Introduction
Radiotherapy is an essential and common treatment
modality used for various malignancies located within the thoracic
region. Thoracic radiotherapy is limited by toxicity to normal lung
tissue in a radiation volume-and dose-dependent manner (1). Clinically, 10–30% of patients with
cancer in the thoracic region suffer radiation-induced lung injury
(RILI) (2,3). Thus, alleviating RILI could improve
the efficacy of cancer treatment and the quality of life for the
patient.
RILI is a complex pathological process that results
in early pneumonitis and late pulmonary fibrosis (4). Radiation to the thorax causes
pulmonary fibrosis by releasing pro-inflammatory cytokines and
activating myofibroblasts, which produce collagen and extracellular
matrix (5). Alveolar epithelial
cells participate in the pathogenesis of pulmonary fibrosis by
producing pro-inflammatory mediators and undergoing
epithelial-to-mesenchymal transition (EMT) (6). Recent studies have suggested that
epithelial cells undergo morphological changes to acquire
fibroblast or myofibroblast markers, such as α-smooth muscle actin
(αSMA), N-cadherin and vimentin, while showing decreased expression
of epithelial markers, such as E-cadherin (7–9).
Geranylgeranlyacetone (GGA) is a nontoxic anti-ulcer
drug that has been reported to induce heat shock protein (HSP)70
(10,11). HSP70 has a cytoprotective property
as an intracellular chaperone by modulating the immune response and
maintaining cellular homeostasis (12–14).
GGA treatment has been shown to protect against various diseases in
animal models and humans via HSP70 induction (15,16).
It was reported that HSP70 may inhibit EMT by inhibiting the
generation of reactive oxygen species (17).
The aim of the present study was to investigate the
protective effect of GGA on RILI via inhibiting radiation-induced
EMT and to provide mechanistic insights into the development of
pharmacological therapeutics to treat or mitigate RILI.
Materials and methods
Animals and GGA treatments
All protocols in this study were approved by the
Institutional Animal Care and Use Committee of the Korean Institute
of Radiological and Medical Sciences (Seoul, Korea; IACUC permit
number: KIRAMS2013-035). Seven-week-old female C57BL/6 mice ~20 g
were obtained from Orient Bio Co. Ltd. (Sungnam, Korea). The mice
were housed for 1 week prior to conducting the experiments and were
randomly assigned to the following three groups: i) Non-irradiated
control (n=7); ii) thoracic irradiation (IR) control (n=9); and
iii) GGA+IR (n=9). The animals were housed at 20±2°C with 50±10%
humidity with a 12/12 h light/dark cycle in a specific
pathogen-free facility and were fed a normal diet and autoclaved
water ad libitum.
GGA was obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). GGA (200 mg/kg) was orally administered 24
and 1 h prior to IR treatment and 24, 48 and 72 h after IR
treatment. Each mouse assigned to the radiation treatment group was
exposed to radiation under anesthesia [30 mg/kg Zoletil (Virbac,
Carros, France) and 10 mg/kg Rompun (Bayer AG, Leverkusen,
Germany)] using an X-Rad320 (Precision X-Ray, East Haven, CT, USA;
filter, 2 mm; aluminium, 42 cm, 260 kV/s, 10 mA, 2.0 Gy/min). The
radiation field size was 20×50 mm for the thoracic irradiation of
the mice. The mice were euthanized 6 months after IR by inhalation
of CO2.
Cell culture and treatments
The L132 human lung epithelial cell line (American
Type Culture Collection, Manassas, VA, USA) was cultured in
Dulbecco's modified Eagle's medium (Welgene, Inc.,
Gyeongsangbuk-do, Korea) with 10% fetal bovine serum (Corning
Incorporated, Corning, NY, USA) and antibiotics (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in the presence
of 5% CO2. GGA was added to the culture medium for 24 h
before IR treatment.
Lung tissue preparation
Six months after IR, all of the mice from the three
groups were euthanized. The lungs of 3 mice from each group were
injected with 1 ml neutralized formalin (Yakuri Pure Chemicals Co.,
Ltd., Kyoto, Japan) and then fixed in formalin for 48 h. The whole
lungs of 3 mice from each group were injected with 1 ml neutralized
formalin, fixed in 10% formalin solution for 48 h and then were
embedded in paraffin for the histological examination. The left
lobes of the lungs (n=4 per control group, and n=6 per IR and
GGA+IR group) were resected and fixed in formalin for histological
analysis, and the right lobes were frozen in liquid nitrogen and
stored at -80°C for western blotting.
Histopathology and immunohistochemical
staining
Paraffin-embedded sections were stained with
hematoxylin and eosin (H&E) or with Masson's Trichrome staining
kit (Sigma-Aldrich, St. Louis, MO, USA). Immunohistochemistry was
conducted using a Vectastain Elite ABC kit (Vector Laboratories
Inc., Burlingame, CA, USA) based on the manufacturer's protocol.
For antigen retrieval, the sections were boiled in citrate buffer
(pH 6.0; 95–98°C) in a water bath for 30 min followed by cooling
for 20 min at room temperature. The sections were then incubated
overnight at 4°C with the following primary antibodies: Mouse
monoclonal anti-8-hydroxy-2′-de-oxyguanosine (8-OHdG) (sc-66036;
1:100; Santa Cruz Biotechnology, Inc.), rabbit polyclonal
anti-pro-SPC (AB3786; 1:500; Millipore, Darmstadt, Germany), mouse
monoclonal anti-E-cadherin (sc-8426; 1:100; Santa Cruz
Biotechnology, Inc.) and mouse monoclonal anti-vimentin (sc-6260;
1:100; Santa Cruz Biotechnology, Inc.), after which the slides were
washed with phosphate-buffered saline (PBS) containing 0.05% Triton
X-100 (Sigma-Aldrich). Then, the sections were incubated with the
corresponding secondary antibody for 30 min and counterstained with
hematoxylin (Sigma-Aldrich). For immunofluorescent staining,
sections stained with primary antibodies were incubated with the
appropriate fluorescently-labeled secondary antibodies (1:250;
Molecular Probes Inc., Eugene, OR, USA) and then counterstained
with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; 3
μM; Sigma-Aldrich). Images obtained using an Olympus BX53
microscope (Olympus Corporation, Tokyo, Japan) equipped with a CCD
camera (ProgRes CF Scan; Jenoptik, Jena, Germany).
Western blot analysis
Lung tissues were physically minced, then proteins
were extracted from the lysates using Pro-prep solution (17081;
Intron Inc., Sungnam, Korea). The concentration of total protein
was quantified using Bio-Rad Laboratories Protein Assay kit II
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins were
separated by electrophoresis on a 6–12.5% sodium dodecyl
sulfate-polyacrylamide gel and were then transferred onto 0.45
μm nitrocellulose membranes (Pall Corporation, Willoughby,
OH, USA) for 2 h at 100 V. The membranes were blocked with 5%
non-fat dry milk in PBS containing 0.1% Tween-20 (Sigma-Aldrich)
for 30 min. The membranes were subsequently incubated overnight at
4°C with the following antibodies: Mouse monoclonal anti-HSP70
(ADI-SPA-810; 1:1,000; Enzo Life Sciences, Farmingdale, NY, USA),
mouse monoclonal anti-E-cadherin (sc-8426; 1:1,000; Santa Cruz
Biotechnology, Inc.), mouse monoclonal anti-vimentin (sc-6260;
1:1,000; Santa Cruz), mouse monoclonal anti-N-cadherin (610920;
1:1,000; BD Biosciences, Franklin Lakes, NJ, USA) and mouse
monoclonal β-actin (A1978; 1:3,000; Sigma-Aldrich). Then membranes
were then washed thoroughly in PBS-0.1% Tween-20 and incubated for
1 h with the following horseradish-conjugated secondary antibodies
from Santa Cruz Biotechnology, Inc. at a dilution of 1:3,000: Goat
anti-mouse IgG (sc-2005), donkey anti-goat IgG (sc-2020), goat
anti-rabbit IgG (sc-2004). Protein bands were visualized by
electrochemiluminescence (G:BOX Chemi XT6; Syngene, Cambridge, UK).
Protein expression was then quantified using a Fluor-S MultiImager
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
for each experiment. Statistical analysis was performed using
one-way analysis of variance and the Tukey post-hoc test for
multiple comparisons with GraphPad Prism version 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
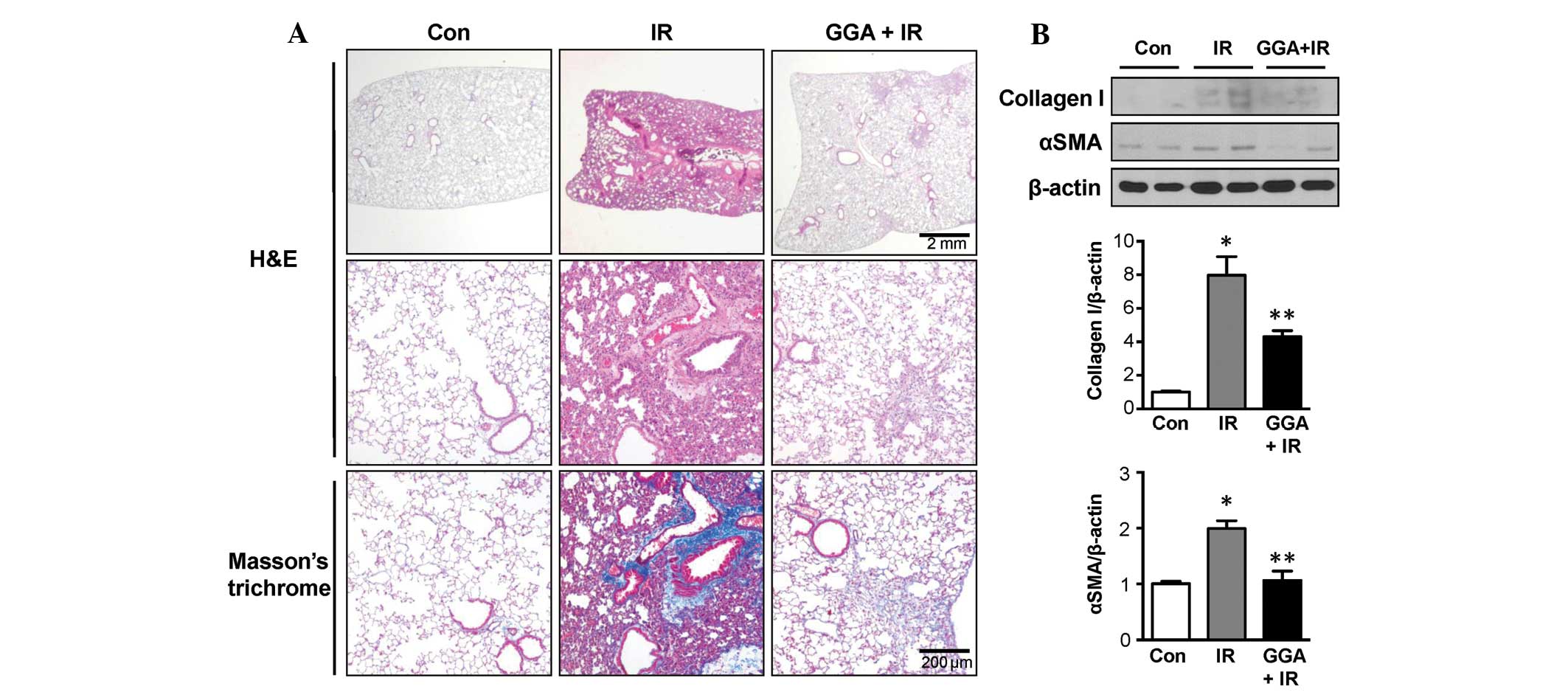
GGA alleviates radiation-induced lung
injury in late phase
At 24 weeks post-IR, Masson's trichrome staining
showed that the IR group experienced marked fibrotic changes and
collagen accumulation in the lung parenchyma. GGA treatment
markedly alleviated late phase RILI, which was reflected by the
reduction in pathological changes to the lung parenchyma (Fig. 1A). Immunoblotting data showed
increased expression of collagen I and αSMA in the IR group when
compared with the control-treated mice. GGA treatment significantly
prevented expression of collagen I and αSMA in the lungs associated
with IR (P<0.05, Fig. 1B).
GGA protects lung epithelial cells
against IR
Twenty-four weeks after treatment, the IR group
showed high expression of 8-OHdG in lung epithelial cells, which
suggests an accumulation of oxidative damage. At this time point,
the IR group also showed increased numbers of pro-SPC-positive
cells, which suggests the transdifferentiation of lung epithelial
cells to a mesenchymal-like phenotype. With GGA treatment, 8-OHdG
expression and the number of pro-SPC-positive cells were
significantly reduced (Fig.
2).
GGA suppresses radiation-induced EMT
signals
Next, the effect of GGA treatment EMT, which is key
in radiation-induced lung injury was examined. Following IR,
immunofluorescence demonstrated decreased expression of E-cadherin,
which is an epithelial marker, and increased expression of
vimentin, which is a mesenchymal marker in the lung tissue
(Fig. 3A). GGA treatment
significantly prevented radiation-induced EMT in the lung 6 months
after IR. These results were confirmed by western blot analysis of
the E-cadherin, vimentin and N-cadherin protein levels in the lung
tissue lysates (Fig. 3B).
HSP70 inhibits radiation-induced EMT in
L132 human lung epithelial cells
GGA treatment upregulated HSP70 expression in L132
cells. Consistent with in vivo data, GGA treatment
significantly inhibited radiation-induced EMT in L132 cells, as
demonstrated by the preserved expression of the epithelial marker
E-cadherin and the reduced upregulation of mesenchymal markers
(vimentin and N-cadherin) (P<0.05, Fig. 4).
 | Figure 4HSP70 expression prevents
radiation-induced EMT marker expression in L132 human lung
epithelial cells. Representative western blotting data for HSP70,
E-cadherin, vimentin, and N-cadherin expression are shown.
Expression of HSP70 was induced by GGA treatment in L132 cells.
L132 cells were harvested 24 h after GGA treatment (0 to 15
μM) and subjected to western blotting, and the expression of
HSP70, E-cadherin, vimentin, and N-cadherin was quantified (n=3,
*P<0.05 vs. 0 μM control,
**P<0.05 vs. IR control). GGA, geranylgeranylacetone;
IR, irradiation; EMT, epithelial-to-mesenchymal transition; HSP70,
heat shock protein 70. |
Discussion
In this study, it was demonstrated that increased
expression of HSP70 in the lung during IR may prevent late signs of
RILI, such as fibrosis. The mice were treated with orally with GGA
(200 mg/kg) during the early stages of radiation injury (24 and 1
h) prior to IR and 24, 48 and 72 h after IR. Increased expression
of HSP70 was also detected in the lungs 24 h after oral GGA
administration by western blotting (data not shown). GGA treatment
resulted in decreased expression of EMT markers and attenuation of
fibrosis in the lungs of mice treated with IR. Based on these
results, inhibiting EMT during the acute phase of radiation
exposure was important for the prevention of RILI.
Radiation-induced cell death is caused by direct DNA
damage and indirect oxidative stress during the acute phase of
radiation. However, late complications of radiation, such as
fibrosis, are progressive and increase the complexity of the
process involved in permanent epithelial cell injury (4). It was demonstrated that GGA
significantly alleviates EMT marker expression and fibrosis in
mouse lungs at the 6 months following IR. GGA is an inducer of
HSP70, which protects cells from various stimuli, such as oxidative
stress. GGA treatment markedly reduced the accumulation of
oxidative damage by IR, which was shown by decreased expression of
8-OHdG. Moreover, Zhang et al (18) demonstrated that HSP70 protects lung
injury from lethal oxidative stress. GGA treatment in mice
decreased the expression of EMT markers pro-SPC and vimentin in
mouse lung tissue. Radiation-induced injury of the lungs leads to
expression of vimentin, a mesenchymal cell marker in lung
epithelial cells (9).
Additionally, the presence of pro-SPC-positive cells with an
expanded interstitium suggests that the lung epithelial cells
become detached from the basement membrane and migrate along with
the extracellular matrix (19).
Therefore, cells displaying an EMT phenotype express pro-SPC, which
is consistent with the loss of epithelial marker expression. These
results suggest that GGA could alleviate radiation-induced lung
fibrosis in mouse lungs by preventing EMT marker expression.
As alveolar epithelial cells perform a critical
function as a barrier against infections as well as in the
inflammatory response, severe injury of the respiratory epithelium
promotes the fibrotic process (20). GGA-treated lung tissue showed
significantly higher expression of E-cadherin protein when compared
with the IR groups. E-cadherin is a calcium-dependent adhesion
molecule expressed on the surface of epithelial cells (21). Normal expression and functional
activity of E-cadherin is critical for the maintenance of tight
junctions between epithelial cells and for the maintenance of
normal function of the paracellular barrier in the airway epithelia
(9). It also confirmed that the
overexpression of HSP70 following GGA treatment during IR,
effectively inhibited the expression of mesenchymal-like phenotypes
and GGA prevented the IR-induced loss of E-cadherin in
vitro. The results from the current study suggest that GGA
directly prevents EMT and indirectly protects lung epithelial
cells.
The present study demonstrates that a protective
effect of GGA on RILI is associated with the inhibition of EMT,
which prevents late fibrosis. Moreover, increased expression of
HSP70 by oral administration of GGA before radiation and during the
acute phase of radiation injury effectively inhibited EMT marker
expression, which appears to be important for the prevention of
lung fibrosis. These results suggest that an HSP70 inducer, such as
GGA, could act as a mitigator or protective agent against RILI. The
current study demonstrated that oral administration of GGA, a non
toxic HSP70-inducing anti-ulcer drug, effectively protects normal
lung tissue against radiation-induced injury. GGA may be a
promising therapeutic target of lung fibrosis subsequent to
radiotherapy. However, further studies are required to clarify the
influence of GGA on cancer treatment.
Acknowledgments
The current study was supported by the National
Research Foundation (NRF) and Ministry of Science, ICT and Future
Planning, Korean Government, through its National Nuclear
Technology Program (grant nos. NRF-2014M2A2A7044825 and
NRF-2013M2A2A7043580).
The English in this document has been checked by at
least two professional editors from American Journal Experts.
References
|
1
|
McDonald S, Rubin P, Phillips TL and Marks
LB: Injury to the lung from cancer therapy: Clinical syndromes,
measurable endpoints and potential scoring systems. Int J Radiat
Oncol Biol Phys. 31:1187–1203. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robnett TJ, Machtay M, Vines EF, McKenna
MG, Algazy KM and McKenna WG: Factors predicting severe radiation
pneu-monitis in patients receiving definitive chemoradiation for
lung cancer. Int J Radiat Oncol Biol Phys. 48:89–94. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hughes-Davies L, Tarbell NJ, Coleman CN,
Silver B, Shulman LN, Linggood R, Canellos GP and Mauch PM: Stage
IA-IIB Hodgkin's disease: Management and outcome of extensive
thoracic involvement. Int J Radiat Oncol Biol Phys. 39:361–369.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cappuccini F, Eldh T, Bruder D, Gereke M,
Jastrow H, Schulze-Osthoff K, Fischer U, Köhler D, Stuschke M and
Jendrossek V: New insights into the molecular pathology of
radiation-induced pneumopathy. Radiother Oncol. 101:86–92. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balli D, Ustiyan V, Zhang Y, Wang IC,
Masino AJ, Ren X, Whitsett JA, Kalinichenko VV and Kalin TV: Foxm1
transcription factor is required for lung fibrosis and
epithelial-to-mesenchymal transition. EMBO J. 32:231–244. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pozharskaya V, Torres-González E, Rojas M,
Gal A, Amin M, Dollard S, Roman J, Stecenko AA and Mora AL: Twist:
A regulator of epithelial-mesenchymal transition in lung fibrosis.
PLoS One. 4:e7559–e7510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hodge S, Holmes M, Banerjee B, Musk M,
Kicic A, Waterer G, Reynolds PN, Hodge G and Chambers DC:
Posttransplant bronchiolitis obliterans syndrome is associated with
bronchial epithelial to mesenchymal transition. Am J Transplant.
9:727–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Almeida C, Nagarajan D, Tian J, Leal SW,
Wheeler K, Munley M, Blackstock W and Zhao W: The role of alveolar
epithelium in radiation-induced lung injury. PLoS One.
8:e536282013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X,
Zhang B, Chen W, Nie J, Wang Z, et al: HSP72 attenuates renal
tubular cell apoptosis and interstitial fibrosis in obstructive
nephropathy. Am J Physiol Renal Physiol. 295:F202–F214. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka K, Tanaka Y, Namba T, Azuma A and
Mizushima T: Heat shock protein 70 protects against
bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol.
80:920–931. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hagiwara S, Iwasaka H, Matsumoto S,
Noguchi T and Yoshioka H: Association between heat stress protein
70 induction and decreased pulmonary fibrosis in an animal model of
acute lung injury. Lung. 185:287–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niu P, Liu L, Gong Z, Tan H, Wang F, Yuan
J, Feng Y, Wei Q, Tanguay RM and Wu T: Overexpressed heat shock
protein 70 protects cells against DNA damage caused by ultraviolet
C in a dose-dependent manner. Cell Stress Chaperones. 11:162–169.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawana K, Miyamoto Y, Tanonaka K, Han-no
Y, Yoshida H, Takahashi M and Takeo S: Cytoprotective mechanism of
heat shock protein 70 against hypoxia/reoxygenation injury. J Mol
Cell Cardiol. 32:2229–2237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirakawa T, Rokutan K, Nikawa T and Kishi
K: Geranylgeranylacetone induces heat shock proteins in cultured
guinea pig gastric mucosal cells and rat gastric mucosa.
Gastroenterology. 111:345–357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niwa Y, Nakamura M, Miyahara R, Ohmiya N,
Watanabe O, Ando T, Kawashima H, Itoh A, Hirooka Y and Goto H:
Geranylgeranylacetone protects against diclofenac-induced gastric
and small intestinal mucosal injuries in healthy subjects: A
prospective randomized placebo-controlled double-blind cross-over
study. Digestion. 80:260–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Bao J, Hao J, Peng Y and Hong F:
HSP70 inhibits high glucose-induced Smad3 activation and attenuates
epithelial-to-mesenchymal transition of peritoneal mesothelial
cells. Mol Med Rep. 10:1089–1095. 2014.PubMed/NCBI
|
|
18
|
Zhang Y, Zhang X, Shan P, Hunt CR, Pandita
TK and Lee PJ: A protective Hsp70-TLR4 pathway in lethal oxidant
lung injury. J Immunol. 191:1393–1403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KK, Kugler MC, Wolters PJ, Robillard
L, Galvez MG, Brumwell AN, Sheppard D and Chapman HA: Alveolar
epithelial cell mesenchymal transition develops in vivo during
pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adamson IY, Young L and Bowden DH:
Relationship of alveolar epithelial injury and repair to the
induction of pulmonary fibrosis. Am J Pathol. 130:377–383.
1988.PubMed/NCBI
|
|
21
|
Takeichi M: Cadherin cell adhesion
receptors as a morphogenetic regulator. Science. 251:1451–1455.
1991. View Article : Google Scholar : PubMed/NCBI
|