Introduction
Premature ovarian failure (POF) occurs in women
before the age of 40, and is characterized by amenorrhea,
infertility, low estrogen levels, high gonadotropin levels and a
lack of mature follicles (1–4). POF
is a common cause of female infertility, the pathogenesis of which
is complex. Various defects, including genetic mutations,
autoimmune disorders, infections and endocrine dysfunction may
result in POF (1–4). Furthermore, patients with POF often
exhibit abnormal karyotypes, follicle-stimulating hormone (FSH)
abnormalities, autoimmune ovarian failure and ovarian reserve
depletion (1–4). The mechanisms underlying POF are
largely unknown; however, previous studies have demonstrated that a
decline in ovarian reserve is closely associated with the
occurrence and development of POF (1–4).
Apoptosis of ovarian granulosa cells (OGCs) is an important
mechanism underlying the decline of ovarian reserve and function
(DORF). OGCs have a supporting and estrogen-mediated regulatory
role in the maturation and development of oocytes, and maintain
hormonal balance in the ovarian niche, in order to promote oocyte
maturation via autocrine and paracrine mechanisms (1–4).
Previous studies have indicated that when OGCs undergo a certain
degree of apoptosis, ovarian atresia is stimulated, which leads to
DORF and POF (2–4). Therefore, in order to maintain
ovarian function, oocyte viability and normal ovulation, high
quality OGCs are required. In 2006, Takahashi and Yamanaka reported
that induced pluripotent stem cells (iPSCs) could be induced to
differentiate from mouse fibroblasts via the addition of four
transcription factors: SRY (sex determining region Y)-box 2 (Sox2),
octamer-binding transcription factor 4 (Oct4), Kruppel-like factor
4 and c-Myc (5). iPSCs share
salient characteristics with embryonic stem cells; however, they
are generated by reprogramming somatic cells through the forced
expression of key transcription factors (2,3,5–9).
iPSCs are capable of self-renewal and pluripotent differentiation,
and express specific biomarkers, including stage-specific embryonic
antigen-4 (SSEA), Oct4, Sox2 and Nanog. In addition, iPSCs express
alkaline phosphatase (AKP) and telomerase at high levels (5,9).
iPSCs not only provide a valuable model for the in vitro
study of the regulation and underlying mechanisms of cell
development, but also provide abundant material for cellular
genetic research (2,3,5–9). In
our previous study, microRNA-17-3p was used to guide the
differentiation of human iPSCs into hormone-sensitive ovarian
surface epithelium (OSE)-like cells in vitro. Ovarian weight
and plasma estradiol (E2) levels were increased over time following
transplantation of these OSE-like cells into a mouse model of POF.
Furthermore, these OSE-like cells survived within the POF mouse
ovaries for ≥14 days, and expressed cytokeratin 7 and estrogen
receptor β proteins (3). These
findings suggested that iPSCs may provide a significant
investigational resource for emerging concepts in cell therapy and
regenerative medicine by exploitation of their pluripotent
characteristics. In the present study, human iPSCs were induced to
differentiate into ovarian granulosa-like cells (OGLCs) in
vitro. Subsequently, these cells were transplanted into POF
mice. The present study aimed to evaluate the therapeutic potential
of such cells for the treatment of POF at the pathological and
molecular level.
Materials and methods
Human iPSCs culture and
differentiation
DYR0100 human iPSCs (SCSP-1301) were obtained from
Professor Xiaoyan Ding (Stem Cell Bank, Chinese Academy of
Sciences, Shanghai, China). All experiments using DYR0100 iPSCs
were approved by the Shanghai University of Traditional Chinese
Medicine Research Ethics Board (Shanghai, China). The DYR0100 iPSCs
were cultured in Dulbecco's modified Eagle's medium:F12 (1:1)
supplemented with 15% KnockOut™ Serum Replacement, 1 mM sodium
pyruvate, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 0.1 mM
beta-mercaptoethanol, penicillin (25 U/ml)-streptomycin (925
mg/ml), Reconstituting Human Basic Fibroblast Growth Factor (15
ng/ml), Reconstituting Human Epidermal Growth Factor (15 ng/ml).
The aforementioned cell culture reagents were purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells were
incubated in a humidified incubator containing 5% CO2 at
37°C. DYR0100 iPSCs were induced to differentiate into OGLCs for 12
days, according to a multistage protocol using appropriate cell
growth factors and hormones (Table
I). Briefly, the iPS cells were stimulated with
all-trans-retinoic acid (atRA) for 48 h, then atRA was removed. The
iPS cells were cultured in fresh medium supplemented with E2, FSH
and human growth hormone (hGH) for 48 h. The iPS cells were then
cultured in fresh medium supplemented with anti-Müllerian hormone
(AMH), estradiol, FSH and hGH for 48 h. Finally, the iPS cells were
cultured in fresh medium supplemented with AMH, estradiol, inhibin
α, inhibin β and transforming growth factor-β for 96 h.
 | Table IHuman growth factors and hormones used
to induce differentiation. |
Table I
Human growth factors and hormones used
to induce differentiation.
| Factor | Company
(location) | Concentration
(ng/ml) |
|---|
| All-trans-retinoic
acid | Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA) | 15 |
| Estradiol | Shanghai XiTang
Biotechnology Co., Ltd., (Shanghai, China) | 20 |
| Anti-Müllerian
hormone | Shanghai XiTang
Biotechnology Co., Ltd. | 20 |
| Follicle stimulating
hormone | Shanghai XiTang
Biotechnology Co., Ltd. | 20 |
| Inhibin α | Shanghai XiTang
Biotechnology Co., Ltd. | 20 |
| Inhibin β | Shanghai XiTang
Biotechnology Co., Ltd. | 20 |
| Transforming growth
factor-β | Gibco; Thermo Fisher
Scientific, Inc. | 15 |
| Human growth
hormone | Gibco; Thermo Fisher
Scientific, Inc. | 10 |
Murine model of POF and in vivo xenograft
experiments
As described in our previous studies (2–4),
hebetic female C57BL/6 mice (n=30; age, 5 weeks) were obtained from
the Shanghai University of Traditional Chinese Medicine with
approval from the Institutional Animal Care and Use Committee. To
generate a murine model of POF, the mice were administered a single
intraperitoneal injection of 70 mg/kg cyclophosphamide
(Sigma-Aldrich, St. Louis, MO, USA). The mice were divided into two
groups: A negative control group (n=15) treated with
phosphate-buffered saline (PBS), and an experimental group (n=15)
transplanted with iPSC-derived OGLCs. A total of 1 week after the
POF models were established, each mouse received an injection of 50
µl cells (~1×107 cells/µl), which were
stained with DiI red fluorescent dye (Beyotime Institute of
Biotechnology, Hangzhou, China), or 50 µl PBS. The final
experiments in both groups were conducted 21 days
post-transplantation. All mice were sacrificed by cervical
dislocation following this treatment and additional experiments
were conducted.
AKP assay
An AKP assay kit (Beyotime Institute of
Biotechnology) was conducted, according to the manufacturer's
protocol. Briefly, the cells were washed twice with PBS and fixed
in 4% paraformaldehyde (Sigma-Aldrich) for 20 min. Dye solution was
added to the cells, which were incubated at 37°C for 60 min until
the AKP-positive cells were stained deep blue. The staining
reaction was terminated following the addition of PBS. Digital
images were captured using a fluorescence microscope (DMI3000;
Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Immunofluorescent staining
As previously described (3,4), the
cultured cells or ovarian tissue sections were washed three times
with PBS and were fixed with 4% paraformaldehyde for 30 min. After
blocking with blocking solution (Beyotime Institute of
Biotechnology), the cells were incubated with primary antibodies
(Table II) overnight at 4°C,
followed by a 30 min incubation with cyanine 3- or fluorescein
isothiocyanate-conjugated goat anti-rabbit immunoglobulin G
antibodies (1:200; cat. no. C0992; Sigma-Aldrich) and 5
µg/ml 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) at room
temperature. Subsequently, the cells and tissues were thoroughly
washed with Tris-buffered saline containing 0.5% Tween-20
(Sigma-Aldrich) and were visualized by fluorescence microscopy
(DMI3000; Leica Microsystems, Inc.).
 | Table IIPrimary antibodies, their source and
dilutions. |
Table II
Primary antibodies, their source and
dilutions.
| Antibody (cat.
no.) | Company
(location) | Dilution |
|---|
| Rabbit
anti-human/mouse Oct 4 (sc-9081) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | 1:100 |
| Rabbit
anti-human/mouse Sox2 (sc-20088) | Santa Cruz
Biotechnology, Inc. | 1:100 |
| Rabbit
anti-human/mouse Nanog (sc-33759) | Santa Cruz
Biotechnology, Inc. | 1:100 |
| Rabbit
anti-human/mouse SSEA-4 (sc-21704) | Santa Cruz
Biotechnology, Inc. | 1:100 |
| Rabbit
anti-human/mouse inhibin α (sc-30146) | Santa Cruz
Biotechnology, Inc. | 1:100 |
| Rabbit
anti-human/mouse inhibin β (sc-50288) | Santa Cruz
Biotechnology, Inc. | 1:100 |
| Rabbit
anti-human/mouse AMH (sc-67287) | Santa Cruz
Biotechnology, Inc. | 1:100 |
| Rabbit
anti-human/mouse FSHR (sc-13935) | Santa Cruz
Biotechnology, Inc. | 1:100 |
Hematoxylin-eosin (H&E) staining
As previously described (3,4),
fresh ovarian tissues were washed three times with PBS and were
fixed with 4% paraformaldehyde for 30 min. The tissue specimens
were then dehydrated in a graded ethanol series, vitrified in
xylene, and paraffin embedded. Serial 6-µm sections were
prepared and stained with H&E. Images were captured using a
DMI3000 microscope (Leica Microsystems, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
Mouse E2 and FSH ELISAs (Shanghai XiTang
Biotechnology Co., Ltd., Shanghai, China) were conducted according
to the manufacturer's protocols and our previous studies (3,4).
Mouse blood plasma was obtained by retro-orbital blood collection
(4). Briefly, a microhematocrit
tube (BD Biosciences, Franklin Lakes, NJ, USA) was inserted into
the mouse lateral canthus at 30 degree angle to the side of the
head. Approximately 100 µl blood was collected from each
mouse into heparin-coated blood collection tubes (BD Biosciences).
Mouse blood was centrifuged at 453 × g at 4°C for 10 min and the
supernatant was collected. Briefly, 100 µl mouse E2 or FSH
standards at the following concentrations: E2, 8,000, 4,000, 2,000,
1,000, 500, 250 and 125 pg/ml; FSH, 10,000, 5,000, 2,500, 1,250,
625, 312 and 156 pg/ml, or diluted mouse plasma were added to
anti-E2 or anti-FSH pre-coated microtest wells and were incubated
for 60 min. The plates were then washed three times with PBS, and
horseradish peroxidase-conjugated antibodies were added, followed
by an enzymatic substrate. Optical absorbance was determined at a
wavelength of 450 nm using a BioTek Synergy Mx plate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Statistical analysis
Each experiment was performed as least three times,
and results are presented as the mean ± standard error of the mean.
Data was analyzed using GraphPad Prism software (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA). Where applicable,
differences were evaluated by Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
iPSCs can be induced to differentiate
into OGLCs in vitro
Pluripotent stem cell biomarkers were detected prior
to induction. Cellular immunostaining detected high expression
levels of AKP, Oct4 and SSEA-4 (Fig.
1A and B). Subsequently, various cell growth factors
(transforming growth factor-β and human growth hormone) and
hormones [E2, anti-Müllerian hormone (AMH), inhibin α and inhibin
β] were used to induce iPSCs to differentiate into OGLCs at various
time points (Fig. 1C). Following
12 days of induction, morphological observations indicated that the
original clone-like iPSCs had successfully differentiated into
fibroblast-like cells (Fig. 1D).
Cell immunofluorescence indicated that prior to induction, iPSCs
expressed high levels of pluripotent stem cell markers, including
Oct4, Sox2, Nanog and SSEA-4, but did not express OGC markers,
including AMH, FSH receptor (FSHR), inhibin α and inhibin β.
Conversely, following induction, the expression levels of OGC
markers were elevated in granulosa-like cells derived from iPSCs
(Fig. 2). These results suggest
that iPSCs may be induced to differentiate into granulosa-like
cells in vitro, following treatment with a combination of
hormones and growth factors.
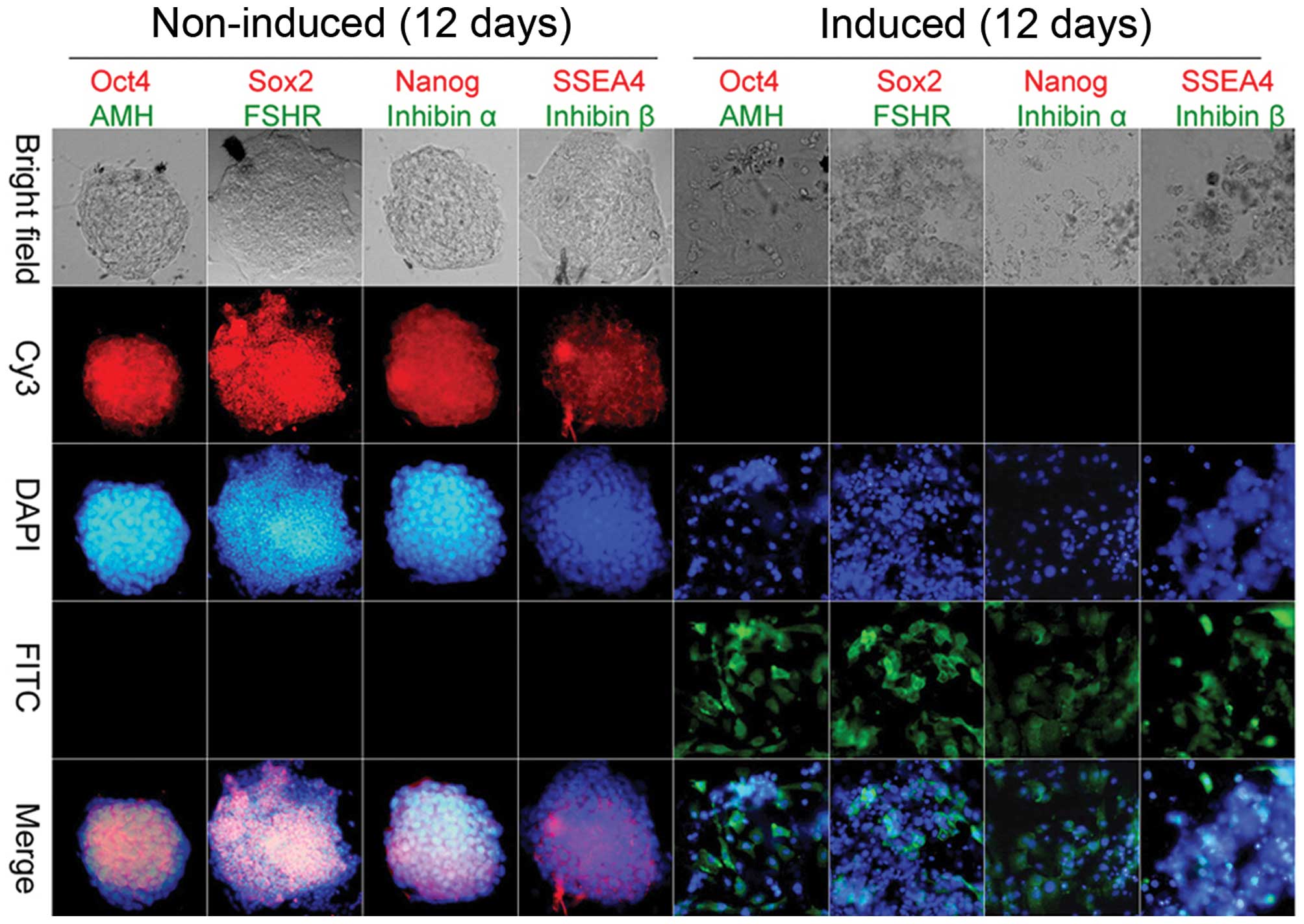 | Figure 2Immunofluorescence staining.
Immunohistochemical staining indicated that ovarian granulosa-like
cells derived from induced pluripotent stem cells strongly
expressed ovarian granulosa cells markers [anti-Müllerian hormone
(AMH), inhibin α, inhibin β and follicle-stimulating hormone
receptor (FSHR)], but not stem cell markers [octamer-binding
transcription factor 4 (Oct4), SRY (sex-determining region Y)-box 2
(Sox2), Nanog and stage-specific embryonic antigen-4 (SSEA4) 12
days post-induction. Each sample was stained for two markers with
the color of the fluorescence indicated by the color of the word.
Original magnification, ×200. Cy3, cyanine 3; DAPI,
4′,6-diamidino-2-phenylindole; FITC, fluorescein
isothiocyanate. |
OGLCs derived from iPSCs can reverse POF
in mice
A total of 21 days post-transplantation the mice
were sacrificed. In the control group (PBS-POF), ovarian
pathological analysis indicated that normal follicles were almost
absent. Ovarian stromal tissue and OGCs exhibited hallmarks of
apoptosis and atrophy. Furthermore, ovarian tissue presented
varying degrees of bleeding and serious follicular atresia
(Fig. 3A). However, compared with
the PBS-POF group, in the experimental group (OGLCs-iPSCs-POF),
ovarian pathological analysis revealed that ovarian tissue
contained some normal mature follicles, with a significant
reduction in the numbers of atretic follicles. In addition, the
growth state of the OGCs was markedly improved (Fig. 3A). Ovarian tissue weight was
increased in the OGLCs-iPSCs-POF group compared with in the PBS-POF
group (P<0.05; Fig. 3B). The
number of atretic follicles in the OGLCs-iPSCs-POF mice was
reduced, as compared with in the PBS-POF mice (P<0.05; Fig. 3C). Furthermore, detection of
hormone levels in murine peripheral blood samples indicated that
the levels of E2 were elevated in the OGLCs-iPSCs-POF group,
whereas the levels of FSH were decreased (Fig. 3D and E; Table III). Furthermore, in the
OGLCs-iPSCs-POF group, immunofluorescence indicated that
iPSC-derived OGLCs transplanted into POF mice (visualized by red
fluorescence) expressed OGC biomarkers (AMH, inhibin α, inhibin β,
FSHR; green fluorescence) in ovarian tissue (Fig. 3F). These results suggest that
iPSCs-derived OGLCs were capable of repairing ovarian injury and
enhancing ovarian function.
 | Figure 3Effects of induced pluripotent stem
cell (iPSC)-derived ovarian granulosa-like cells (OGLCs)
transplanted into a mouse model of premature ovarian failure (POF).
(A) Hematoxylin-eosin staining and pathological analysis revealed
that iPSC-derived OGLCs improved the ovarian tissue niche, reduced
the number of atretic follicles, and increased the number of normal
follicles. OGLCs-iPSCs-POF, POF mice transplanted with iPSC-induced
OGLCs (experimental group); PBS-POF, POF mice treated with PBS
(control group). Original magnification, ×200. (B) Ovarian tissue
weight was elevated in the OGLCs-iPSCs-POF group compared with in
the control group. (C) Number of atretic follicles in the
OGLCs-iPSCs-POF group was significantly reduced. (D) Plasma
estradiol (E2) levels were determined by enzyme-linked
immunosrobent assay (ELISA); E2 levels were higher in the
OGLCs-iPSCs-POF group compared with in the PBS-POF group. (E)
Plasma follicle-stimulating hormone (FSH) levels were determined by
ELISA; FSH levels were lower in the OGLCs-iPSCs-POF group compared
with in the PBS-POF group. Data are presented as the mean ±
standard error of the mean. *P<0.05 vs. the PBS-POF
group; n=15. (F) Immunofluorescence assay indicated that
iPSC-derived OGLCs transplanted into POF mice (red fluorescence)
expressed OGC biomarkers (green fluorescence) in ovarian tissue.
White arrows represent cells with red fluorescence merged with
green fluorescence. Original magnification, ×200. (G) iPSCs
induction and transplantation process. AMH, anti-Müllerian hormone;
Oct4, octamer-binding transcription factor 4; Sox2, SRY
(sex-determining region Y)-box 2; FSHR, FSH receptor; FITC,
fluorescein isothiocyanate; RFP, red fluorescent protein; DAPI,
4′,6-diamidino-2-phenylindole. |
 | Table IIIOvarian weight, hormone levels and the
number of atretic follicles. |
Table III
Ovarian weight, hormone levels and the
number of atretic follicles.
| Parameter | OGLCs-iPSCs-POF | PBS-POF |
|---|
| E2 (pg/ml) | 158.6±42.2a | 87.45±19.0 |
| FSH (pg/ml) | 435.7±184.1a | 971.9±218.5 |
| Ovarian weight
(mg) | 3.7±1.1a | 2.3±1.0 |
| Atretic
follicles | 4±2a | 9±2 |
Discussion
DORF is a typical characteristic and initial stage
of POF (1). Ovarian reserve and
function encapsulates the quantity and quality of follicles in the
ovarian cortex, follicular growth capacity, ovarian development and
oocyte formation (1–4). In addition, ovarian reserve and
function is a measure of female reproductive endocrine
functionality and fertility potential (1–4). The
quality of OGCs directly influences ovarian reserve and function,
and poor quality OGCs may eventually lead to the occurrence of POF
(1–4). OGCs are the most immediate factor
influencing ovarian function and follicular maturation (2–4). The
present study aimed to determine whether ovarian tissue
functionality can be enhanced or even restored following
transplantation of OGCs (1–4). The
present study evaluated techniques to induce differentiation of
iPSCs into OGLCs in vitro. The results indicated that iPSCs
could be induced to differentiate into OGLCs following treatment
with numerous cell growth factors and hormones. Post-induction,
iPSC-derived OGLCs exhibited the morphology of fibroblast cells,
rather than that of embryonic stem cell clones. Furthermore,
iPSC-derived OGLCs strongly expressed OGC biomarkers, but did not
express markers characteristic of iPSCs. Subsequently, iPSC-derived
OGLCs were transplanted into POF mice, and were revealed to express
OGC biomarkers in ovarian tissue, improve ovarian tissue maturation
status and further maintain follicular growth. These findings
suggested that iPSC-derived OGLCs may be capable of re-establishing
ovarian function. In addition, although the number of OGLCs
observed in the ovarian tissues of POF mice were low, they served a
role in ovarian repair. Growth hormones are crucial for the
maintenance of ovarian follicular development, including AMH,
inhibin α and inhibin β. The prevalence and maturation of follicles
is not only modulated by the pituitary gonadal axis, but is also
influenced by specific cytokines in the ovarian niche. AMH, inhibin
α and inhibin β are members of the transforming growth factor-β
superfamily, which are secreted by OGCs and exert an important role
in ovarian maturation and recruitment. In addition, iPSCs-derived
OGLCs did not induce teratoma formation and were relatively
quiescent in vivo in the POF mice (Fig. 3G).
The results of the present study indicated that
iPSC-derived OGLCs exhibited complete differentiation in
vitro and were stable when transplanted into normal mice. In
conclusion, the present study provides a novel insight regarding
the treatment of POF. iPSC-derived OGLCs were able to enhance
ovarian function in a murine model of POF, resulting in a reversal
of ovarian dysfunction and thereby promoting follicular
maturation.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81273794)
to Jin Zheng. In addition, this work was supported by grants from
the National Natural Science Foundation of China (grant no.
81202811), the Project funded by China Postdoctoral Science
Foundation (grant no. 2014M550250), and the Shanghai Municipal
Health Bureau Fund (grant no. 20124320) to Te Liu. The authors
declare no potential conflicts of interest.
References
|
1
|
Vujović S, Ivović M, Tancić-Gajić M,
Marina L, Barać M, Arizanović Z, Nenezić A, Ivanisević M, Micić J,
Sajić S and Micić D: Premature ovarian failure. Srp Arh Celok Lek.
140:806–811. 2012. View Article : Google Scholar
|
|
2
|
Liu T, Huang Y, Guo L, Cheng W and Zou G:
CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive
and proliferate in the ovary long-term in a mouse model of
chemotherapy-induced premature ovarian failure. Int J Med Sci.
9:592–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu T, Qin W, Huang Y, Zhao Y and Wang J:
Induction of estrogen-sensitive epithelial cells derived from
human-induced pluripotent stem cells to repair ovarian function in
a chemotherapy-induced mouse model of premature ovarian failure.
DNA Cell Biol. 32:685–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Huang Y, Zhang J, Qin W, Chi H,
Chen J, Yu Z and Chen C: Transplantation of human menstrual blood
stem cells to treat premature ovarian failure in mouse model. Stem
Cells Dev. 23:1548–1557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kiskinis E and Eggan K: Progress toward
the clinical application of patient-specific pluripotent stem
cells. J Clin Invest. 120:51–59. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu T, Chen Q, Huang Y, Huang Q, Jiang L
and Guo L: Low microRNA-199a expression in human amniotic
epithelial cell feeder layers maintains human-induced pluripotent
stem cell pluripotency via increased leukemia inhibitory factor
expression. Acta Biochim Biophys Sin (Shanghai). 44:197–206. 2012.
View Article : Google Scholar
|
|
8
|
Liu T, Cheng W, Huang Y, Huang Q, Jiang L
and Guo L: Human amniotic epithelial cell feeder layers maintain
human iPS cell pluripotency via inhibited endogenous microRNA-145
and increased Sox2 expression. Exp Cell Res. 318:424–434. 2012.
View Article : Google Scholar
|
|
9
|
Liu T, Zou G, Gao Y, Zhao X, Wang H, Huang
Q, Jiang L, Guo L and Cheng W: High efficiency of reprogramming
CD34+ cells derived from human amniotic fluid into
induced pluripotent stem cells with Oct4. Stem Cells Dev.
21:2322–2332. 2012. View Article : Google Scholar : PubMed/NCBI
|

















