Introduction
Osteoarthritis (OA) is the most common form of
progressive joint disease in the elderly, which has a reported
prevalence of 34.18% in women and 30.18% in men, aged 65 years or
older, in the United States of America (1). A previous study conducted in Beijing,
China, revealed a higher prevalence of OA in Chinese women of a
similar age (46.16%) and a comparable prevalence in Chinese men
(27.16%) (2). OA is associated
with degenerative changes in the joints, along with joint diseases
caused by loss of the cartilage matrix (3). Furthermore, previous studies have
highlighted the role of inflammation in the pathogenesis of OA
(4,5). Elevated expression levels of
pro-inflammatory cytokines and mediators, including interleukin
(IL)-1, IL-6, tumor necrosis factor (TNF)-α and nitric oxide (NO)
are present in the serum and synovial fluid of OA patients
(6,7). Anti-inflammatory agents, such as
IL-1β inhibitor, diacerein and TNF-α inhibitors, infliximab and
etanercept have demonstrated promising clinical efficacy for the
treatment of OA (8–10). However, to the best of our
knowledge, there have been no reports of therapeutic strategies
that could simultaneously engage multiple inflammatory mediators
and alleviate inflammation-induced tissue damage.
Recent studies have extended the functional scope of
glutamate beyond neuronal tissues to a wide range of tissue and
cell types, exhibiting an autocrine or paracrine effect (11–13).
McNearney et al (14)
revealed increased levels of glutamate, and pro-inflammatory
cytokines and chemokines in the synovial fluid of patients with
active arthritis. Glutamate interacts specifically with the
N-methyl-D-aspartate (NMDA) receptor and activates the downstream
signaling cascade (15). Flood
et al (16) proposed the
role of NMDA receptor in inflammatory joint damage in rheumatoid
arthritis. In an animal study, injecting glutamate into the knee
joint of rats led to thermal hyperalgesia and mechanical allodynia,
which was alleviated by subsequent injections of NMDA receptor
antagonists (17). Magnesium is an
NMDA receptor antagonist, and an articular injection has been shown
to improve the degree of pain following arthroscopic surgery and
improve experimental rat bone arthritis (18,19).
Results from the above-mentioned studies indicate the therapeutic
potential of glutamate/NMDA receptor signaling in OA. Ketamine is a
non-competitive antagonist of the NMDA receptor, and has been
widely administered as an anesthetic and pain killer (20,21).
Recent studies indicate that ketamine exhibited potent
anti-inflammatory activity in various inflammatory disease models
when administered at or below clinical dosages. Ketamine
antagonizes the NMDA receptor to regulate calcium influx, increases
the intracellular concentration of cyclic adenosine monophosphate,
inhibits the formation of oxygen radicals and inducible NO synthase
(iNOS) following polymorphonuclear activation, and modulates the
production of various pro-inflammatory mediators (21). Mechanistic studies indicate that
ketamine directly inhibits the expression of nuclear factor
(NF)-κB, which is a master regulator of pro-inflammatory cytokine
transcriptions (22). In addition,
ketamine exhibits protective effects against oxidative stress by
inhibiting iNOS activity and decreasing nitrite/nitrate levels
(23). These findings highlight
the therapeutic potential of ketamine in inflammatory disorders,
including OA.
In the present study, a rabbit OA model was
established by immobilizing the knee joint, as previously described
(24), and the efficacy of
different doses of ketamine in ameliorating the inflammatory
response was evaluated. In addition, the current study investigated
the anti-inflammatory role of ketamine in regulating multiple
inflammatory cytokines and signaling molecules for possible
therapeutic application in OA.
Materials and methods
Animals and reagents
Thirty skeletally mature, male, New Zealand white
rabbits (weight, 2.5–3 kg) were obtained from the Experimental
Animal Center of Guiyang Medical School (Guiyang, China) and were
acclimated for one week prior to the experimental procedures. All
animal procedures were approved by the Institutional Ethics
Committee.
The ketamine used in the present study was a 1:1
racemic mixture of two enantiomers, which was purchased from
Yichang Humanwell Pharmaceutical Co., Ltd. (Yichang, China).
Enzyme-linked immunosorbent assay (ELISA) kits for IL-10 and TNF-α,
and the anti-NF-κB p65 antibody were purchased from Shanghai Bogoo
Biotechnology Co., Ltd. (Shanghai, China). The
3,3′-diaminobenzidine (DAB) Substrate kit was obtained from
ZSGB-Bio Co., Ltd. (Beijing, China), and Alcian blue/periodic
acid-Schiff (AB/PAS) Stain kit was obtained from Huanyu Jinying
Technology Co., Ltd. (Beijing, China).
Rabbit OA model and ketamine
treatment
Twenty-four rabbits were randomly selected to
establish the OA model. The left knee joint was immobilized (3 cm
above the rear ankle to 1.5 cm below the groin) for six weeks using
plaster bandages, with the knee flexed at 30–40°. Dorsalis pedis
pulses were detected on each side. Plaster tightness was examined
for three consecutive days after creating the model and were
adjusted as appropriate. The rabbits were housed in separate cages
and forced to move or exercise occasionally. Six rabbits were left
untreated and served as a normal control.
After six weeks, the plaster was removed and rabbits
were randomly allocated into four groups: Normal saline,
Ket60, Ket100 and Ket200. Saline
or various concentrations of ketamine (60, 100 and 200
µmol/l) were injected into the articular cavity in 0.5-ml
volumes, twice a week for four weeks. One week after the last
injection, sample collection was conducted, then all rabbits were
sacrificed under intravenous anesthesia with 25% urethane (4 ml/kg;
Shanghai-Rui Biological Technology Co., Ltd., Shanghai, China) by
air injection (20 ml) into an ear marginal vein.
Sample collection
The thigh was rotated and an incision was made on
the inner side in order to peel back the skin. The quadriceps were
dissected 0.5 cm above the knee joint, and separated from the femur
and patella. The synovial membrane under the patella was removed
using ophthalmic scissors (Shanghai Yuyan Instruments Co., Ltd.,
Shanghai, China) and rinsed in ice-cold phosphate-buffered saline
solution (Shanghai-Rui Biological Technology Co., Ltd.), and fixed
in 10% neutral buffered formalin (BioSynTech, Beijing, China) for
24 h followed by dehydration, clearing, wax infiltration and
embedding in paraffin (Junruishengwu Technology Corporation,
Shanghai, China) for further histological examination. The samples
were 0.5 × 1 × 0.5 cm in size.
The femoral condyle and a portion of the cartilage
from the knee joint were removed, rinsed and immediately fixed in
10% neutral buffered formalin for 24 h. Samples were decalcified
prior to histological examination.
Synovial fluid was obtained from the knee joint by
injecting 0.5 ml saline solution followed by aspiration, which was
performed three times. Synovial fluid (~0.8–1 ml) was extracted and
samples were centrifuged at 2147 × g for 10 min. The supernatants
were obtained and stored at −70°C until further use.
General and histopathological
examination
The knee joint was observed for signs of synovitis,
including joint effusion and swelling. Pathological changes in the
articular surface of the medial femoral condyle were evaluated
under an Olympus CX41 biological microscope (Olympus Corporation,
Tokyo, Japan). Samples were scored according to the following
criteria (25): 0, Smooth
articular surface with normal color; i) rough articular surface
with gray color and small cracks; ii) eroded articular surface with
cartilage lesions penetrating the middle layer; iii) ulcers on
articular surface with cartilage lesions penetrating the deep
layer; and iv) complete loss of cartilage with exposed subchondral
bone. Paraffin-embedded tissue samples were sectioned into
3–5-µm thick slices using a DQP-9010 rotary slicer (Shanghai
Huayan Equipment Co., Ltd., Shanghai, China). The synovial membrane
was stained with hematoxylin and eosin (H&E; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China), and mounted
with neutral balata (Shanghai-Rui Biological Technology Co., Ltd.);
the pathological changes were evaluated by two independent
observers. The cartilage was stained using H&E and AB/PAS, and
mounted with neutral balata. Samples were scored by two independent
observers according to Mankin's score system (26) and the mean was taken as the final
score.
ELISA of IL-10 and TNF-α in the synovial
fluid
IL-10 and TNF-α concentration levels in the synovial
fluid were measured using specific ELISA kits according to the
manufacturer's instructions. Absorbance (optical density value) at
a wavelength of 450 nm was determined using a UV-2600 microplate
reader (Shimadzu Corporation, Kyoto, Japan).
Immunohistochemical analysis of
NF-κB
Immunohistochemical expression of NF-κB in the
cartilage was determined using the Labeled Streptavidin Biotin
method. Samples were evaluated based on positive DAB staining in
the cytoplasm and nucleus of chondrocytes: Weak positive, pale
yellow stain; positive, yellow stain; medium positive, brown stain;
strong positive, tan stain. Eight fields (magnification, ×400) from
each section were randomly selected and the positive rate (%) was
determined by the percentage of positively-stained cells. Results
were assessed by an investigator and two experienced
technicians.
Statistical analysis
Data were expressed as arithmetic or geometric mean
± standard deviation. Means of the two groups were compared using
Levene's test of homogeneity of variance. Homogeneous variance was
analyzed by one-way analysis of variance, and pairwise comparison
between groups was made by Fisher's least significant difference
test. Dunnett's T3 test was performed when there was lack of
homogeneity of variances. Positive rates were compared by
χ2-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Evaluation of the rabbit OA model
A rabbit OA model was established by knee
immobilization, as described in previous studies (24,27).
Following six weeks of immobilization, varying degrees of thigh
muscle atrophy and knee stiffness were observed with the loss of
the majority of active and passive ranges of motion. Typical OA
pathological changes were identified, including hyperemia, swelling
and edema in the joint capsule and synovial tissue, as well as
joint effusion, rough and dull articular surfaces and cartilage
lesions; indicating the successful establishment of the OA
model.
Ketamine attenuates OA
Various doses (60, 100 and 200 µmol/l) of
ketamine were intra-articularly injected into rabbits in the
Ket60, Ket100 and Ket200 groups
following immobilization. After four weeks of treatment, rabbits in
the normal saline group demonstrated the highest gross scores
(3.67±0.52); exhibiting prominent joint effusion and swelling,
eroded articular surfaces and cartilage lesions. Rabbits in the
Ket60 group had marginally lower gross scores
(3.17±0.75); while rabbits in the Ket100 and
Ket200 groups exhibited the lowest gross scores
(2.0±0.00 and 1.33±0.82, respectively), and showed mild joint
swelling and a small degree of effusion (Fig. 1).
Synovial membrane samples from all four groups were
stained with H&E and the histopathological changes were
evaluated. Pronounced inflammatory cell infiltration was observed
in the synovial membrane samples from the normal saline group
(Fig. 2A). The number of
infiltrating inflammatory cells decreased with the increase in
ketamine dosage, indicating the attenuation of inflammation
(Fig. 2B–D).
H&E staining revealed that the structure and
organization of chondrocytes were markedly disrupted and partially
replaced by proliferating tissues in the normal saline group
(Fig. 3A). Despite obvious
chondrocyte proliferation in the Ket60 group, the
chondrocyte structure was highly visible with featuring tidemarks
(Fig. 3B). Morphology of the
articular cartilage was further improved in the Ket100
group, as demonstrated by the normal layer structure observed
(Fig. 3C). In the
Ket200 group, only mild deformation and disorganization
were observed in chondrocytes of the upper layer without any
changes in the middle or lower layers (Fig. 3D).
AB/PAS staining was performed to indicate
cartilaginous tissues. As shown in Fig. 4, the extent of blue staining of
cartilaginous tissue samples was significantly reduced in the
normal saline group, but revealed a dose-dependent increase
following treatment with ketamine. Finally, unlike the normal
control group without knee immobilization, rabbits in the normal
saline group exhibited the highest Mankin's scores, which indicated
the most severe cartilage lesions (Fig. 5). These scores decreased in the
ketamine-treated groups (6.7±0.9, 5.8±0.6 and 4.1±0.7 for
Ket60, Ket100, and Ket200,
respectively) in a dose-dependent manner, indicating that ketamine
treatment ameliorated the joint damage associated with OA.
Ketamine regulates the expression levels
of IL-10, TNF-α and NF-κB
ELISA and immunohistochemistry revealed
significantly elevated pro-inflammatory cytokine, TNF-α expression
levels in the synovial fluid of rabbits with immobilization-induced
OA (normal saline group; 22.69±1.23), but not in the normal control
group (Fig. 6A; 0.00±0.00).
Ketamine treatment significantly decreased TNF-α levels in a
dose-dependent manner (from 18.78±1.51 to 12.53±1.34 in the
Ket60 and Ket200 groups, respectively). By
contrast, the expression levels of anti-inflammatory cytokine,
IL-10 were lower in OA rabbits (0.38±0.11) when compared with those
of the normal controls (0.49±0.13); however a dose-dependent
increase was exhibited in the ketamine-treated groups (Fig. 6B; 0.54±0.09 to 0.81±0.19 in the
Ket60 and Ket200 groups, respectively). The
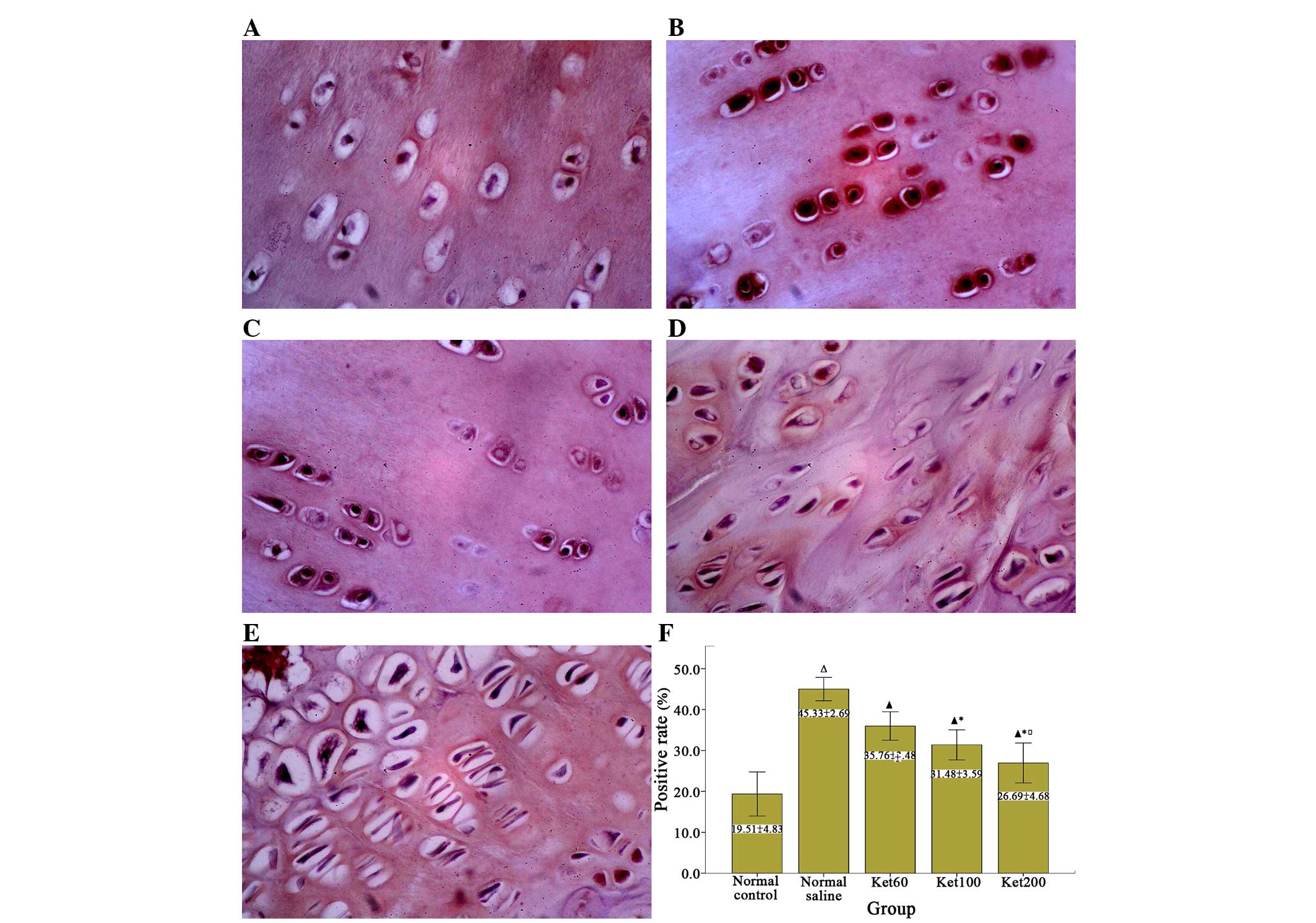
NF-κB expression level in the articular cartilage was determined
using anti-NF-κB p65 antibodies. Minimal NF-κB p65 subunit
expression levels were detected in chondrocytes of the normal
control group, but were markedly elevated in OA rabbits of the
normal saline group (Fig. 7A and
B). Following intra-articular injection of ketamine, a
dose-dependent reduction of NF-κB p65 expression was observed
(Fig. 7C–F; 35.76±2.69 to
26.69±4.68 in the Ket60 and Ket200 groups,
respectively), which indicated that ketamine downregulated the
expression of pro-inflammatory cytokines and mediators, while
promoting the expression of anti-inflammatory cytokines.
Discussion
Current therapeutic strategies for OA are not
curative, and are limited to providing symptomatic relief and pain
control. Commonly used non-steroidal anti-inflammatory therapeutic
agents are associated with adverse gastrointestinal and
cardiovascular side-effects. Previous studies regarding the
pathogenesis of OA remain inconclusive (28,29),
which is hindering the development of effective therapeutic agents
for OA. Risk factors for the pathogenesis and progression of OA
include age, mechanical injury to the joint, increased expression
of metalloproteinases associated with destruction of the cartilage
matrix, and impaired repair mechanisms (30–32).
Recently, the role of inflammation in the onset and development of
OA has been reported in animal and human studies (29). Thus, anti-inflammatory strategies
are considered to be an effective approach. In the present study, a
rabbit OA model was used to demonstrate the dose-dependent effect
of ketamine in ameliorating pathological changes in the knee joint
and modulating the expression levels of inflammatory mediators.
A well-established rabbit model was produced in the
current study by knee immobilization, as previously described
(27). Plaster bandages were used
to immobilize the knee joint, and OA was induced in the flexed
position. The underlying principle was associated with the
contracture of the joint and muscle, increase in the load-bearing
surface, and changes in biomechanics of the cartilage; leading to
cartilage degeneration. Okazaki et al (33) found that articular cartilage
degeneration occurred as early as one week after the rabbit knee
was immobilized in extension. Furthermore, Kojima et al
(34) demonstrated permanent and
irreversible changes in cartilage and synovial membrane following
four weeks of knee immobilization at full flexion. Compared with
other experimental OA models involving therapeutic agent injection
or surgical trauma, the knee immobilization model better simulated
the natural process of OA pathogenesis. In the present study, knee
immobilization for six weeks led to morphological and structural
changes in articular cartilage and synovial membrane, along with
infiltrating inflammatory cells and elevated levels of
pro-inflammatory mediators including IL-1, IL-6, TNF-α and NO,
which are typical of OA.
The glutamate/NMDA receptor signaling pathway
mediates OA progression. In a rat model of collagenase-induced OA,
intra-articular injection of magnesium sulfate, a non-competitive
antagonist of the NMDA receptor, inhibited chondrocyte apoptosis
and attenuated cartilage degeneration and synovitis (35). In patients undergoing arthroscopic
knee surgery, intra-articular magnesium sulfate injection
alleviated postoperative pain (36). In addition, previous studies
revealed altered NMDA receptor signaling in OA mediated by specific
NMDA receptor, NMDA receptor subtype 2B subunit expression in
chondrocytes (37). However,
clinical applications of NMDA receptor blockers in managing and
treating OA is limited by serious side-effects, such as
hallucinations, memory loss and motor function defects. As an
antagonist and allosteric inhibitor of the NMDA receptor, ketamine
has been widely administered in clinical practice. In addition to
conventional anesthetic and analgesic effects, it manifests
anti-inflammatory activity, preserves brain function and relieves
bronchial spasm (21,38). Furthermore, intra-articular
injection generally requires smaller doses and the plasma
concentration of ketamine is particularly low, which greatly
reduces the incidence of adverse reactions in the central nervous
system (39). Ketamine appears to
be a strong candidate for treating OA. Sakai et al (22) revealed that
lipopolysaccha-ride-induced NF-κB activation was inhibited by
pre-treatment with 0.1–1 µmol/l ketamine in A172 human
glioblastoma cells. In vivo experiments revealed efficient
inhibition of NF-κB activity with an intravenous ketamine dose of
2.5–10 mg/kg, resulting in a plasma concentration of 60–80
µmol/l, which is similar to the dosage used in clinical
practice (40,41). To the best of our knowledge, the
present study represents the first investigation of ketamine in the
potential treatment of OA in a rabbit model, and three different
doses of ketamine were investigated. The results indicated that the
anti-inflammatory effect of ketamine in OA was dose-dependent, and
the highest efficiency was observed at a 200-µmol/l
intra-articular dose. Further studies are required to determine the
duration of the anti-inflammatory effects of ketamine and assess
its long-term effects.
The findings of the current study demonstrated the
efficacy of targeting inflammatory mediators in OA pathogenesis.
TNF-α has been demonstrated to be essential in cartilage
degeneration. Synovitis usually prevails in the early stages of OA,
and TNF-α is detected in the majority of synovial fluid samples,
indicating that TNF-α is one of the earliest inflammatory cytokines
involved in OA onset (7,42). TNF-α activates multinucleated giant
cells and stimulates synovial cells to produce prostaglandins and
other inflammatory mediators, contributing to matrix degradation,
chondrocyte apoptosis and cartilage metabolic disorder; thus,
resulting in the pathogenesis and progression of OA (43). In the current study, TNF-α was not
detected in the normal control rabbits, but high expression levels
of TNF-α were identified in the synovial fluid of OA rabbits.
Ketamine effectively reduced the concentration of the inflammatory
cytokine, TNF-α. Similarly, NF-κB activation is considered to be
important in mediating inflammatory responses associated with OA.
Previous studies have evaluated the role of NF-κB inhibitors in
preventing chondrocyte apoptosis and ameliorating articular
cartilage damage; however, the efficacy of these inhibitors has not
been extensively investigated in OA animal models (44). The current results revealed an
inverse association between ketamine injection and NF-κB p65
expression levels in cartilage samples from OA rabbits,
demonstrating the potential of ketamine administration to regulate
the NF-κB signaling pathway and thus preventing OA development.
IL-10 is a major anti-inflammatory cytokine that
modulates inflammatory responses in various types of disease,
including multiple sclerosis, systemic lupus erythematosus and
inflammatory bowel disease (45,46).
Furthermore, IL-10 directly inhibits the production of
pro-inflammatory cytokines (47),
including TNF-α in the joint cavity in multiple experimental models
of arthritic animals (48,49). In the OA rabbit model of the
present study, IL-10 expression levels in the synovial fluid were
significantly reduced; however, upon ketamine treatment, the IL-10
levels increased in a dose-dependent manner. The underlying
molecular mechanism, however, remains unknown and further
investigation is required.
In conclusion, the pathogenesis of OA is a
particularly complicated process, which is mediated by chondrocyte
apoptosis, matrix destruction, cartilage degeneration, metabolic
disorder and altered signal transduction. The results of the
current study provide support for anti-inflammatory therapeutic
strategies, using ketamine, for the treatment of OA. However,
additional investigations are required to establish the optimal
therapeutic dosage of ketamine in OA, and to elucidate the
molecular mechanisms underlying the regulation of inflammation.
Controlled multicenter clinical trials are required to provide an
evidence-based therapeutic strategy for possible clinical
application.
Acknowledgments
The present study was supported by the Science and
Technology Foundation of Guizhou Province, China [grant no.
(2011)2244].
References
|
1
|
Felson DT, Naimark A, Anderson J, Kazis L,
Castelli W and Meenan RF: The prevalence of knee osteoarthritis in
the elderly. The framingham osteoarthritis study. Arthritis Rheum.
30:914–918. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Xu L, Nevitt MC, Aliabadi P, Yu
W, Qin M, Lui LY and Felson DT: Comparison of the prevalence of
knee osteoarthritis between the elderly Chinese population in
Beijing and whites in the United States: The Beijing osteoarthritis
study. Arthritis Rheum. 44:2065–2071. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuettner KE and Cole AA: Cartilage
degeneration in different human joints. Osteoarthritis Cartilage.
13:93–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelletier JP, Martel-Pelletier J and
Abramson SB: Osteoarthritis, an inflammatory disease: Potential
implication for the selection of new therapeutic targets. Arthritis
Rheum. 44:1237–1247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abramson SB, Attur M, Amin AR and Clancy
R: Nitric oxide and inflammatory mediators in the perpetuation of
osteoarthritis. Curr Rheumatol Rep. 3:535–541. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mcinnes IB and Liew FY: Cytokine
networks-towards new therapies for rheumatoid arthritis. Nat Clin
Pract Rheumatol. 1:31–39. 2005. View Article : Google Scholar
|
|
7
|
Smith MD, Triantafillou S, Parker A,
Youssef PP and Coleman M: Synovial membrane inflammation and
cytokine production in patients with early osteoarthritis. J
Rheumatol. 24:365–371. 1997.PubMed/NCBI
|
|
8
|
Dougados M, Combe B, Braun J, Landewé R,
Sibilia J, Cantagrel A, Feydy A, Van Der Heijde D, Leblanc V and
Logeart I: A randomised, multicentre, double-blind,
placebo-controlled trial of etanercept in adults with refractory
heel enthesitis in spondyloarthritis: The HEEL trial. Ann Rheum
Dis. 69:1430–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fioravanti A, Fabbroni M, Cerase A and
Galeazzi M: Treatment of erosive osteoarthritis of the hands by
intra-articular infliximab injections: A pilot study. Rheumatol
Int. 29:961–965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Louthrenoo W, Nilganuwong S, Aksaranugraha
S, Asavatanabodee P and Saengnipanthkul S; Thai Study Group: The
efficacy, safety and carry-over effect of diacerein in the
treatment of painful knee osteoarthritis: A randomised,
double-blind, NSAID-controlled study. Osteoarthritis Cartilage.
15:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Skerry TM and Genever PG: Glutamate
signalling in non-neuronal tissues. Trends Pharmacol Sci.
22:174–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hinoi E, Takarada T, Ueshima T,
Tsuchihashi Y and Yoneda Y: Glutamate signaling in peripheral
tissues. Eur J Biochem. 271:1–13. 2004. View Article : Google Scholar
|
|
13
|
Kalariti N, Pissimissis N and Koutsilieris
M: The glutamatergic system outside the CNS and in cancer biology.
Expert Opin Investig Drugs. 14:1487–1496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mcnearney T, Baethge BA, Cao S, Alam R,
Lisse JR and Westlund KN: Excitatory amino acids, TNF-alpha, and
chemokine levels in synovial fluids of patients with active
arthropathies. Clin Exp Immunol. 137:621–627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laube B, Hirai H, Sturgess M, Betz H and
Kuhse J: Molecular determinants of agonist discrimination by NMDA
receptor subunits: Analysis of the glutamate binding site on the
NR2B subunit. Neuron. 18:493–503. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flood S, Parri R, Williams A, Duance V and
Mason D: Modulation of interleukin-6 and matrix metalloproteinase 2
expression in human fibroblast-like synoviocytes by functional
ionotropic glutamate receptors. Arthritis Rheum. 56:2523–2534.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lawand NB, Willis WD and Westlund KN:
Excitatory amino acid receptor involvement in peripheral
nociceptive transmission in rats. Eur J Pharmacol. 324:169–177.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bondok RS and Abd El-Hady AM:
Intra-articular magnesium is effective for postoperative analgesia
in arthroscopic knee surgery. Br J Anaesth. 97:389–392. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee CH, Wen ZH, Chang YC, Huang SY, Tang
CC, Chen WF, Hsieh SP, Hsieh CS and Jean YH: Intra-articular
magnesium sulfate (MgSO4) reduces experimental osteoarthritis and
nociception: Association with attenuation of N-methyl-D-aspartate
(NMDA) receptor subunit 1 phosphorylation and apoptosis in rat
chondrocytes. Osteoarthritis Cartilage. 17:1485–1493. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Irifune M, Shimizu T, Nomoto M and Fukuda
T: Ketamine-induced anesthesia involves the N-methyl-D-aspartate
receptor-channel complex in mice. Brain Res. 596:1–9. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loix S, De Kock M and Henin P: The
anti-inflammatory effects of ketamine: State of the art. Acta
Anaesthesiol Belg. 62:47–58. 2011.PubMed/NCBI
|
|
22
|
Sakai T, Ichiyama T, Whitten CW, Giesecke
AH and Lipton JM: Ketamine suppresses endotoxin-induced NF-kappaB
expression. Can J Anaesth. 47:1019–1024. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gokcinar D, Ergin V, Cumaoglu A, Menevse A
and Aricioglu A: Effects of ketamine, propofol, and ketofol on
proinflammatory cytokines and markers of oxidative stress in a rat
model of endotoxemia-induced acute lung injury. Acta Biochim Pol.
60:451–456. 2013.PubMed/NCBI
|
|
24
|
Videman T: Experimental osteoarthritis in
the rabbit: Comparison of different periods of repeated
immobilization. Acta Orthop Scand. 53:339–347. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pelletier JP, Jovanovic D, Fernandes JC,
Manning P, Connor JR, Currie MG, Di Battista JA and
Martel-Pelletier J: Reduced progression of experimental
osteoarthritis in vivo by selective inhibition of inducible nitric
oxide synthase. Arthritis Rheum. 41:1275–1286. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteoarthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|
|
27
|
Langenskiöld A, Michelsson JE and Videman
T: Osteoarthritis of the knee in the rabbit produced by
immobilization. Attempts to achieve a reproducible model for
studies on pathogenesis and therapy. Acta Orthop Scand. 50:1–14.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Felson DT: Developments in the clinical
understanding of osteoarthritis. Arthritis Res Ther. 11:2032009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sokolove J and Lepus CM: Role of
inflammation in the pathogenesis of osteoarthritis: Latest findings
and interpretations. Ther Adv Musculoskelet Dis. 5:77–94. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldring MB and Goldring SR: Articular
cartilage and subchondral bone in the pathogenesis of
osteoarthritis. Ann N Y Acad Sci. 1192:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woessner JF Jr and Gunja-Smith Z: Role of
metalloproteinases in human osteoarthritis. J Rheumatol Suppl.
27:99–101. 1991.PubMed/NCBI
|
|
32
|
Blaney Davidson EN, Scharstuhl A, Vitters
EL, Van Der Kraan PM and Van Den Berg WB: Reduced transforming
growth factor-beta signaling in cartilage of old mice: Role in
impaired repair capacity. Arthritis Res Ther. 7:R1338–R1347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okazaki R, Sakai A, Ootsuyama A, Sakata T,
Nakamura T and Norimura T: Apoptosis and p53 expression in
chondrocytes relate to degeneration in articular cartilage of
immobilized knee joints. J Rheumatol. 30:559–566. 2003.PubMed/NCBI
|
|
34
|
Kojima S, Hoso M, Watanabe M, Matsuzaki T,
Hibino I and Sasaki K: Experimental joint immobilization and
remobilization in the rats. J Phys Ther Sci. 26:865–871. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee CH, Wen ZH, Chang YC, Huang SY, Tang
CC, Chen WF, Hsieh SP, Hsieh CS and Jean YH: Intra-articular
magnesium sulfate (MgSO4) reduces experimental osteoarthritis and
nociception: Association with attenuation of N-methyl-D-aspartate
(NMDA) receptor subunit 1 phosphorylation and apoptosis in rat
chondrocytes. Osteoarthritis Cartilage. 17:1485–1493. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bondok RS and Abd El-Hady AM:
Intra-articular magnesium is effective for postoperative analgesia
in arthroscopic knee surgery. Br J Anaesth. 97:389–392. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ramage L, Martel MA, Hardingham GE and
Salter DM: NMDA receptor expression and activity in osteoarthritic
human articular chondrocytes. Osteoarthritis Cartilage.
16:1576–1584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Agrawal A and Shrivastava J: Intravenous
ketamine for refractory bronchospasm precipitated by H1N1
infection. Front Pediatr. 2:242014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akhondzade R, Pipelzade MR, Gousheh MR,
Sarrafan N and Mahmoodi K: Comparison of the analgesic effect of
intra-articular and extra-articular injection of morphine and
ketamine compound in arthrotomy lower limb surgery under spinal
anesthesia. Pak J Med Sci. 30:942–945. 2014.PubMed/NCBI
|
|
40
|
Idvall J, Ahlgren I, Aronsen KR and
Stenberg P: Ketamine infusions: Pharmacokinetics and clinical
effects. Br J Anaesth. 51:1167–1173. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wieber J, Gugler R, Hengstmann JH and
Dengler HJ: Pharmacokinetics of ketamine in man. Anaesthesist.
24:260–263. 1975.PubMed/NCBI
|
|
42
|
Venn G, Nietfeld JJ, Duits AJ, Brennan FM,
Arner E, Covington M, Billingham ME and Hardingham TE: Elevated
synovial fluid levels of interleukin-6 and tumor necrosis factor
associated with early experimental canine osteoarthritis. Arthritis
Rheum. 36:819–826. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Botha-Scheepers S, Watt I, Slagboom E, De
Craen AJ, Meulenbelt I, Rosendaal FR, Breedveld FC, Huizinga TW and
Kloppenburg M: Innate production of tumour necrosis factor alpha
and interleukin 10 is associated with radiological progression of
knee osteoarthritis. Ann Rheum Dis. 67:1165–1169. 2008. View Article : Google Scholar
|
|
44
|
Saklatvala J: Inflammatory signaling in
cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use
of inhibitors for research into pathogenesis and therapy of
osteoarthritis. Curr Drug Targets. 8:305–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Beebe AM, Cua DJ and de Waal Malefyt R:
The role of interleukin-10 in autoimmune disease: Systemic lupus
erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth
Factor Rev. 13:403–412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tilg H, van Montfrans C, van den Ende A,
Kaser A, van Deventer SJ, Schreiber S, Gregor M, Ludwiczek O,
Rutgeerts P, Gasche C, et al: Treatment of Crohn's disease with
recombinant human interleukin 10 induces the proinflammatory
cytokine interferon gamma. Gut. 50:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
de Waal Malefyt R, Abrams J, Bennett B,
Figdor CG and De Vries JE: Interleukin 10(IL-10) inhibits cytokine
synthesis by human monocytes: An autoregulatory role of IL-10
produced by monocytes. J Exp Med. 174:1209–1220. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lubberts E, Joosten LA, Van Den Bersselaar
L, Helsen MM, Bakker AC, Xing Z, Richards CD and Van Den Berg WB:
Intra-articular IL-10 gene transfer regulates the expression of
collagen-induced arthritis (CIA) in the knee and ipsilateral paw.
Clin Exp Immunol. 120:375–383. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lechman ER, Jaffurs D, Ghivizzani SC,
Gambotto A, Kovesdi I, Mi Z, Evans CH and Robbins PD: Direct
adenoviral gene transfer of viral IL-10 to rabbit knees with
experimental arthritis ameliorates disease in both injected and
contralateral control knees. J Immunol. 163:2202–2208.
1999.PubMed/NCBI
|