Introduction
Implantation occurs during a specific period of the
menstrual cycle, known as the window of implantation (between day 6
and day 10 of the cycle, following the luteinizing hormone surge),
and is dependent on a synchronized dialogue between the embryo and
endometrium. This dialogue is mediated by specific biochemical
factors, including hormones, growth factors, enzymes, integrins and
cytokines (1–3).
Leukemia inhibitory factor (LIF), which is a
multifunctional protein that belongs to the interleukin 6 cytokine
family, exerts numerous regulatory actions on various domains of
cellular function (4). LIF was
initially reported to induce macrophage differentiation in M1
murine myeloid leukemic cells, and to suppress their proliferation
in vitro (5). LIF was later
examined in transgenic mice, and was identified as the first
necessary cytokine for implantation (6,7).
Furthermore, LIF expression has been detected in both the embryo
and endometrium, and its role expands from blastocyst development
and endometrial differentiation to blastocyst attachment and
invasion of the endometrium (4,8).
LIF exerts its actions by interacting with its
receptor, which is a heterodimer composed of two transmembrane
proteins, LIF receptor (LIF-R) and glycoprotein 130 (gp130)
(9–11). LIF-R selectively interacts with
LIF, whereas gp130 may also interact with other cytokines. LIF is
initially connected to LIF-R with low-affinity binding, which in
turn induces dimerization with gp130, leading to a high affinity
receptor (1,4,12,13).
Development of the heterodimer receptor induces numerous
intracellular signaling pathways, including the
phosphatidylinositol 3 kinase, mitogen activated protein kinase,
and janus kinase/signal transducer and activator of transcription
pathways, through which LIF performs its numerous actions (14–17).
The presence of LIF and LIF-R in endometrial cells,
alongside alterations in their expression levels during the
menstrual cycle, supports their decisive role in the normal
implantation process (18,19). During the proliferative phase, LIF
and LIF-R endometrial expression is reduced; however, after
ovulation there is a gradual increase in LIF and LIF-R levels,
which continues until the end of the menstrual cycle. LIF
expression is maximized in endometrial cells during the mid luteral
phase (8,20,21).
LIF concentration is maximized between days 7 and 12 post
ovulation, whereas the levels of LIF-R and gp130 have been reported
to peak between days 19 and 25 of the menstrual cycle (22–24).
The increased expres sion of LIF and its receptors during the mid
secretory phase coincides with the implantation window, thus
indicating the significance of this cytokine for endometrial
receptivity (25).
Despite the fact that a decisive role has been
recognized for LIF in animal implantation, few studies have
compared LIF expression patterns between fertile and infertile
women. Furthermore, a review of the literature indicates that no
data is available regarding the expression patterns of LIF-R in the
epithelial and stromal cells of infertile women during the
implantation window. Therefore, the main aim of the present study
was to compare LIF and LIF-R endometrial expression between
infertile and fertile women during the implantation window.
Materials and methods
Study design and subjects
The patients were recruited from 3rd Department of
Obstetrics and Gynecology of Aristotle University of Thessaloniki
and IAKENTRO Infertility Treatment Center (Thessaloniki, Greece).
The present analysis is a prospective observational case control
study, which was performed between March 2013 and March 2015. The
patient group consisted of infertile women, whereas the control
group consisted of fertile women. Infertile women were defined as
patients that had failed to achieve a clinical pregnancy after ≥12
months of regular unprotected sexual intercourse. Fertile women
were defined as subjects with at least one live newborn, who had
not presented with signs or symptoms of infertility following their
last childbirth. Exclusion criteria for both groups included: Age
>42 years old, history of gynecological surgical procedures in
the cervix and uterus, endometrial hyperplasia, polyps,
gynecological cancer, and cervical intra epithelial dysplasia.
Fertile women with a history of miscarriage and ectopic pregnancies
were also excluded. Informed consent was obtained from all of the
women participating in the present study. The present study was
approved by the Institutional Review Board and Ethical Committee of
Aristotle University of Thessaloniki (Thessaloniki, Greece).
Description of intervention
All women underwent persistent ultrasound
evaluation, in order to determine their day of ovulation.
Transvaginal ultrasound was performed from the 8th
menstrual day on a daily basis, and the maximum diameter of the
predominant follicle was measured. The day during which the maximum
diameter of the follicle was detected, which on the next day was
followed by elimination or hetero geneity of clear ultrasound
limits was considered the ovulation day. The cycle was considered
as ovulatory only if a follicle with a mean diameter >18 mm was
observed, otherwise the subject was excluded from the study.
All women fulfilling the inclusion criteria of the
present study had an endometrial biopsy 7 or 8 days after
ovulation. Endometrial biopsy was performed using a Pipelle de
Cornier®(Prodimed, Neuilly en Thelle, France). All
biopsies were performed by the same physician (Y.P). Endometrial
tissue was added to 10% formalin solution and immunohisto chemistry
(IHC) was performed by a specialized pathologist. The pathologist
was unaware of the sample origin (fertile/infer tile) and the
menstrual day of the biopsy (blind examiner).
IHC
Each specimen was fixed in 10% buffered formalin
solution for 12 h at room temperature. The specimens were prepared
according to the routine procedure: Overnight dehydration in an
automated closed type tissue processor, followed by paraffin
embedding. Serial 3.5 µm sections were cut from each
paraffin block using a rotary microtome, and were set in positively
charged SuperFrost microscope slides. These slides were used for
immunohistochemical staining, whereas another plain microscope
slide was stained with hematoxylin and eosin (Atom Scientific,
Manchester, UK). The positively charged slides were deparaffinized
in an incu bator at 64.5°C for 45 min. Immunostaining was performed
using an automated immunostainer (Bond; Leica Biosystems Ltd.,
Newcastle, UK). A kit was used with the immunostainer for the
detection of primary antibodies (Bond Polymer Refine Detection;
Leica Biosystems Ltd.), which contained 3.0% hydrogen peroxide,
polymer penetration enhancer (Post Primary), polymer horseradish
peroxidase anti mouse/rabbit immunoglobulin G, 3,3′
diaminobenzidine tetrahydrochloride and hematoxylin. The
deparaffinization was performed with incubation of the slides for 1
h in 60°C, prior to the procedure. Using the immunohistochemical
kit provided the slides were incubated with
H2O2 for 5 min, followed by application of
the optimal antibody for 10 min (LIF in pH9 and LIFR in pH6),
incubated with the post primary antibody solution for 10 min, with
the polymer for 10 min, with DAB for 10 min and stained with
hematoxylin for 5 min. At the end of the protocol, the slides were
hydrated through ascending alcohols, cleared with xylene and
mounted. Rabbit polyclonal antibodies were used for the detection
of LIF (cat. no. HPA018844; Sigma Aldrich, St. Louis, MO, USA) and
LIF-R expression (cat. no. sc 659; C 19; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA,).
Histological dating was assessed according to the
histo logical criteria outlined by Noyes et al (26). A sample was considered as out of
phase when the histological dating differed >3 days from the
chronological dating. IHC staining was assessed by optical
microscopy (DM1000; Leica Microsystems GmbH, Weltzar, Switzerland).
Liver, kidney and lung tissues were used as control samples.
Endometrial samples were considered positive when the cells were
stained brown. The percentage of positive cell staining was
measured in every sample. Staining intensity was evaluated using a
score scale between 0 and 3: Score 0, no staining; 1, mild
staining; 2, moderate staining; and 3, intense staining. H score
was defined as Σxi (i+1) of positive cell percentage and staining
intensity (27). The H score is a
method of assessing the extent of nuclear immunoreactivity. The
score is obtained by the formula: 3 x percentage of strongly
staining nuclei + x percentage of moderately staining nuclei +
percentage of weakly staining nuclei, giving a range of 0 to 300.
These parameters were examined separately for epithelial and
stromal cells. Scoring of all tissues was performed blindly by the
same physician (S.M.).
Independent variables and epidemiological
characteristics
The epidemiological characteristics of the women
included in the present study were examined. Obstetrical history of
the women was examined, including gravidity, parity, mode of
delivery for fertile women, and number of potential miscarriages
and abortions. For the infertile women, the exact cause of
infertility, and previous attempts at in vitro fertilization
and their outcome were examined. Menstrual day on which the biopsy
was performed, the interval between day of ovulation and day of
biopsy, and endometrial thickness at biopsy were also recorded.
Primary and secondary outcomes
Primary outcomes were defined as the percentage of
positive cellular staining, the intensity of staining, and the H
score of LIF and LIF-R expression in the epithelial and stromal
cells of fertile and infertile women. Secondary outcomes included
the endometrial dating of obtained samples, as well as the rate of
out of phase endometrial tissues in the two study groups.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA). Mean values, standard deviation and
standard error of the mean were estimated for continuous variables,
whereas categorical variables were expressed as percentages.
Numerical vari ables of the present study were tested for normality
using the Kοlmogorov-Smirnov test. Independent samples t test was
used for the comparison of normally distributed variables, and
Mann-Whitney test was used for the comparison of non-normally
distributed variables. Fisher's exact test (χ2
criterion) was used to analyze the categorical parameters of this
study. Both primary and secondary outcomes were compared between
the fertile and infertile women (groups 1 and 2). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Overall, 20 fertile and 40 infertile women were
initially included in the present study. Ovulation was confirmed in
17 fertile and 33 infertile women. Adequate tissue was obtained
from 15 fertile and 30 infertile women. A flowchart of the patients
included in the present study is presented in Fig. 1.
Mean age was 32.8±6.0 years for fertile women, and
37.6±3.7 years for infertile women. The parameters of gynecological
history were similar between the two groups, and epidemiological
characteristics for both groups are presented in Table I.
 | Table IEpidemiological characteristics of the
women included in the present analysis. |
Table I
Epidemiological characteristics of the
women included in the present analysis.
| Parameters | Fertile
group
(n=15) | Infertile
group
(n=30) | P value |
|---|
| Personal
characteristics | | | |
| Age
(years)a | 32.8±6.0 | 37.6±3.7 | 0.02 |
| Height (m)a | 1.68±0.1 | 1.63±0.07 | 0.04 |
| Weight
(kg)a | 69.3±3.6 | 65.5±8.0 | 0.32 |
| Gynecological
history | | | |
| Menarche
(years)a | 12.8±0.8 | 12.9±1.8 | 0.87 |
| Menstrual cycle
(days)a | 28.6±2.7 | 27.7±1.5 | 0.28 |
| Menstruation
(days)a | 4.0±0.8 | 4.6±1.0 | 0.14 |
| Obstetrical
history | | | |
| Gravidityb | 2.6 (1–5) | 0.6 (0–3) | <0.001 |
| Parityb | 1.8 (1–3) | – | – |
|
Miscarriageb | – | 0.6 (0–3) | – |
| Abortionb | 0.9 (0–3) | – | – |
| Cause of
infertility | | | |
| Poor ovarian
responsec | – | 16 (53.3) | – |
| Tubal
factorc | – | 7 (23.3) | – |
| Unexplained
infertilityc | – | 7 (23.3) | – |
| Infertility
history | | | |
| Interval from
infertility diagnosis (years)b | – | 4.7 (1–14) | – |
| Previous ART
effortsb | – | 2.7 (0–16) | – |
| Previous IUI
effortsb | – | 0.6 (0–4) | – |
| Previous IVF
effortsb | – | 1.8 (0–15) | – |
| Previous Natural
Cycle IVFb | – | 0.4 (0–6) | – |
Endometrial biopsy characteristics
Menstrual day of ovulation was 12.3±1.4 for fertile
women, and 14.1±1.8 for infertile women (P=0.002). The interval
between ovulation and biopsy was comparable between the two groups
(P=0.17). Menstrual day at biopsy obtainment was 19.3±1.6 for the
fertile group, and 21.3±1.8 for the infertile group (P=0.001).
Endometrial thickness was significantly lower in infertile women
(8.8±1.7 mm) compared with in the fertile controls (10.6±2.9 mm)
(P=0.02). Characteristics of the endometrial biopsy are presented
in Table II.
 | Table IIEndometrial tissue
characteristics. |
Table II
Endometrial tissue
characteristics.
| Parameters | Fertile
group
(n=15) | Infertile
group
(n=30) | P value |
|---|
| Day of
ovulation | 12.3±1.4 | 14.1±1.8 | 0.002 |
| Menstrual day at
biopsy | 19.3±1.6 | 21.3±1.8 | 0.001 |
| Ovulation to biopsy
interval | 7.0±0.4 | 7.2±0.4 | 0.17 |
| Endometrial
thickness at biopsy | 10.6±2.9 | 8.8±1.7 | 0.02 |
Primary outcomes
The expression of LIF and LIF-R was significantly
lower in the epithelial cells of infertile women compared with the
fertile controls. No significant differences were detected
regarding the expression of LIF and LIF-R in the stromal cells
between the two groups.
LIF expression was detected in a significantly
higher percentage of epithelial cells in the fertile group compared
with the infertile group (P=0.05). Intensity of staining was
comparable between the two groups (P=0.21); however, H score for
epithelial LIF expression was 105.7±28.5 in fertile women, as
compared with 61.2±15.0 in infertile women (P=0.05). Regarding LIF
expression in stromal cells, no significant difference was detected
between the fertile and infertile women (P=0.95).
The percentage of cells positively stained for LIF-R
and staining intensity were significantly lower in the epithelial
cells of infertile women (P=0.04 and P=0.002, respectively). In
addition, LIF-R H-score for epithelial cells was significantly
reduced in infertile women (128.4±11.2) compared with fertile
controls (189.2±19.5) (P=0.006). Regarding LIF-R expression in
stromal cells, the H score was higher in fertile women; however,
the difference did not reach statistical significance (P=0.10).
Positive cellular percentage and staining intensity were comparable
between the two groups (P=0.19 and P=0.29, respectively). Primary
outcomes of the study are presented in Table III.
 | Table IIIPrimary outcomes of the present
study. |
Table III
Primary outcomes of the present
study.
| Parameters | Fertile
group
(n=15) | Infertile
group
(n=30) | P value |
|---|
| LIF | | | |
| Epithelial
cells | | | |
| Positive nuclei
percentage | 42.9±9.9 | 24.9±5.5 | 0.05a |
| Intensity of
staining | 2.3±0.2 | 1.9±0.2 | 0.21a |
| H score | 105.7±28.5 | 61.2±15.0 | 0.05a |
| Stromal cells | | | |
| Positive nuclei
percentage | 64.6±5.9 | 63.6±3.9 | 0.89 |
| Intensity of
staining | 2.5±0.2 | 2.6±0.1 | 0.52a |
| H score | 155.0±18.5 | 153.4±13.6 | 0.95 |
| LIF receptor | | | |
| Epithelial
cells | | | |
| Positive nuclei
percentage | 76.3±5.5 | 63.0±4.3 | 0.04a |
| Intensity of
staining | 2.5±0.1 | 1.9±0.9 | 0.002a |
| H score | 189.2±19.5 | 128.4±11.2 | 0.006 |
| Stromal cells | | | |
| Positive nuclei
percentage | 75.0±3.1 | 67.6±3.6 | 0.19a |
| Intensity of
staining | 2.6±0.1 | 2.4±0.1 | 0.29a |
| H score | 198.3±11.9 | 162.8±13.6 | 0.10 |
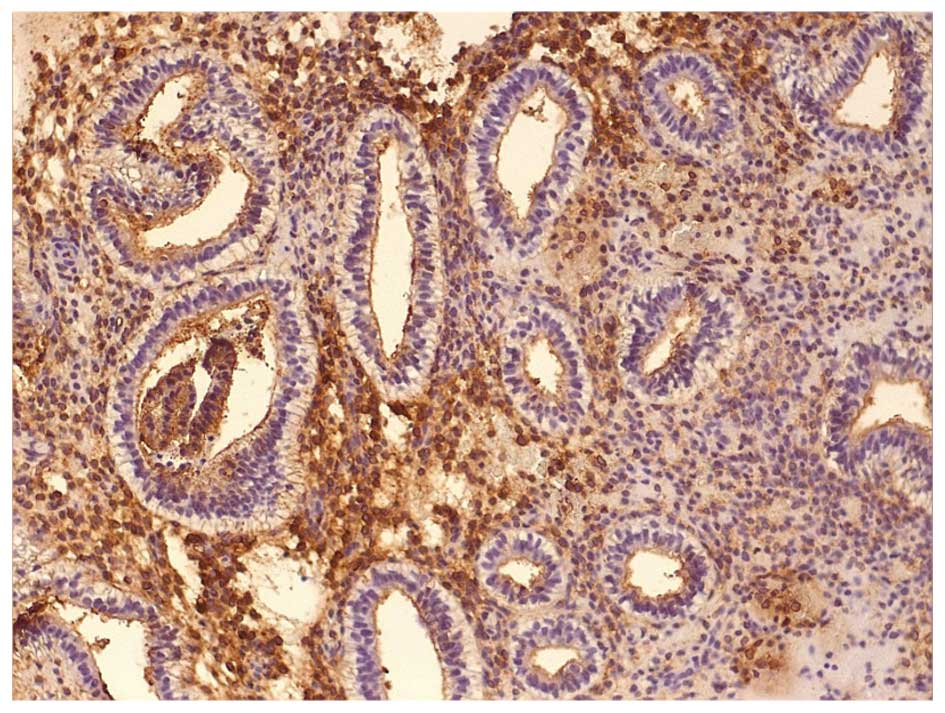
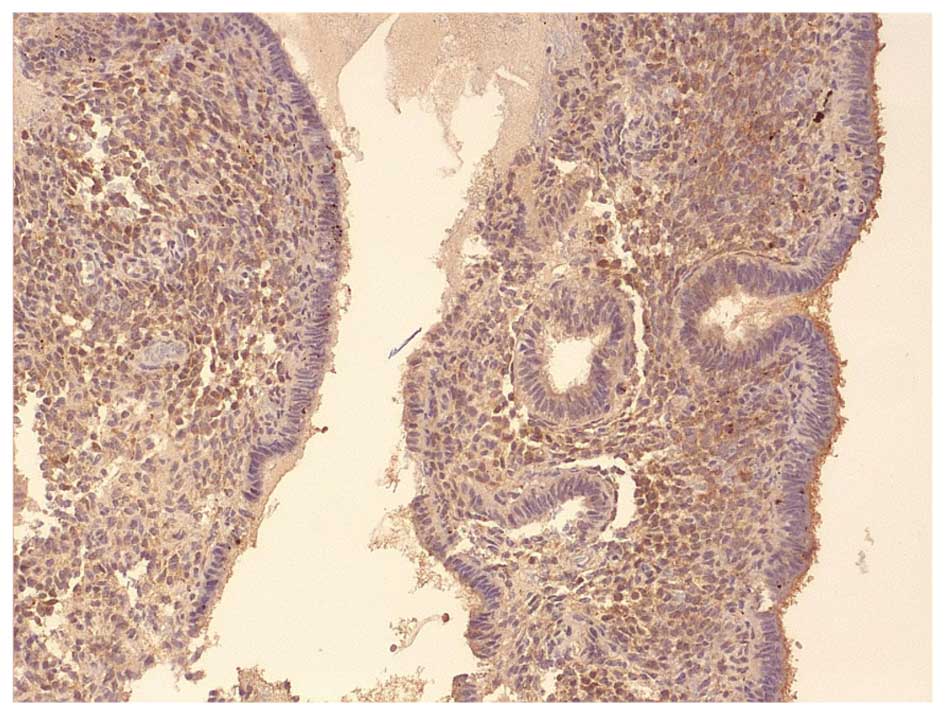
Images of IHC staining of LIF and LIF-R expression
in the epithelial and stromal cells of fertile and infertile women
are presented in Figs. 2Figure 3Figure 4–5.
Secondary outcomes
The difference between normal histological dating
according to menstrual day, and observed endometrial histological
dating was significantly higher in the infertile group (P=0.02). In
addition, there was a higher rate of out of phase endometrial
tissues in the infertile group (66.7%) compared with in the fertile
control group (26.7%) (P=0.01). Secondary outcomes of the present
study are presented in Table
IV.
 | Table IVSecondary outcomes of the study. |
Table IV
Secondary outcomes of the study.
| Parameters | Fertile
group
(n=15) | Infertile
group
(n=30) | P value |
|---|
| Endometrial
datinga | 16.6±0.7 | 16.9±0.7 | 0.83 |
| Biopsy day dating
differenceb | −2.3±0.9 | −5.3±0.7 | 0.02 |
| Out of phase
tissuesc | 4 (26.7) | 20 (66.7) | 0.01 |
| Endometrial
thickness at biopsya | 10.6±2.9 | 8.8±1.7 | 0.02 |
Discussion
The present study demonstrated that LIF and LIF-R
expression is significantly lower in the epithelial endometrial
cells of infertile women, as compared with in fertile women.
Furthermore, LIF-R expression may be impaired in the stromal cells
of infertile women; however, this hypothesis requires further
investigation in a larger sample size.
A review of the literature revealed a discrepancy
regarding the expression of LIF in the epithelial endometrial cells
of infertile women. Numerous studies have detected lower levels of
this cytokine in the epithelial cells of infertile women compared
with fertile women. Mariee et al (28) analyzed 15 endometrial biopsies from
fertile women, and 45 from infertile women with unexplained
infertility and multiple implantation failure (MIF), and reported
that LIF expression was significantly decreased in the epithelium
of infertile women. Similar observations were made by Wu et
al (29) in a total of 30
endometrial biopsies, and by Dimitriadis et al (30) in a total of 15 biopsies from women
with unexplained infertility and endometriosis, respectively.
Decreased LIF expression has also been reported in studies using
quantitative polymerase chain reaction or enzyme linked
immunosorbent assay analyses of either endometrial tissue or
uterine flushing samples (31,32).
Conversely, previous studies have reported similar
LIF expression between the epithelial cells of fertile and
infertile women. Xu et al (33) studied LIF expression in 30
infertile women who suffered from recurrent pregnancy loss, and
observed no significant difference in epithelial endometrial cell
expression compared with the fertile control group. However,
recurrent pregnancy loss alone should not be considered proof of
infertility, since embryo attachment, invasion and implantation
have successfully occurred in these cases. Furthermore, miscarriage
that occurs after the 6th week of gestation is usually
caused by factors not related to the endometrium. Similar LIF
expression between epithelial and stromal cells has also been
observed by Mikolajczyk et al (34) in a study that compared the results
from 14 infertile women with endometriosis and 21 fertile controls.
However, uterine flushing, and not endometrial biopsy, was used in
this previous study, thus providing a potential explanation for the
different results obtained. Endometrial flushing may only contain
exfoliated epithelial cells, whereas an endometrial biopsy contains
both epithelial and stromal cells obtained in their functional
condition.
The LIF intracellular signaling pathway is disturbed
in not all, but in some cases of female infertility, dependent on
the cause of infertility. Aghajanova et al (35) reached the conclusion that LIF
intracellular signaling is predominantly affected in cases of
unexplained infertility with MIF. In a subsequent study, the same
author observed that deficient LIF expression is not a constant
finding in infertile women; however, increased expression is
indicative of endometrial receptivity (36). Therefore, it was concluded that
evaluation of LIF expression on its own may not be sufficient for
definitive conclusions regarding implantation achievement, even in
women with unexplained infertility. Further research is required to
assess the exact LIF expression patterns in various infertility
sub-groups.
Numerous studies regarding LIF expression in the
endometrium of infertile women have been performed; however, less
studies have been conducted regarding the expression patterns of
LIF-R. As previously stated, the basic condition of LIF action is
its connection with LIF-R as a primary step to create a
high-affinity binding heterodimer. However, a review of the
literature revealed no study that directly compared LIF-R
expression between fertile and infertile women during the
implantation window. Cullinan et al (18) studied the expression patterns of
LIF-R in the proliferative and secretory phases, concluding that
there is a possible autocrine/paracrine interaction between LIF and
LIF-R at the luminal epithelium. In addition, in a hamster study
performed by Ding et al (37) a significant role for LIF-R was
identified in uterine receptivity and implantation. The present
study is one amongst few that has observed significantly decreased
levels of LIF-R in the epithelial cells of infertile women,
alongside reduced LIF levels (38,39).
Furthermore, as the decreased expression levels of LIF-R in stromal
cells in infertile women was significant; therefore, future
research should be performed to clarify LIF-R expression patterns
in stromal cells. These results suggested that the key factor for
implantation is not LIF expression, but the synchronized expression
of adequate LIF-R, in order to achieve normal implantation.
The present study is not devoid of limitations. A
potential confounding variable may be the heterogeneity of the
infertile patients with regards to the cause of infertility.
However, the authors of the present study believe that expression
patterns of various cytokines and molecules associated with the
implantation process should be initially studied in the general
infertile population, followed by in the specific sub-groups of
infertility, particularly in those with unexplained infertility.
The present analysis reported the results of a prospective study,
including a large sample size, in the domain of reproductive
immunology. Furthermore, the present study may be the first to
report on the significant alteration of LIF-R levels in the
endometrium of infertile women. Expansion of the study into a
larger number of patients, and evaluation of LIF and LIF-R
expression in the various sub groups of infertility will hopefully
lead to safer and more reliable conclusions regarding the potential
pathogenetic role of these molecules in infertility.
In conclusion, the present analysis demonstrated
that LIF and LIF-R expression was decreased in the epithelial cells
of infertile women. This observation further underlines the
predominant role of LIF-R in endometrial receptivity. Further
studies elucidating the expression patterns of cytokines in various
sub-groups of infertility may define their exact etiopathogenetic
role in endometrial receptivity. Investigation into the expression
patterns of LIF and LIF-R may ideally lead to the development of
tests that could assess endometrial receptivity, in order to
improve implantation rates in assisted reproductive technology.
Acknowledgments
The present study was supported by the IKY
Fellowships of Excellence for Postgraduate Studies in Greece
Siemens Program.
References
|
1
|
Dimitriadis E, Menkhorst E, Salamonsen LA
and Paiva P: Review: LIF and IL11 in trophoblast endometrial
interactions during the establishment of pregnancy. Placenta.
31(Suppl): S99–S104. 2010. View Article : Google Scholar
|
|
2
|
Koot YE and Macklon NS: Embryo
implantation: Biology, evaluation, and enhancement. Curr Opin
Obstet Gynecol. 25:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tabibzadeh S and Babaknia A: The signals
and molecular pathways involved in implantation, a symbiotic
interaction between blastocyst and endometrium involving adhesion
and tissue invasion. Hum Reprod. 10:1579–1602. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paiva P, Menkhorst E, Salamonsen L and
Dimitriadis E: Leukemia inhibitory factor and interleukin 11:
Critical regulators in the establishment of pregnancy. Cytokine
Growth Factor Rev. 20:319–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gearing DP, Gough NM, King JA, Hilton DJ,
Nicola NA, Simpson RJ, Nice EC, Kelso A and Metcalf D: Molecular
cloning and expression of cDNA encoding a murine myeloid leukaemia
inhibitory factor (LIF). EMBO J. 6:3995–4002. 1987.PubMed/NCBI
|
|
6
|
Stewart CL, Kaspar P, Brunet LJ, Bhatt H,
Gadi I, Köntgen F and Abbondanzo SJ: Blastocyst implantation
depends on maternal expression of leukaemia inhibitory factor.
Nature. 359:76–79. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart CL: The role of leukemia
inhibitory factor (LIF) and other cytokines in regulating
implantation in mammals. Ann NY Acad Sci. 734:157–165. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aghajanova L: Leukemia inhibitory factor
and embryo human implantation. Ann NY Acad Sci. 1034:176–183. 2004.
View Article : Google Scholar
|
|
9
|
Singh M, Chaudry P and Asselin E: Bridging
endometrial receptivity and implantation: Network of hormones,
cytokines, and growth factors. J Endocrinol. 210:5–14. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sánchez Cuenca J, Martín JC, Pellicer A
and Simón C: Cytokine pleiotropy and redundancy gp130 cytokines in
human implantation. Immunol Today. 20:57–59. 1999. View Article : Google Scholar
|
|
11
|
Classen Linke I, Müller Newen G, Heinrich
PC, Beier HM and von Rango U: The cytokine receptor gp130 and its
soluble form are under hormonal control in human endometrium and
decidua. Mol Hum Reprod. 10:495–504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gearing DP, Thut CJ, VandeBos T, Gimpel
SD, Delaney PB, King J, Price V, Cosman D and Beckmann MP: Leukemia
inhibitory factor receptor is structurally related to the IL 6
signal transducer, gp130. EMBO J. 10:2839–2848. 1991.PubMed/NCBI
|
|
13
|
Gearing DP, Comeau MR, Friend DJ, Gimpel
SD, Thut CJ, McGourty J, Brasher KK, King JA, Gillis S, Mosley B,
et al: The IL 6 signal transducer, gp130: An oncostatin M receptor
and affinity converter for the LIF receptor. Science.
255:1434–1437. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Auernhammer CJ and Melmed S: Leukemia
inhibitory factor neuroimmune modulator of endocrine function.
Endocr Rev. 21:313–345. 2000.PubMed/NCBI
|
|
15
|
Heinrich PC, Behrmann I, Müller Newen G,
Schaper F and Graeve L: Interleukin 6 type cytokine signalling
through the gp130/Jak/STAT pathway. Biochem J. 334:297–314. 1998.
View Article : Google Scholar
|
|
16
|
Duval D, Reinhardt B, Kedinger C and Boeuf
H: Role of suppressors of cytokine signaling (Socs) in leukemia
inhibitory factor (LIF) dependent embryonic stem cell survival.
FASEB J. 14:1577–1584. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng JG, Chen JR, Hernandez L, Alvord WG
and Stewart CL: Dual control of LIF expression and LIF receptor
function regulate Stat3 activation at the onset of uterine
receptivity and embryo implantation. Proc Natl Acad Sci USA.
98:8680–8685. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cullinan EB, Abbondanzo SJ, Anderson PS,
Pollard JW, Lessey BA and Stewart CL: Leukemia inhibitory factor
(LIF) and LIF receptor expression in human endometrium suggests a
potential autocrine/paracrine function in regulating embryo
implantation. Proc Natl Acad Sci USA. 93:3115–3120. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vogiagis D, Marsh MM, Fry RC and
Salamonsen LA: Leukaemia inhibitory factor in human endometrium
throughout the menstrual cycle. J Endocrinol. 148:95–102. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tawfeek MA, Eid MA, Hasan AM, Mostafa M
and El Serogy HA: Assessment of leukemia inhibitory factor and
glycoprotein 130 expression in endometrium and uterine flushing: A
possible diagnostic tool for impaired fertility. BMC Womens Health.
12:102012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharkey AM, King A, Clark DE, Burrows TD,
Jokhi PP, Charnock Jones DS, Loke YW and Smith SK: Localization of
leukemia inhibitory factor and its receptor in human placenta
throughout pregnancy. Biol Reprod. 60:355–364. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laird SM, Tuckerman EM, Dalton CF, Dunphy
BC, Li TC and Zhang X: The production of leukaemia inhibitory
factor by human endometrium: Presence in uterine flushings and
production by cells in culture. Hum Reprod. 12:569–574. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aghajanova L, Stavreus Evers A, Nikas Y,
Hovatta O and Landgren BM: Coexpression of pinopodes and leukemia
inhibitory factor, as well as its receptor, in human endometrium.
Fertil Steril. 79(Suppl 1): S808–S814. 2003. View Article : Google Scholar
|
|
24
|
Lass A, Weiser W, Munafo A and Loumaye E:
Leukemia inhibitory factor in human reproduction. Fertil Steril.
76:1091–1096. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei T, Yang ZQ, Xia T, Gan L, Chen XD,
Yuan JH and Zhu Y: Stage-specific expression of leukaemia
inhibitory factor and its receptor in rabbit pre implantation
embryo and uterine epithelium during early pregnancy. Reprod Domest
Anim. 39:13–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noyes RW, Hertig AI and Rock J: Dating the
endometrial biopsy. Am J Obstet Gynecol. 122:262–263.
1975.PubMed/NCBI
|
|
27
|
Ellis YO, Pider SE and Lee A: Tumors of
the breast. 2nd Edition. Diagnostic Histopathology of Tumors.
Fletcher C: Churchill Livingstone; Elsevier, London, UK: pp.
1057–1070. 2007
|
|
28
|
Mariee N, Li TC and Laird SM: Expression
of leukaemia inhibitory factor and interleukin 15 in endometrium of
women with recurrent implantation failure after IVF; correlation
with the number of endometrial natural killer cells. Hum Reprod.
27:1946–1954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu M, Yin Y, Zhao M, Hu L and Chen Q: The
low expression of leukemia inhibitory factor in endometrium:
Possible relevant to unexplained infertility with multiple
implantation failures. Cytokine. 62:334–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dimitriadis E, Stoikos C, Stafford Bell M,
Clark I, Paiva P, Kovacs G and Salamonsen LA: Interleukin 11
receptoralpha and leukemia inhibitory factor are dysregulated in
endometrium of infertile women with endometriosis during the
implantation window. J Reprod Immunol. 69:53–64. 2006. View Article : Google Scholar
|
|
31
|
Alizadeh Z, Shokrzadeh N, Saidijam M and
Sanoee MF: Semi quantitative analysis of HOXA11, leukemia
inhibitory factor and basic transcriptional element binding protein
1 mRNA expression in the mid secretory endometrium of patients with
endometriosis. Iran Biomed J. 15:66–72. 2011.
|
|
32
|
Hambartsoumian E: Endometrial leukemia
inhibitory factor (LIF) as a possible cause of unexplained
infertility and multiple failures of implantation. Am J Reprod
Immunol. 39:137–143. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu B, Sun X, Li L, Wu L, Zhang A and Feng
Y: Pinopodes, leukemia inhibitory factor, integrin β3 and mucin 1
expression in the peri implantation endometrium of women with unex
plained recurrent pregnancy loss. Fertil Steril. 98:389–395. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mikolajczyk M, Wirstlein P and Skrzypczak
J: Leukaemia inhibitory factor and interleukin 11 levels in uterine
flushings of infertile patients with endometriosis. Hum Reprod.
21:3054–3058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aghajanova L, Altmäe S, Bjuresten K,
Hovatta O, Landgren BM and Stavreus Evers A: Disturbances in the
LIF pathway in the endometrium among women with unexplained
infertility. Fertil Steril. 91:2602–2610. 2009. View Article : Google Scholar
|
|
36
|
Aghajanova L: Update on the role of
leukemia inhibitory factor in assisted reproduction. Curr Opin
Obstet Gynecol. 22:213–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding T, Song H, Wang X, Khatua A and Paria
BC: Leukemia inhibitory factor ligand receptor signaling is
important for uterine receptivity and implantation in golden
hamsters (Mesocricetus auratus). Reproduction. 135:41–53. 2008.
View Article : Google Scholar
|
|
38
|
Subramani E, Madogwe E, Ray CD, Dutta SK,
Chakravarty B, Bordignon V, Duggavathi R and Chaudhury K:
Dysregulated leukemia inhibitory factor and its receptor regulated
signal transducers and activators of transcription 3 pathway:A
possible cause for repeated implantation failure in women with
dormant genital tuberculosis? Fertil Steril. pii: S0015-0282
02184-6. 2016.Epub ahead of print. View Article : Google Scholar
|
|
39
|
Moberg C, Bourlev V, Ilyasova N and
Olovsson M: Endometrial expression of LIF and its receptor and
peritoneal fluid levels of IL 1α and IL 6 in women with
endometriosis are associated with the probability of pregnancy.
Arch Gynecol Obstet. 292:429–437. 2015. View Article : Google Scholar : PubMed/NCBI
|