, 10, 50,
100 and 200 µg/ml) for 12, 24 and 48 h, cell viability was
determined using a tetrazolium bromide assay. Data are expressed as
the mean ± standard deviation (n=6), #, ▲P<0.05 and
**, ##, ▲▲P<0.01, vs. control. |
Hyperoside protects melanocytes against
H2O2-induced apoptosis
Oxidative damage to human melanocytes is one of the
inducing factors causing vitiligo (19). The results of the Annexin
V/propidium iodide staining in the present study showed that
treatment of the melanocytes with H2O2 (200
µM) resulted in a significant increase in apoptotic rates,
compared with the control group (Fig.
3). Pretreatment with hyperoside (2, 10 and 50 µg/ml)
for 2 h led to a notable decrease in the apoptotic rates of the
melanocytes, compared with the H2O2-treated
group. These results indicated the protective effects of hyperoside
against H2O2-induced apoptosis in
melanocytes.
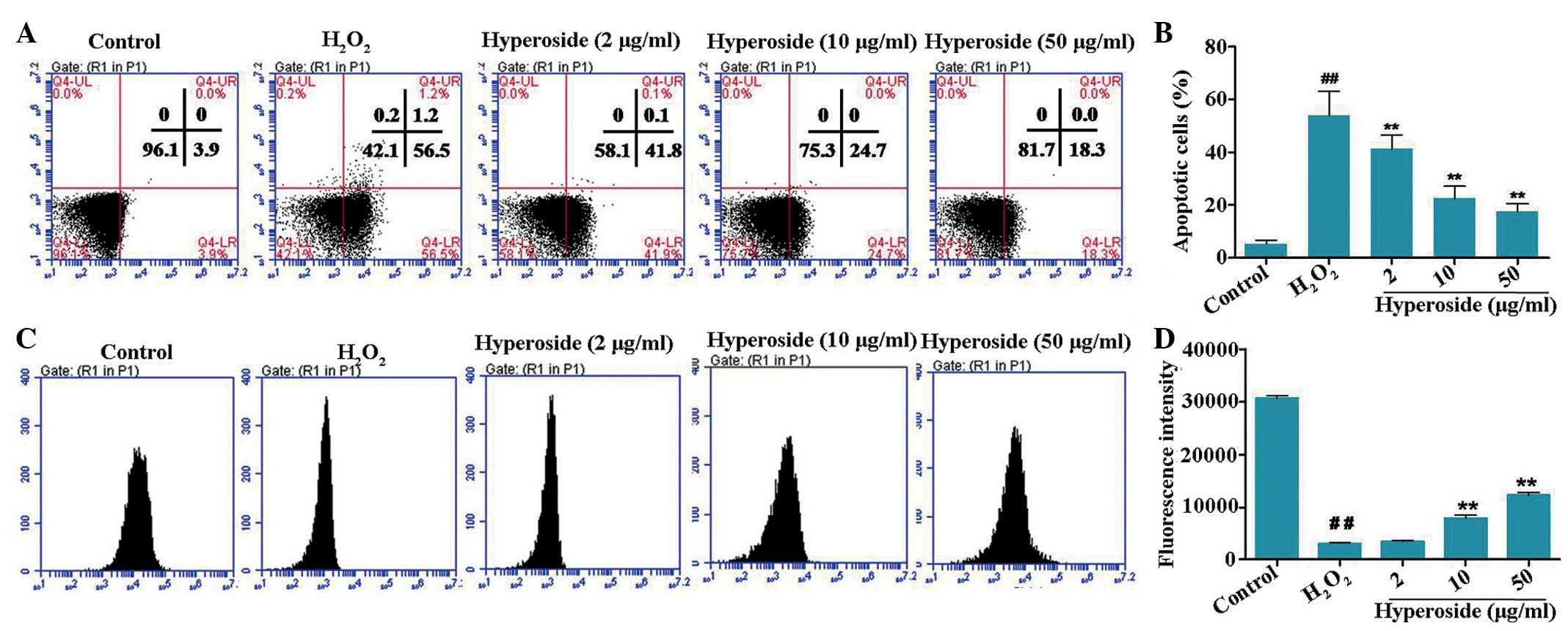 | Figure 3Effects of hyperoside on
H2O2-induced apoptosis and MMP of human
primary melanocytes. (A and B) Melanocytes were treated with
different concentrations of hyperoside (0, 2, 10 and 50
µg/ml) for 2 h, and then exposed to
H2O2 (200 µM) for 24 h. An Annexin V
assay was used for apoptosis detection. (C and D) Melanocytes were
treated with different concentrations of hyperoside (0, 2, 10 and
50 µg/ml) for 2 h, anf then exposed to
H2O2 (200 µM) for 6 h. A Rhodamine-123
assay was used for MMP detection. Data are presented as the mean ±
standard deviation (n=3). ##P<0.01, vs. control;
**P<0.01, vs. H2O2-treated
melanocytes. H2O2, hydrogen peroxide; MMP,
mitochondrial membrane potential. |
The breakdown in MMP is an early stage of the
apoptotic process. In the present study, the effects of hyperoside
on the MMP were evaluated using a Rho-123 assay. As shown in
Fig. 3B, a notable reduction in
the MMP was observed in the H2O2-treated
human primary melanocytes. Compared with the
H2O2-treated control cells, 1.91-fold and
3.45-fold increases in MMP were detected in the 10 and 50
µg/ml hyperoside pretreatment groups, respectively.
Hyperoside mediates the expression levels
of Bcl-2, Bax and caspase 3 in H2O2-induced
melanocytes
The relative expression levels of Bcl-2/Bax and
caspase 3 are crucial in the process of cell apoptosis (20,21).
In the present study, the mRNA and protein expression levels of
Bcl-2/Bax and caspase 3 were measured using RT-qPCR and Western
blot analyses, respectively. As shown in Fig. 4A, the expression of Bcl-2 was
downregulated, and the expression of Bax was upregulated, in the
melanocytes exposed to H2O2. Hyperoside
effectively increased the relative mRNA expression levels of Bcl-2
and decreased those of Bax. As shown in Fig. 4B, the mRNA expression levels of
caspase 3 were enhanced in the melanocytes exposed to
H2O2, whereas hyperoside (2, 10 and 50
µg/ml) led to a dose-dependent reduction in the mRNA
expression of caspase 3, by 17.23, 64.78 and 79.23%, respectively.
As shown in Fig. 4C and D, the
relative protein expression levels of Bcl-2/Bax were decreased by
H2O2 treatment, whereas hyperoside treatment
effectively upregulated the levels of Bcl-2/Bax, in a
dose-dependent manner. On examination of the protein levels of
caspase 3 by Western blotting, the protein expression levels
increased significantly following exposure to
H2O2, and decreased significantly following
hyperoside treatment (Fig.
4B).
 | Figure 4Effect of hyperoside on the
expression levels of Bcl-2, Bax and caspase 3 in
H2O2-treated melanocytes. (A and B)
Melanocytes were treated with different concentrations of
hyperoside (0, 2, 10 and 50 µg/ml) for 2 h, and then exposed
to H2O2 (200 µM) for 3 h. Reverse
transcription-quantitative polymerase chain reaction analysis was
used to determine the mRNA expression levels of Bcl-2, Bax and
caspase 3. (C–E) Following the treatment with
H2O2 for 6 h, the protein levels of Bcl-2,
Bax and caspase 3 were detected using Western blotting. Data are
presented as the mean ± standard deviation (n=6),
##P<0.01, vs. control; *P<0.05 and
**P<0.01 vs. H2O2-treated
melanocytes. Bcl-2, B cell lymphoma-2; Bax, Bcl-2-associated X
protein; H2O2, hydrogen peroxide. |
Hyperoside regulates PI3K/AKT and MAPK
signaling H2O2-treated melanocytes
PI3K/AKT and MAPK signaling are crucial in cell
apoptosis, proliferation, differentiation and various cellular
functions (22,23). The phosphorylation of AKT exerts a
protective effect in cell apoptosis, whereas the phosphorylation of
p38 MAPK stimulates the process of apoptosis (24,25).
In the present study, Western blot analysis was performed to
evaluate the phosphorylation of AKT and p38. As shown in Fig. 5A and B, the levels of p-AKT/AKT in
the melanocytes treated with H2O2 were
markedly lower, compared with those in the control, and the
expression levels of p-AKT/AKT in the groups pretreated with
different doses of hyperoside (2, 10 and 50 µg/ml) were
increased by 83.3, 103.1 and 236.5%, respectively, compared with
those of the H2O2-treated group. By contrast,
the expression of p-p38/p38 was increased on exposure to
H2O2, and reduced following hyperoside
treatment (Fig. 5A and B).
 | Figure 5Effect of hyperoside on the
expression levels of p-AKT and p-p38 in
H2O2-treated melanocytes. (A and B)
Melanocytes were treated with different concentrations of
hyperoside (0, 2, 10 and 50 µg/ml) for 2 h, and then exposed
to H2O2 (200 µM) for 6 h. Western blot
analysis was performed to identify the protein levels of p-AKT,
AKT, p-p38 and p38 in the menlanocytes, and GAPDH was detected as a
sample loading control. Data are presented as the mean ± standard
deviation (n=3). ##P<0.01, vs. control;
**P<0.01, vs. H2O2-treated
melanocytes. p-, phosphorlyated; H2O2,
hydrogen peroxide. |
Discussion
The repair of injured melanocytes is one of the most
important driving forces in the treatment of vitiligo. As recorded
in the Chinese Pharmacopoeia (2005, 2010), Cuscutae semen shows
beneficial effect in vitiligo treatment, which is also contained
within the prescribed Chinese herbal compound, Chi Tu Ding, which
is used extensively for the treatment of leucoderma (14). Wang et al reported that an
ethanol fraction from Cuscutae semen significantly affected
melanogenesis by regulating the enzymatic activity of tyrosinase in
zebrafish (26). Ma et al
(27) demonstrated that the
ethanol extract of Cuscutae semen was effective in inducing the
adhesion and migration of melanocytes, and offered potential in the
treatment of vitiligo treatment. However, the pharmacodyamic
material basis of Cuscutae semen in the vitiligo treatment remains
to be elucidated. In the present study, six compounds from
Cuscuta australis were obtained, and hyperoside was found to
exhibit marked effects on the induction of melanogenesis in human
primary melanocytes.
Melanocytes are considered to be more vulnerable to
the damaging effects of oxidative stress, compared with
keratinocytes and fibroblasts (4–6), and
oxidative stress is one of inducing factors causing vitiligo. In
the present study, it was shown that hyperoside significantly
reduced the apoptosis of cultured human melanocytes treated with
H2O2. PI3K/AKT and MAPK signaling are
reported to be important regulators of cell apoptosis. The
phosphorylation of AKT exerts protective effects in cell apoptosis,
whereas the phosphorylation of p38 MAPK stimulates the process of
apoptosis (28,29). H2O2-treatment
significantly decreased the phosphorylation of AKT, but increased
the phosphorylation of p38. Pretreament with hyperoside partially
reversed these effects of on the phosphorylation of AKT and p38.
Taken together, these data demonstrated that hyperoside protected
the human primary melanocytes against
H2O2-induced apoptosis via the regulation of
PI3K/AKT and p38 signaling.
Mitochondrial dysfunction caused by oxidative stress
can result in a decease in MMP levels (30). In the present study, the MMP levels
of the H2O2-treated melanocytes pretreated
with hyperoside were notably increased, comparison with those of
the H2O2-only treated melanocytes. The loss
of MMP causes an increase in the permeability of the MMP, followed
by the release of pro-apoptotic molecules, including cytochrome
c. The release of cytochrome c from the mitochondria
interacts with ATP, Apaf-1 and caspase 9, and subsequently
activates caspase 3, which consequently elicits caspase-dependent
apoptotic cell death (31). In the
present study, the mRNA and protein expression levels of casepase 3
in the H2O2-treated melanocytes with
hyperoside pretreatment were significantly decreased, compared with
those in the H2O2-treated melanocytes without
pretreatment. These results indicated that hyperoside showed
protective effects towards the human primary melanocytes from
oxidative damage by inhibiting the mitochondrial apoptotic
pathway.
Taken together, the results of the present study
lead to the hypothesis that hyperoside protects melanocytes against
oxidative damage by activating AKT, inhibiting p38 phosphorylation
and suppressing mitochondrial apoptosis signaling,. These findings
may provide further insight into vitiligo therapy (Fig. 6), and hyperoside may be a useful
therapeutic agent in the treatment of vitiligo.
Acknowledgments
This study was supported by the China Postdoctoral
Science Foundation (grant no. 2014M562671) and the National Natural
Science Foundation (grant no. 81201243).
References
|
1
|
Ruiz-Argüelles A, Brito GJ,
Reyes-Izquierdo P, Pérez-Romano B and Sánchez-Sosa S: Apoptosis of
melanocytes in vitiligo results from antibody penetration. J
Autoimmun. 29:281–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silverberg NB: Recent advances in
childhood vitiligo. Clin Dermatol. 32:524–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guerra L, Dellambra E, Brescia S and
Raskovic D: Vitiligo: Pathogenetic hypotheses and targets for
current therapies. Curr Drug Metab. 11:451–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu CF, Hu CL, Chiang SS, Tseng KC, Yu RC
and Pan TM: Beneficial preventive effects of gastric mucosal lesion
for soy-skim milk fermented by lactic acid bacteria. J Agric Food
Chem. 57:4433–4438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoogduijn MJ, Cemeli E, Ross K, Anderson
D, Thody AJ and Wood JM: Melanin protects melanocytes and
keratinocytes against H2O2-induced DNA strand breaks through its
ability to bind Ca2+. Exp Cell Res. 294:60–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valverde P, Manning P, Todd C, McNeil CJ
and Thody AJ: Tyrosinase may protect human melanocytes from the
cytotoxic effects of the superoxide anion. Exp Dermatol. 5:247–253.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laddha NC, Dwivedi M, Mansuri MS, Gani AR,
Ansarullah M, Ramachand AV, Dalai S and Begum R: Vitiligo:
Interplay between oxidative stress and immune system. Exp Dermatol.
22:245–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He X, Yang W, Ye M, Wang Q and Guo D:
Differentiation of Cuscuta chinensis and Cuscuta australis by
HPLC-DAD-MS analysis and HPLC-UV quantitation. Planta Med.
77:1950–1957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang JL, Liu BY and Ma KW: Traditional
chinese medicine. The Lancet. 372:1938–1940. 2008. View Article : Google Scholar
|
|
10
|
Yang HM, Shin HK, Kang YH and Kim JK:
Cuscuta chinensis extract promotes osteoblast differentiation and
mineralization in human osteoblast-like MG-63 cells. J Med Food.
12:85–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yen FL, Wu TH, Lin LT, Cham TM and Lin CC:
Nanoparticles formulation of Cuscuta chinensis prevents
acetaminophen-induced hepatotoxicity in rats. Food Chem Toxicol.
46:1771–1777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
China Pharmacopoeia Committee: Chinese
pharmacopoeia. Chinese Medicine Science and Technology Press;
Beijing, China: pp. 7402005
|
|
13
|
State Pharmacopoeia Committee: Chinese
pharmacopoeia. Chinese Medicine Science and Technology Press;
Beijing: pp. 70–71. 2010
|
|
14
|
Jang JY, Kim HN, Kim YR, Choi YH, Kim BW,
Shin HK and Choi BT: Aqueous fraction from Cuscuta japonica seed
suppresses melanin synthesis through inhibition of the p38
mitogen-activated protein kinase signaling pathway in B16F10 cells.
J Ethnopharmacol. 141:338–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye M, Yan Y, Ni X and Qiao L: Studies on
the chemical constituents of the herba of Cuscuta chinensis. Zhong
Yao Cai. 24:339–341. 2001.In Chinese.
|
|
16
|
Ye M, Yan YN, Qiao L and Ni XM: Studies on
chemical constituents of Cuscuta chinensis. Zhongguo Zhong Yao Za
Zhi. 27:115–117. 2002.In Chinese.
|
|
17
|
Baek A, Park HJ, Na SJ, Shim DS, Moon JS,
Yang Y and Choi YC: The expression of BAFF in the muscles of
patients with dermatomyositis. J Neuroimmunol. 249:96–100. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
VanGuilder HD, Vrana KE and Freeman WM:
Twenty-five years of quantitative PCR for gene expression analysis.
Biotechniques. 44:619–626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schallreuter KU, Moore J, Wood JM, et al:
In vivo and in vitro evidence for hydrogen peroxide (H2O2)
accumulation in the epidermis of patients with vitiligo and its
successful removal by a UVB-activated pseudocatalase. The journal
of investigative dermatology. Symposium proceedings/the Society for
Investigative Dermatology, Inc and European Society for
Dermatological Research. 4:91–96. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li CP, Li JH, He SY, Li P and Zhong XL:
Roles of Fas/Fasl, Bcl-2/Bax, and Caspase-8 in rat nonalcoholic
fatty liver disease pathogenesis. Genet Mol Res. 13:3991–3999.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of
breast cancer cells to paclitaxel. Asian Pac J Cancer Prev.
15:8617–8622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
CV SB, Babar SM, Song EJ, Oh E and Yoo YS:
Kinetic analysis of the MAPK and PI3K/Akt signaling pathways. Mol
Cells. 25:397–406. 2008.
|
|
23
|
Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH,
Kim BW, Choi HY, Jeong MY and Cho SG: Interplay between PI3K/Akt
and MAPK signaling pathways in DNA-damaging drug-induced apoptosis.
Biochim Biophys Acta. 1763:958–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang TJ, An J, Chen XH, Deng QD and Yang
L: Assessment of Cuscuta chinensis seeds effect on melanogenesis:
Comparison of water and ethanol fractions in vitro and in vivo. J
Ethnopharmacol. 154:240–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma H, Zhang X, Mu K, Feng J, Niu X, Liu C
and Dang Q: Effects of herb extracts on melanocyte adhesion and
migration. Zhongguo pifu xingbingxue zazhi. 18:526–527. 2004.In
Chinese.
|
|
28
|
Sabine VS, Crozier C, Brookes CL, Drake C,
Piper T, van de Velde CJ, Hasenburg A, Kieback DG, Markopoulos C,
Dirix L, et al: Mutational analysis of PI3K/AKT signaling pathway
in tamoxifen exemestane adjuvant multinational pathology study. J
Clin Oncol. 32:2951–2958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toda M, Kuo CH, Borman SK, Richardson RM,
Inoko A, Inagaki M, Collins A, Schneider K and Ono SJ: Evidence
that formation of vimentin mitogen-activated protein kinase (MAPK)
complex mediates mast cell activation following FcεRI/CC chemokine
receptor 1 cross-talk. J Biol Chem. 287:24516–24524. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cavalcanti BC, da Costa PM, Carvalho AA,
Rodrigues FA, Amorim RC, Silva EC, Pohlit AM, Costa-Lotufo LV,
Moraes MO and Pessoa C: Involvement of intrinsic mitochondrial
pathway in neosergeolide-induced apoptosis of human HL-60 leukemia
cells: The role of mitochondrial permeability transition pore and
DNA damage. Pharm Biol. 50:980–993. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang WJ, Bi LY, Li ZZ, Zhang X and Ye Y:
Formononetin induces the mitochondrial apoptosis pathway in
prostate cancer cells via downregulation of the IGF-1/IGF-1R
signaling pathway. Pharm Biol. 2013.PubMed/NCBI
|




















