Introduction
Mesenchymal stem cells (MSCs) are multipotent
progenitor cells that have the capacity for self-renewal and
differentiation into osteocytes, adipocytes and other cells
(1). MSCs have been used in
preclinical models for tissue engineering of bone, cartilage,
muscle, marrow stroma, tendon, fat and other connective tissues
(2–4). In addition, MSCs secrete a broad
spectrum of bioactive macromolecules that are immunomodulatory and
serve to structure regenerative microenvironments in fields of
tissue injury (5). Human
mesenchymal stem cells have previously been isolated and
characterized from the gingiva, and gingiva-derived stem cells have
been applied for tissue engineering purposes (6). Moreover, stem cells may be obtained
intraorally, and gingiva is a readily accessible tissue source with
a relatively high quantity of obtainable tissue (7).
Tacrolimus is a 23-member macrolide lactone
(molecular weight, 803.5 Da) with potent immunosuppressive activity
that is effective in the prophylaxis of organ rejection following
liver, heart, kidney and small bowel transplantation (8). Tacrolimus has a narrow therapeutic
window (9) and it is important to
choose the right dose (10).
Clinically, the doses of tacrolimus administered following kidney,
heart and liver transplantation are 0.15–0.3, 0.05–0.075 and
0.10–0.2 mg/kg/day, respectively (11–14).
In addition, whole blood tacrolimus concentrations were observed to
be 10–15 ng/ml during months 1–3 and 5–12 ng/ml during months 4–12
with the initial tacrolimus dose of 0.15–0.2 mg/kg/day (14,15).
Tacrolimus has been shown to exert a variety of
actions on bone metabolism (16).
It was reported that tacrolimus causes bone loss when administered
systemically (17,18). However, other studies have shown
that tacrolimus promotes osteogenic differentiation (19,20).
Conversely, another study demonstrated that tacrolimus was not
associated with osteogenic differentiation of human heart-derived
MSCs (21).
Limited information is currently available regarding
the effects of tacrolimus on dental tissue, and none is available
on its effects on human mesenchymal stem cells derived from the
gingiva. The aim of the present study was to evaluate the effects
of a broad range of concentrations of tacrolimus on the morphology
and viability of human stem cells derived from the gingiva.
Materials and methods
Isolation and culture of stem cells
derived from the gingiva
Healthy gingival tissue samples were collected from
healthy patients (mean age, 51.8±18.1 years; 2 male and 2 female)
and this study was reviewed and approved by the Institutional
Review Board of Seoul St. Mary's Hospital, College of Medicine, The
Catholic University of Korea (Seoul, Korea; approval no.
KC11SISI0348); informed consent was obtained from all patients. The
resected gingival tissues were immediately placed in sterile
phosphate-buffered saline (PBS; Welgene Inc., Daegu, Korea) with
100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) at 4°C. The gingival tissue was
de-epithelialized, minced into 1–2 mm2 fragments, and
digested in 0.2 µm filtered α-Minumum Essential Medium (MEM;
HyClone; GE Healthcare Life Sciences, Chalfont, UK) with
collagenase IV (Sigma-Aldrich). The cells were incubated at 37°C in
a humidified incubator with 5% CO2 and 95%
O2. After 24 h, the non-adherent cells were washed with
PBS, and adherent cell were administered fresh medium and replaced
every 2–3 days. A previous report demonstrated that these cells
showed colony-forming abilities, plastic adherence, and
multilineage differentiation (osteogenic, adipogenic, chondrogenic)
potency (22). The cells expressed
CD44, CD73, CD90, and CD105, but did not express CD14, CD45, CD34,
and CD19 in flow cytometry (22).
Evaluation of cellular morphology
The cells were plated at a density of
2.0×103 cells/well in 96-well plates. The cells were
incubated in α-MEM containing 15% fetal bovine serum (Gibco; Thermo
Fisher Scientific Inc., Waltham, MA, USA), 100 U/ml penicillin and
100 µg/ml streptomycin in the presence of tacrolimus at
final concentrations ranging of 0 (control), 0.001, 0.01, 0.1, 1,
10 and 100 µg/ml. Tacrolimus was dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich) and filter-sterilized. Equal
quantities of DMSO were added to each culture sample to offset the
influence of this dissolving vehicle. The morphology of the cells
was viewed under an inverted microscope (Leica DM IRM, Leica
Microsystems, Wetzlar, Germany) on days 1, 3, 5 and 7. The images
were saved as JPEGs.
Determination of cell viability
The cell viability analysis was performed on days 1,
3, 5 and 7. 2-(2-methoxy-4-nitrophe
nyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H tetrazolium,
monosodium salt (WST-8 from Cell Counting kit-8; Dojindo, Tokyo,
Japan) was added to the culture and the cells were incubated for 3
h at 37°C. Viable cells were identified using the CCK-8 assay,
which relies on the ability of mitochondrial dehydrogenases to
oxidize WST-8 to a formazan product. The spectrophotometric
absorbance at 450 nm was measured using a microplate reader (BioTek
Instruments Inc., Winooski, VT, USA). The tests were performed in
triplicate.
Alizarin Red S staining
Cell cultures obtained on days 5 and 7 were washed
twice with PBS, fixed with 70% ethanol and rinsed twice with
deionized water. Cultures were stained with Alizarin Red S (Wako
Pure Chemical Industries, Ltd., Osaka, Japan) and evaluated with a
microscope (Leica DM IRB; Leica Microsystems). To remove
non-specifically bound stain, cultures were washed three times with
deionized water and once with PBS for 15 min at ambient
temperature. Bound dye was solubilized in 10 mM sodium phosphate
containing 10% cetylpyridinium chloride (Sigma-Aldrich) and
quantified spectrophotometrically at 560 nm (PowerWave XS2; BioTek
Instruments, Inc., Winooski, VT, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation of the experiments. A one-way analysis of variance with
post-hoc test was performed to determine the differences between
the groups using a commercially available program (SPSS 12 for
Windows, SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Evaluation of cell morphology
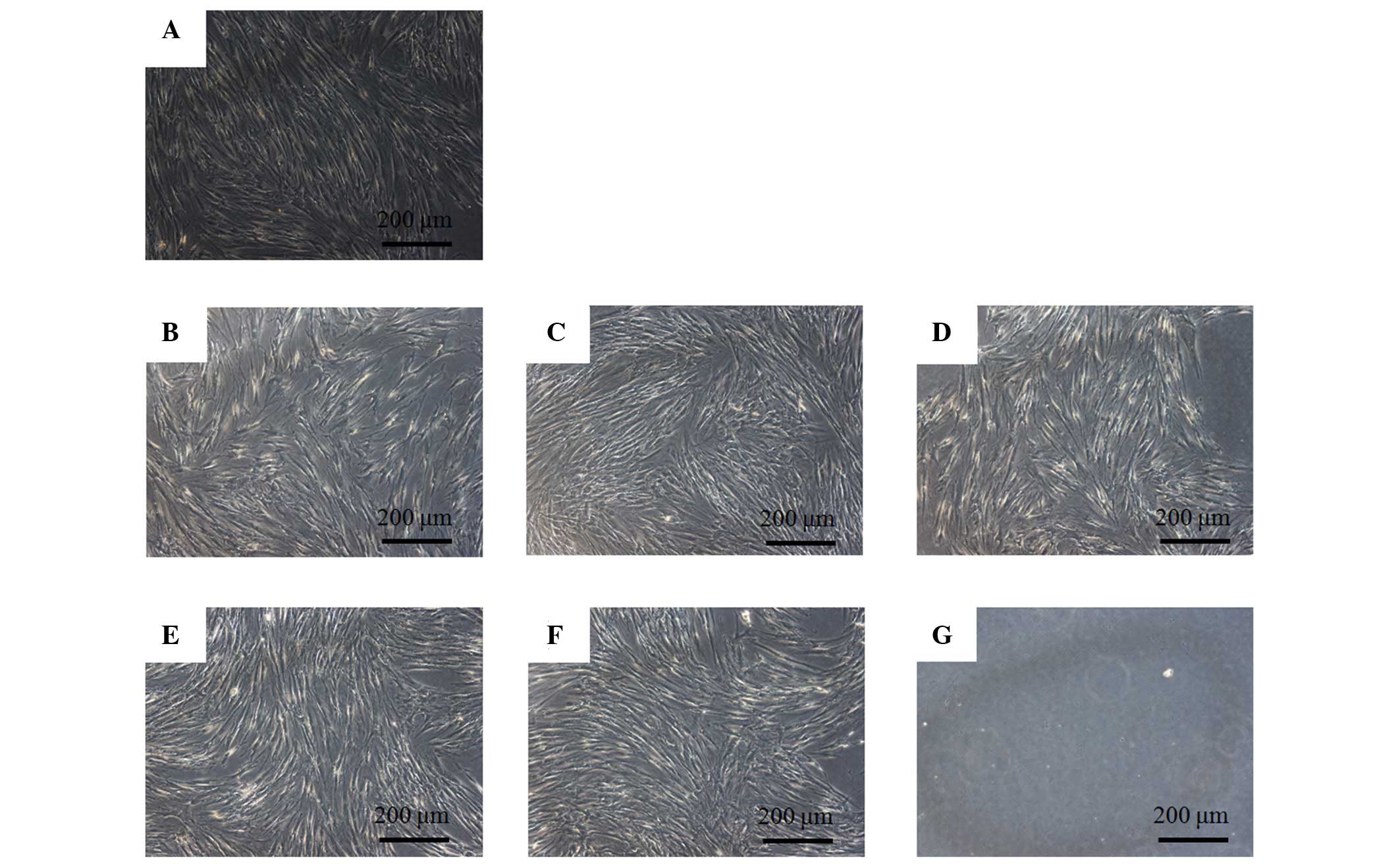
The control group showed normal fibroblast
morphology on day 1 (Fig. 1). The
shapes of the cells treated with 0.001, 0.01, 0.1, 1 and 10
µg/ml tacrolimus were similar to those of the control group.
However, the 100 µg/ml group was markedly different when
compared with the control group. The shapes of the cells in the 100
µg/ml group were rounder, and fewer cells were present. The
morphology of the cells on day 3 is shown in Fig. 2. An increased number of cells were
observed in each group and the shapes of the cells in the tested
groups were similar to those in the control group. The results for
cells on days 5 and 7 are shown in Figs. 3 and 4, respectively. Noticeable differences
were observed in the 100 µg/ml group.
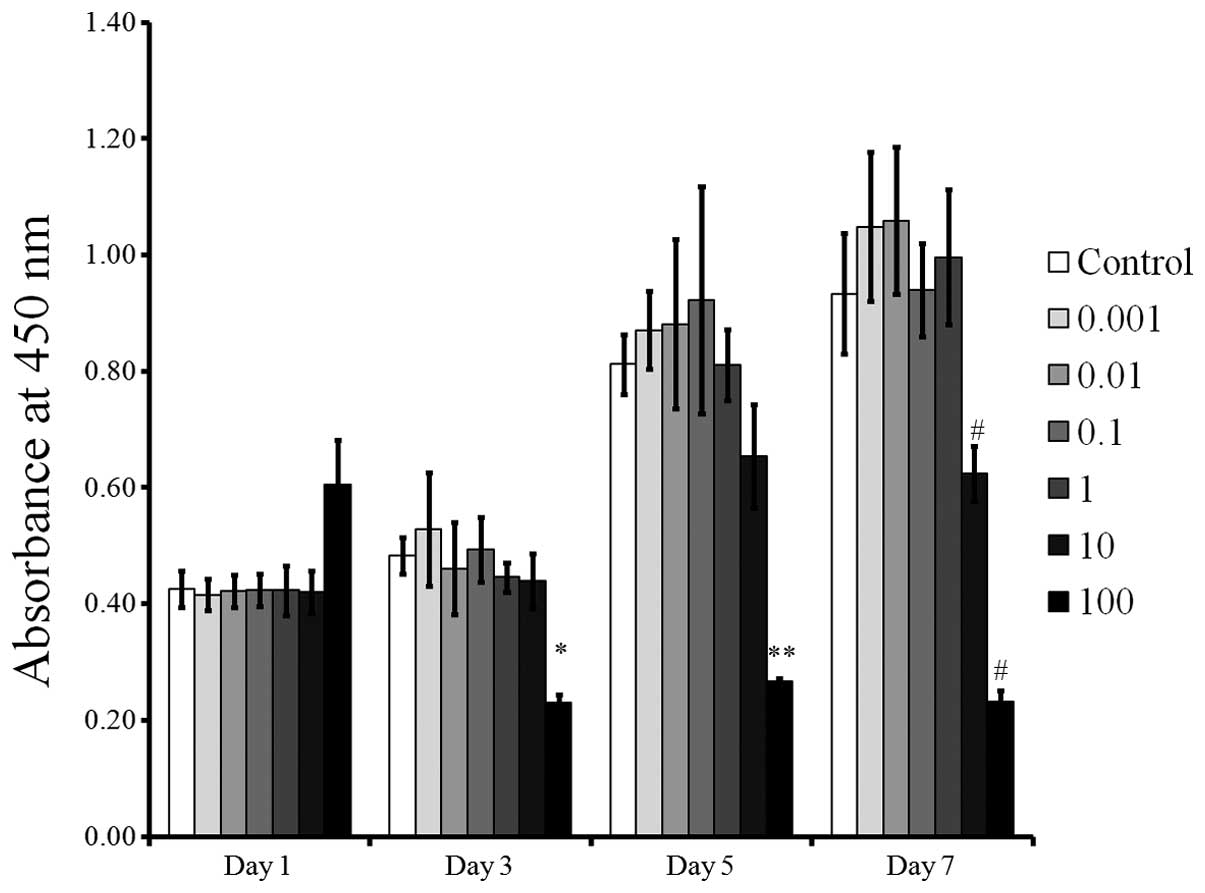
Cellular viability
The CCK-8 results demonstrated cellular viability on
days 1, 3, 5 and 7 are shown in Fig.
5. All groups except the 10 and 100 µg/ml group showed
relatively increased cell proliferation over time. The cultures
grown in the presence of tacrolimus at 0.001, 0.01, 0.1, 1 and 10
µg/ml did not show any statistically significant differences
compared with the control at days 1, 3 and 5 using the CCK-8 assays
(P>0.05). However, growth in the presence of tacrolimus at a
concentration of 100 µg/ml resulted in decreases in the
CCK-8 values at days 3, 5 and 7 (P<0.05).
Mineralization assay
Mineralized extracellular deposits were minimally
observed after Alizarin Red S staining on day 5 (Fig. 6). Increased mineralized deposits
were noted on day 7 (Fig. 7). The
quantitative results regarding bound dye on days 5 and 7 are shown
in Fig. 8. The cultures grown in
the presence of 0.001, 0.01, 0.1 and 1 µg/ml tacrolimus
exhibited increased mineralized deposits compared with the control
on day 5. The relative values of the mineralization at 0.001, 0.01,
0.1 and 1 µg/ml of tacrolimus were 110.9±10.7%, 102.1±8.4%,
106.5±8.0%, and 111.8±6.7%, respectively when the result of the
control group on day 5 was considered to be 100% (100.0±9.1%). A
significant decrease in mineralization was observed in the 100
µg/ml group on day 5 in comparison with the control group
(P<0.05). The results for day 7 showed that treatment with
tacrolimus at concentrations 0.001–1 µg/ml led to increase
mineralized deposits compared with the control group. The relative
value of the mineralization at 0.001, 0.01, 0.1 and 1 µg/ml
of tacrolimus were 118.0±11.2, 123.6±12.3, 118.3±4.5 and
104.4±5.9%, respectively, when the result of the control group on
day 7 was considered 100% (100.0±5.4%). A statistically significant
decrease was observed in the 100 µg/ml group on day 7 in
comparison with the control group (P<0.05).
Discussion
Immunosuppressants have provided great improvement
in organ transplantation by suppressing the rejection of
allografts; this has increased the survival rate of organ
transplant patients (23).
Tacrolimus (FK506) is a widely-used, well-known immunosuppressant
used following kidney or heart transplantation, and has been
recognized as effective in promoting the growth of bone grafts
(24). The present study was
performed in order to investigate the effects of tacrolimus on
proliferation and osteoblastic differentiation of mesenchymal stem
cells in vitro.
This study determined the effects of tacrolimus on
the morphology, cell viability and mineralization following
treatment with 0.001–100 µg/ml tacrolimus. The cells exposed
to tacrolimus at concentrations of 0.001–10 µg/ml exhibited
a similar fibroblastic spindle shape. Short-term application of
tacrolimus did not result in morphologic changes at final
concentrations ranging from 0.001 to 10 µg/ml.
Cellular viability was determined using a CCK-8
assay, which is based on mitochondrial enzyme reduction of the
WST-8 and spectrophotometric quantification of the water-soluble
formazan dye generated (25). This
study showed that tacrolimus at the tested concentrations was not
identified to exhibit a significant effect on the viability of stem
cells derived from the gingiva at final concentrations ranging from
0.001 to 10 µg/ml. However, tacrolimus at 100 µg/ml
decreased cell viability on days 3, 5 and 7.
Osteogenic differentiation can be evaluated by
Alizarin red S staining (26). The
presence of calcium in cellular deposits was confirmed by Alizarin
Red S staining with cetylpyridinium chloride for quantification
(27). Tacrolimus at 0.001, 0.01
and 1 µg/ml resulted in the highest degree of mineralized
nodule formation on day 7. A previous study showed that tacrolimus
at 0.04 and 0.4 µg/ml enhanced osteoblastic differentiation
of rat mesenchymal stem cells (20). Co-stimulation with tacrolimus (1.0
µg/ml) and bone morphogenetic protein-9 (100 ng/ml) induced
marked osteoblastic differentiation of dedifferentiated fat cells,
which were isolated from mature adipocytes using the ceiling
culture method and exhibit similar characteristics to mesenchymal
stem cells (28). In the present
study, the highest differentiation was achieved at 0.01
µg/ml; however, a previous study demonstrated that
remarkable osteoblast differentiation was achieved at 1.0
µg/ml (20). The
differences may be explained by the different type and stage of the
cells, the culturing time period and the culture system (29,30).
A previous study demonstrated that tacrolimus
promoted the early stage of osteoinduction (24). The mechanism underlying osteogenic
differentiation has not yet been fully elucidated; however, it has
been suggested that the osteogenic effect of tacrolimus may involve
bone morphogenetic protein signaling (16). A previous report suggested that
tacrolimus promoted osteogenic differentiation by activating BMP
receptors through interacting with FK506-binding protein 12
(16). Tacrolimus enhanced the
positive effects of bone morphogenetic proteins on alikaline
phosphatase activity and osteocalcin in mRNA (19). The effects of the combination
treatment with bone morphogenetic protein and tacrolimus acted in a
dose- and time-dependent manner (19).
In conclusion, the present study demonstrated that
treatment with tacrolimus at the tested concentrations ranging from
0.001 to 10 µg/ml did not result in significant changes in
the viability of stem cells derived from gingiva, but increased
osteogenic differentiation of the stem cells. Further studies
related to this phenomenon in an in vivo model are necessary
in order to ascertain greater understanding.
Acknowledgments
This study was supported by the 2015 Yeungnam
University, Basic Science Research Program (grant nos.
2014R1A1A1002536 and 2015R1A2A2A04005616) and the Medical Research
Center Program (grant no. 2015R1A5A2009124) through the National
Research Foundation of Korea (NRF) funded by the Ministry of
Science, ICT and Future Planning.
References
|
1
|
Beyer Nardi N and da Silva Meirelles L:
Mesenchymal stem cells: Isolation, in vitro expansion and
characterization. Handb Exp Pharmacol:. 249–282. 2006. View Article : Google Scholar
|
|
2
|
Chen WC, Péault B and Huard J:
Regenerative translation of human blood-vessel-derived MSC
precursors. Stem Cells Int. 2015:3751872015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng S, Huang R, Wang J, Zhang S, Chen Z,
Wu S, Jiang Y, Peng Q, Cai X and Lin Y: Miscellaneous animal models
accelerate the application of mesenchymal stem cells for cartilage
regeneration. Curr Stem Cell Res Ther. 9:223–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Landa-Solís C, Granados-Montiel J,
Olivos-Meza A, Ortega-Sánchez C, Cruz-Lemini M, Hernández-Flores C,
Chang-González ME, García RG, Olivos-Díaz B, Velasquillo-Martínez
MC, et al: Cryopreserved CD90+ cells obtained from mobilized
peripheral blood in sheep: a new source of mesenchymal stem cells
for preclinical applications. Cell Tissue Bank. July 29–2015.Epub
ahead of print.
|
|
5
|
Caplan AI: Adult mesenchymal stem cells
for tissue engineering versus regenerative medicine. J Cell
Physiol. 213:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SI, Yeo SI, Kim BB, Ko Y and Park JB:
Formation of size-controllable spheroids using gingiva-derived stem
cells and concave microwells: Morphology and viability tests.
Biomed Rep. 4:97–101. 2016.PubMed/NCBI
|
|
7
|
Jeong SH, Lee JE, Kim BB, Ko Y and Park
JB: Evaluation of the effects of Cimicifugae Rhizoma on the
morphology and viability of mesenchymal stem cells. Exp Ther Med.
10:629–634. 2015.PubMed/NCBI
|
|
8
|
Tamura S, Ohike A, Ibuki R, Amidon GL and
Yamashita S: Tacrolimus is a class II low-solubility
high-permeability drug: The effect of P-glycoprotein efflux on
regional permeability of tacrolimus in rats. J Pharm Sci.
91:719–729. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scholten EM, Cremers SC, Schoemaker RC,
Rowshani AT, van Kan EJ, den Hartigh J, Paul LC and de Fijter JW:
AUC-guided dosing of tacrolimus prevents progressive systemic
overexposure in renal transplant recipients. Kidney Int.
67:2440–2447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Felipe CR, Garcia C, Moreira S, Olsen N,
Silva HT and Pestana OM: Choosing the right dose of new
immunossuppressive drugs for new populations: Importance of
pharmacokinetic studies. Transplant Proc. 33:1095–1096. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Hooff JP, Boots JM, van Duijnhoven EM
and Christiaans MH: Dosing and management guidelines for tacrolimus
in renal transplant patients. Transplant Proc. 31:54S–57S. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taylor DO, Barr ML, Radovancevic B,
Renlund DG, Mentzer RM Jr, Smart FW, Tolman DE, Frazier OH, Young
JB and VanVeldhuisen P: A randomized, multicenter comparison of
tacrolimus and cyclosporine immunosuppressive regimens in cardiac
transplantation: Decreased hyperlipidemia and hypertension with
tacrolimus. J Heart Lung Transplant. 18:336–345. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Busuttil RW, Klintmalm GB, Lake JR, Miller
CM and Porayko M: General guidelines for the use of tacrolimus in
adult liver transplant patients. Transplantation. 61:845–847. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Staatz CE and Tett SE: Clinical
pharmacokinetics and pharmacodynamics of tacrolimus in solid organ
transplantation. Clin Pharmacokinet. 43:623–653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laskow DA, Neylan JF III, Shapiro RS,
Pirsch JD, Vergne-Marini PJ and Tomlanovich SJ: The role of
tacrolimus in adult kidney transplantation: A review. Clin
Transplant. 12:489–503. 1998.PubMed/NCBI
|
|
16
|
Kugimiya F, Yano F, Ohba S, Igawa K,
Nakamura K, Kawaguchi H and Chung UI: Mechanism of osteogenic
induction by FK506 via BMP/Smad pathways. Biochem Biophys Res
Commun. 338:872–879. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park KM, Hay JE, Lee SG, Lee YJ, Wiesner
RH, Porayko MK and Krom RA: Bone loss after orthotopic liver
transplantation: FK 506 versus cyclosporine. Transplant Proc.
28:1738–1740. 1996.PubMed/NCBI
|
|
18
|
Stempfle HU, Werner C, Echtler S, Assum T,
Meiser B, Angermann CE, Theisen K and Gärtner R: Rapid trabecular
bone loss after cardiac transplantation using FK506
(tacrolimus)-based immunosuppression. Transplant Proc.
30:1132–1133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang L, Ebara S, Kawasaki S, Wakabayashi
S, Nikaido T and Takaoka K: FK506 enhanced osteoblastic
differentiation in mesenchymal cells. Cell Biol Int. 26:75–84.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Byun YK, Kim KH, Kim SH, Kim YS, Koo KT,
Kim TI, Seol YJ, Ku Y, Rhyu IC and Lee YM: Effects of
immunosuppressants, FK506 and cyclosporin A, on the osteogenic
differentiation of rat mesenchymal stem cells. J Periodontal
Implant Sci. 42:73–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoogduijn MJ, Crop MJ, Korevaar SS,
Peeters AM, Eijken M, Maat LP, Balk AH, Weimar W and Baan CC:
Susceptibility of human mesenchymal stem cells to tacrolimus,
mycophenolic acid and rapamycin. Transplantation. 86:1283–1291.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and
Park JB: Isolation and characterization of human mesenchymal stem
cells from gingival connective tissue. J Periodontal Res.
50:461–467. 2015. View Article : Google Scholar
|
|
23
|
Lechler RI, Sykes M, Thomson AW and Turka
LA: Organ transplantation - how much of the promise has been
realized? Nat Med. 11:605–613. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaihara S, Bessho K, Okubo Y, Sonobe J,
Kusumoto K, Ogawa Y and Iizuka T: Effect of FK506 on osteoinduction
by recombinant human bone morphogenetic protein-2. Life Sci.
72:247–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Utheim TP, Raeder S, Utheim ØA, Cai Y,
Roald B, Drolsum L, Lyberg T and Nicolaissen B: A novel method for
preserving cultured limbal epithelial cells. Br J Ophthalmol.
91:797–800. 2007. View Article : Google Scholar
|
|
26
|
Bang SM, Moon HJ, Kwon YD, Yoo JY, Pae A
and Kwon IK: Osteoblastic and osteoclastic differentiation on SLA
and hydrophilic modified SLA titanium surfaces. Clin Oral Implants
Res. 25:831–837. 2014. View Article : Google Scholar
|
|
27
|
Wang B, Bi M, Zhu Z, Wu L and Wang J:
Effects of the antihypertensive drug benidipine on osteoblast
function in vitro. Exp Ther Med. 7:649–653. 2014.PubMed/NCBI
|
|
28
|
Nakamura T, Shinohara Y, Momozaki S,
Yoshimoto T and Noguchi K: Co-stimulation with bone morphogenetic
protein-9 and FK506 induces remarkable osteoblastic differentiation
in rat dedifferentiated fat cells. Biochem Biophys Res Commun.
440:289–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: An in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park JB, Zhang H, Lin CY, Chung CP, Byun
Y, Park YS and Yang VC: Simvastatin maintains osteoblastic
viability while promoting differentiation by partially regulating
the expressions of estrogen receptors α. J Surg Res. 174:278–283.
2012. View Article : Google Scholar
|