Introduction
Glioma is the most common primary malignant tumor of
the central nervous system. Neuroectodermal tumors account for
~40–60% of central nervous system tumors (1). Complete surgical excision of lesions
is difficult, as the boundaries between the glioma and surrounding
brain tissue are unclear due to the active infiltrative growth
along nerve fibers, which is observed in the majority of gliomas.
The poor tolerance of brain tissue to radiation reduced sensitivity
of malignant gliomas to radiotherapy. Systemic chemotherapy is
associated with high whole-body toxicity and the
blood-cerebrospinal fluid barrier restricts the penetration of
anti-cancer therapeutic agents. Although these methods produce a
limited curative effect, peripheral tumors cannot be effectively
eradicated. Clinical and imaging studies have demonstrated that
neurogliomas, particularly malignant gliomas, are prone to
recurrence, with the majority recurring within 0.5–3.0 cm of the
primary tumor (2). Only 5.5% of
patients diagnosed with glioblastoma in the United States in 1980
exhibited a survival time >5 years (3). Surgical resection, radiotherapy and
chemotherapy are ineffective in controlling disease progression,
and do not markedly improve the prognosis, spontaneous generation
or development of glioma. Thus, it is necessary to develop novel
auxiliary treatments for glioma, to control the growth and
recurrence of tumors, and to prolong survival time.
As the current comprehensive treatment includes
surgical resection, radiotherapy and chemotherapy, it is difficult
to treat malignant gliomas. To prevent tumor recurrence,
therapeutic agents are administered directly to the tumor, to the
lumen of the tumor or into surgically created resection cavities.
Therapeutic strategies include injection of killer cells activated
by lymphokines, interleukin-2 (IL-2), chemotherapeutics, embedded
slow-release chemotherapy pills and monoclonal antibodies marked
with radioisotopes (4). Although
these methods are currently in clinical use, their effects are
unsatisfactory. With advances in molecular biology and tumor
immunology, certain novel therapeutic strategies have been
proposed. In particular, biological therapy is garnering increasing
attention due to its low toxicity and few side effects. The
anti-carcinoma effect mediated by killer cell immunoglobulin-like
receptor (KIR) ligand-mismatch in natural killer (NK) cell surface
receptors has been successfully applied in allogeneic bone marrow
transplantation (5,6). Certain previous studies have
demonstrated that this effect can be applied in transplants for the
treatment of leukemia and for solid tumors (5,7).
However, few studies have focused on the use of
allogeneic NK cells to treat neuroglioma. As neuroglioma is
associated with frequent recurrence and treatment is difficult, the
present study aimed to identify an effective method for the
biological treatment of neuroglioma by observing the cytotoxic
activity of allogeneic NK cells on U251 glioma cells and to
investigate the mechanism of action in vitro.
Materials and methods
Subjects and cell culture
The U251 glioma and K562 human chronic myeloid
leukemia cell lines were donated by the Institute of Genetics at
Xiangya Medical College of South Central University (Changsha,
China). Peripheral blood samples were obtained from 7 healthy
volunteers (4 women, 3 men; age, 25–36 years; median age, 32
years). The current study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of South Central University. Written informed consent was obtained
from all participants.
U251 and K562 cells were cultured in complete
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an
incubator at 37°C with 5% CO2 and sufficient moisture.
The medium was replaced every 2 days and the cellular morphology
was observed daily using an inverted DM IL microscope (Leica
Microsystems, Inc., Buffalo Grove, IL, USA).
Cell cloning and observation
The number of cloned U251 cells (>50 cells) was
counted using Giemsa staining (Sigma-Aldrich, St. Louis, MO, USA)
under a microscope (Nikon TMS; Nikon, Tokyo, Japan). Briefly, cells
were washed with phosphate buffered saline, fixed with formaldehyde
and washed again prior to incubation with Giemsa staining solution.
After 30 min of staining, cells were washed and allowed to dry.
Colony formation efficiency was calculated as follows: Colony
formation efficiency (%) = colony no. / inoculated cell no. ×
100.
U251 cells in the logarithmic growth phase were
passaged (1:2) and a cell suspension was created by adding a small
quantity (2 ml) of fixation liquid (methanol) to the cells
following pretreatment. The cells were Giemsa-stained and sealed,
then the chromosome number was counted in 15 cells and changes in
the chromosome structure were observed.
Cell separation, purification and
culture
A total of 50 ml peripheral venous blood, containing
heparin anticoagulant (Shanghai Chemical Reagent Co., Ltd.,
Shanghai, China), was obtained from the healthy volunteers. The
precipitate was separated via centrifugation (1,000 × g) using a
lymphocyte separation medium (relative ratio, 1.077 g/l; Litton
Bionetics, Kensington, MD, USA). Cells were then suspended in
phosphate-buffered saline and the number of cells was counted using
a Countstar automatic cell counter (Biomen Biosystems Co., Ltd.,
Guangzhou, China). Purification of NK cells was performed using the
method described by Xu et al (8).
NK cells were suspended in RPMI-1640 cell culture
medium with 10% FBS, 100 µg/ml penicillin and 100
µg/ml streptomycin (both purchased from Gibco; Thermo Fisher
Scientific, Inc.). The concentration of NK cells was adjusted to
1×106 cells/ml and the cells were seeded in 24-well
culture plates (1 ml/well) and 1,000 µg/ml recombinant human
(rh)IL-2 (Shenyang Sunshine Pharmaceutical Co., Ltd., Shenyang,
China) was added. The medium was changed every 2 days and
simultaneously supplemented with an equal concentration of rhIL-2.
Cellular morphology was observed daily under the inverted
microscope.
Cell activity measurement
Cell activity was determined using a lactate
dehydrogenase (LDH) detection kit (Promega Corporation, Madison,
WI, USA), according to the manufacturer's instructions. Briefly,
samples (0.5–1 ml) were collected at regular time points between 12
and 24 h from the culture. Samples were then centrifuged at 250 × g
and the supernatant was removed. Then, the samples were titrated in
a microtiter plate in the appropriate culture medium by serial
dilution with a final volume of 100 µl/well. A total of 100
µl/well Solution C (reaction mixture) was then added to each
well and incubated for up to 30 min at room temperature. The
absorbance of the samples at 490 or 492 nm was measured using a
microplate reader (iMark; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
The primers used in the current study (Table I) were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China) based on previously published
sequences (8). Total RNA was
isolated using TRIzol (Thermo Fisher Scientific, Inc.), and the
concentration and purity of the RNA were determined based on the
A260/A280 ratio by the NanoDrop spectrophotometer (Thermo Fisher
Scientific, Inc.). cDNA was synthesizes from 2 µg total RNA
using an AMV Reverse Transcriptase First-strand cDNA Synthesis Kit
(GE Healthcare Life Sciences, Chalfont, USA) according to the
manufacturer's protocol, which was used to detect the mRNA
expression levels of the MHC class I polypeptide-related sequence A
and B ligands of natural killer group 2, member D (NKG2D) in U251
cells. LightCycler-Fast Start DNA SYBR Green I mix (Takara, Tokyo,
Japan) and 500 nmol/l of each primer were used in a final volume of
20 µl. The reaction was initiated at 95°C for 10 min,
followed by 40 cycles of denaturation at 95°C for 10 sec, annealing
for 10 sec at 69°C, and extension at 72°C for 15 sec. A DNA ladder
(cat. no. D514C; Takara) and ethidium bromide (cat. no. 1203-10;
BioVision, Milpitas, CA, USA) were used in electrophoresis. The
presence of amplified PCR products was confirmed using a 1% agarose
gel followed by UV visualization following ethidium bromide
staining.
 | Table IPrimer sequences used in the present
study. |
Table I
Primer sequences used in the present
study.
| Gene | Forward primer | Reverse primer | Product size
(bp) |
|---|
| MICA |
5′-GTGCCCCAGTCCTCCAGAGCTCAG-3′ |
5′-GTGGCATCCCTGTGGTCACTCGTC-3′ | 635 |
| MICB |
5′-GGCGTCAGGATGGGGTATCTTTGA-3′ |
5′-GGCAGGAGCAGTCGTGAGTTTGCC-3′ | 690 |
| ULBP1 |
5′-CTGCAGGCCAGGATGTCTTGTGAG-3′ |
5′-TGAGGGTGGTGGCCATGGCCTTGG-3′ | 319 |
| ULBP2 |
5′-CTGCAGGCAAGGATGTCTTGTGAG-3′ |
5′-TGAGGGTGGTGGCTGTGGCCCTGA-3′ | 327 |
| ULBP3 |
5′-CTGCAGGTCAGGATGTCTTGTGAG-3′ |
5′-TGAGGGTGGTGGCTATGGCTTTGG-3′ | 321 |
| β-actin |
5′-AGCCATGTACGTAGCCAT-3′ |
5′-TTTGATGTCACGCACGATTT-3′ | 232 |
Quantification of MICA/B and UL16 binding
proteins 1–3 (ULBP1-3) on the cell surface
Expression levels of NKG2D ligands on the surface of
U251 cells, including MICA/B and ULBP1-3, were determined using an
EPICS XL flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) with
an indirect immunofluorescence labeling method. Briefly, whole
blood was labelled with combinations of monoclonal antibodies
conjugated to fluorescein isothiocyanate (FITC), phycoerythrin, and
either peridinin chlorophyll protein (PerCP), cychrome, or
Tricolour. Isotype-matched fluorochrome-conjugated antibodies
served as controls (CD3 FITC, CD45RO FITC, CD27 FITC, CD19
phycoerythrin, CD27 phycoerythrin, CD4 phycoerythrin, CD8
phycoerythrin, CD45 PerCP, CD4 PerCP, CD8 cychrome, CD45RO
cychrome, CD16+CD56 FITC phycoerythrin, IgM FITC, IgD FITC and CD19
Tricolour; BD Biosciences, Oxford, UK). The resulting three-colour
cell staining was analyzed using an Epic XL flow cytometer (Beckman
Coulter, Inc.). Cells were sorted with a Beckman Coulter Epics
Altra with Expo32 software (verxion ADC 1.1C; Beckman Coulter,
Inc.).
Cytotoxic activity of NK cells
The primary mouse monoclonal AMO-1 mAb antibody,
specific for MICA and anti-MICA/B-PE (clone 6D4; 1:500; cat. no.
NB100-78034PE), was purchased from Novus Biologicals (Littleton,
CO, USA). The anti-ULBP 1-3 monoclonal antibodies used, human
ULBP-1 MAB (clone 170818; 1:500; cat. no. MAB1380), human
ULBP-2/5/6 MAb (clone 165903; 1:500; cat. no. MAB1298) and human
ULBP-3 MAb (clone 166510; 1:500; cat. no. MAB1517) were purchased
from R&D Systems, Inc. (Minneapolis, MN, USA). Each monoclonal
antibody was incubated with K562 and U251 cells for 15 min at room
temperature, then the solution was added to the NK cells. The
cytotoxic activity of the NK cells was measured using an LDH
detection kit. All antibodies were purchased from R&D Systems,
Inc.
Statistical analysis
All data are presented as the mean ± standard
deviation. An unpaired t-test was used to analyze differences
between two groups and one-way analysis of variance was used to
estimate differences among groups (the least significant difference
method was used to perform multiple comparisons between groups).
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
version 12.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Colony formation
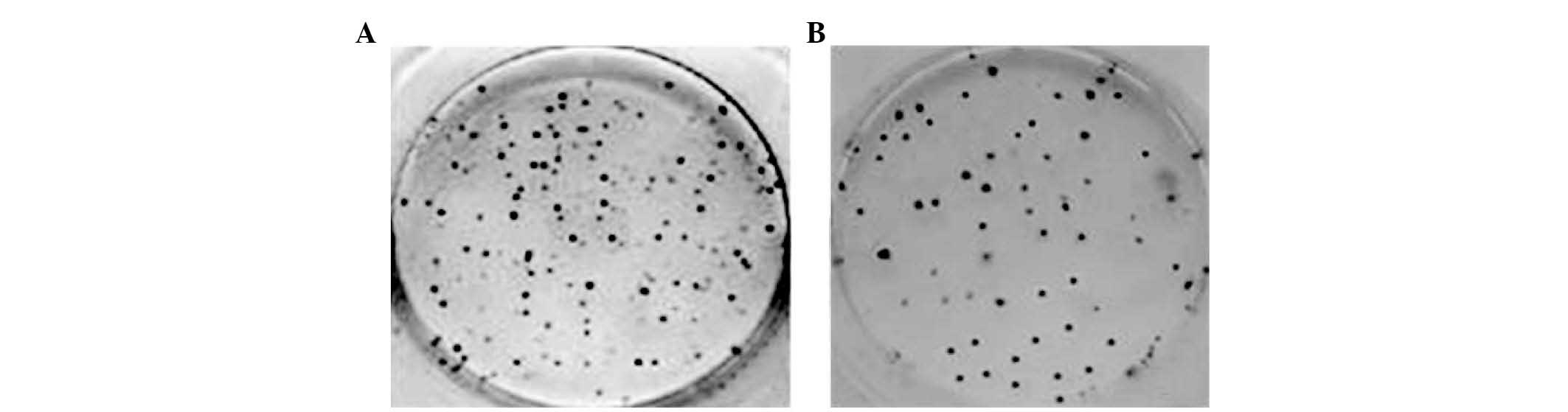
U251 cells formed colonies in culture and the colony
forming efficiency was estimated by counting the number of colonies
of each group in 6-well culture dishes. At day 7 and 14, colony
forming efficiencies were 26.07±2.70 and 38.44±2.68%, respectively
(Fig. 1).
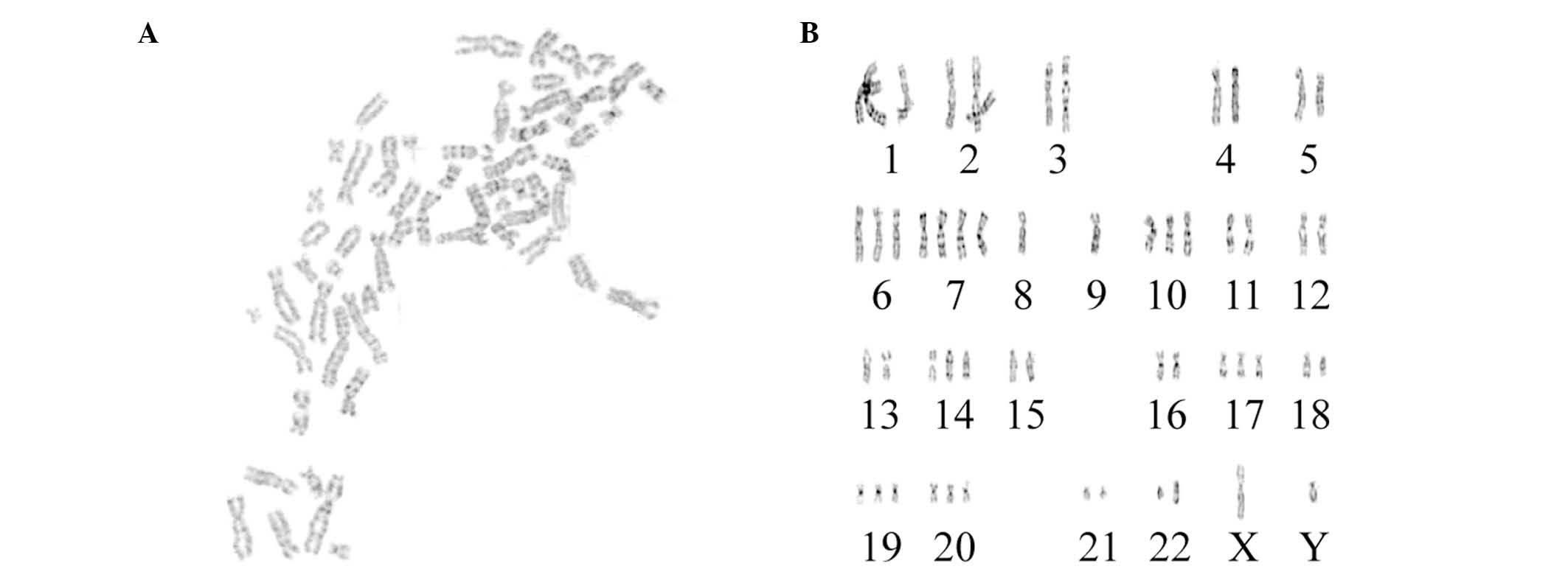
Karyotype analysis
The karyotypes of 15 cells were determined under a
microscope. All chromosomes were hyperdiploid, and the chromosome
number ranged from 52 to 61 (Fig.
2); 33.3% contained 56 chromosomes, 20.0% contained 60
chromosomes, 13.3% contained 56 or 61 chromosomes, and 6.7%
contained 52, 58 or 55 chromosomes. Chromosome with the two arms
were present in 83.3% of cells, indicating that the U251 cells were
malignant.
Purity of NK cells
Flow cytometry analysis indicated that the purity of
cluster of differentiation (CD)−, CD16+ and
CD56+ cells (NK cells) was 89.56±2.37% following
separation.
Cytotoxic activity of NK cells on K562
and U251 cells
The LDH method was used to determine the cytotoxic
effect of NK cells on K562 and U251 cells. The results showed that
there was significant difference between the cytotoxicity of NK
cells on K562 and U251 cells at each effector (E):target (T) ratio
by pairwise comparison (P>0.05; Figs. 3 and 4); the cytotoxic effect differed
significantly between the different E:T ratios (P<0.05). Thus,
NK cells demonstrated significant cytotoxic activity on the two
cell types and the cytotoxic activity increased as the E:T ratio
increased.
Expression levels of NKG2D ligands
The expression levels of NKG2D ligands, including
MICA, MICB, ULBP1, ULBP2 and ULBP3, on the surface of U251 cells
were marginally reduced compared with K562 cells. The highest
expression level observed was ULBP3 (45.2%) and the lowest was
ULBP1 (21.9%; Fig. 5). The
expression levels of these ligands did not differ significantly
between the two cell types (P>0.05; Table II).
 | Table IIExpression levels of MICA/B and
ULBP1-3 on U251 and K562 cell surfaces. |
Table II
Expression levels of MICA/B and
ULBP1-3 on U251 and K562 cell surfaces.
| Cell line | Expression level (%)
|
|---|
| MICA | MICB | ULBP1 | ULBP2 | ULBP3 |
|---|
| K562 | 57.3±2.47 | 46.8±2.41 | 43.3±1.98 | 65.3±3.06 | 53.9±2.66 |
| U251 | 34.2±1.35 | 40.4±1.27 | 22.7±1.12 | 39.4±1.43 | 45.4±1.75 |
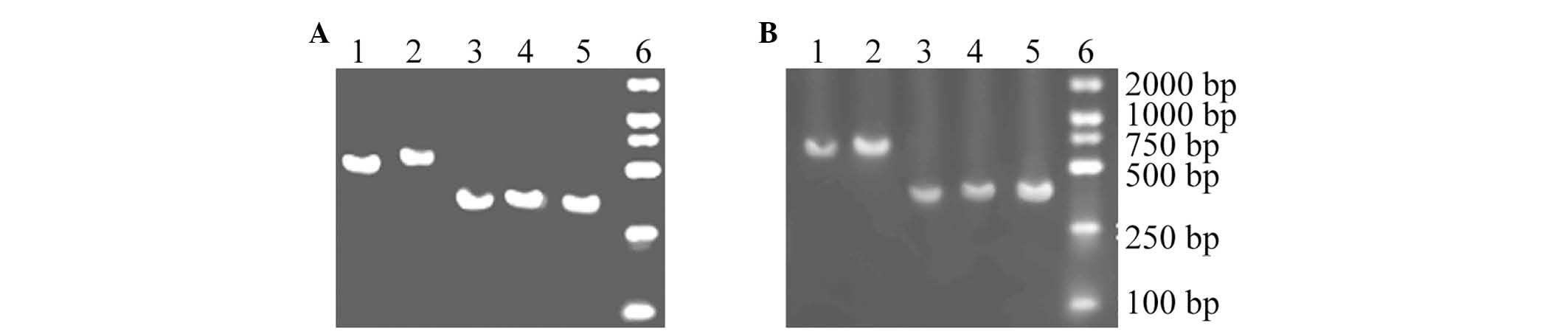
RT-PCR
For RT-PCR analysis, five fragments of consistent
length were amplified from the U251 and K562 cells, indicating that
these products are mRNAs that encode the NKG2D ligands in U251 and
K562 cells (Fig. 6).
Changes to the cytotoxic activity of NK
cells
As demonstrated in Table III, when the E:T ratio was 20:1,
AMO-1, BMO-1, M295, M310 and M551 bound to the relevant molecules
on the K562 and U251 cell surfaces. Compared with the cytotoxic
activity prior to blocking MICA/B and ULBP1-3, a significant
difference was detected between the activity of NK cells on K562
and U251 cells (P<0.05). These results indicate that NKG2D
ligands may be involved in the cytotoxic activity of NK cells on
K562 and U251 cells.
 | Table IIICytotoxic activity of natural killer
cells on K562 and U251 cells when the effector:target ratio is 20:1
blocking MICA/B and ULBP1-3. |
Table III
Cytotoxic activity of natural killer
cells on K562 and U251 cells when the effector:target ratio is 20:1
blocking MICA/B and ULBP1-3.
| Cell line | Cytotoxic activity
(%)
|
|---|
| AMO-1 | BMO-1 | M295 | M310 | M551 |
|---|
| K562 | 49.82±1.74 | 50.26±2.41 | 52.42±1.58 | 50.74±2.16 | 51.57±1.66 |
| U251 | 29.01±0.72 | 30.83±0.91 | 37.87±0.82 | 31.45±1.46 | 39.42±1.07 |
Discussion
NK cells are an important lymphocyte subpopulation
and a primary factor involved in innate immunity. The activation
and function of NK cells is determined by cell-surface activating
and inhibitory receptors. The 'missing self' hypothesis was
proposed in 1990 (9), which stated
that NK cells selectively recognize and kill cells lacking, or with
lower expression levels of, major histocompatibility complex I (MHC
I) molecules via a specific mechanism, although NK cells do not
kill healthy cells.
NK cell-activating receptors are divided into three
classes: KIRs, C type lectin receptors and non-MHC class I-specific
activating NK cell receptors. KIRs are predominantly expressed on
the surface of NK cells and certain T cells. The gene encoding KIRs
is located on the long arm of human chromosome 19 (19q) (10). C type-lectin receptors are a type
of MHC-I receptor in the C-type lectin superfamily. The receptor is
composed of a dimer of CD94 and NKG2 type II transmembrane
proteins, and the gene encoding these receptors is located on the
short arm of chromosome 12 (12p) (10,11).
Expression of NKG2D does not require CD94 or combination with CD94,
and it can combine with MICA or B to activate the cytotoxic
activity of NK cells, mediated by the polypeptide DNAX-activating
protein 10 (DAP10) (12). Unlike
MICA and MICB, the NKG2D-DAP10 complex also recognizes and combines
with retinoic acid early-inducible protein 1 and human
cytomegalovirus glycoprotein ULBP, which acts as an anchor junction
with glycosyl phosphatidyl inositol in target cells (13). Three NK cell stimulating receptors
that are non-MHC dependent belong to the Ig superfamily (NKP46,
NKP44 and NKP30) and are known to be natural cytotoxicity receptors
(14). The ability of NK cells to
kill target cells mediated by these three receptors is associated
with cell surface receptor density. The current study primarily
demonstrates the cytotoxic activity of NK cells on U251 glioma
cells and the primary underlying mechanisms.
In the present study, the sensitive K562 cell line
served as a positive control and the LDH cytotoxic release assay
was used to detect the cytotoxic activity of allogeneic NK cells on
U251 cells at different E:T ratios in vitro. The current
study demonstrated that NK cells exhibited significant cytotoxic
activity against the two cell types and that the cytotoxic effect
increased as the E:T ratio increased. The mRNA expression of MICA/B
and ULBP genes was detected in K562 and U251 cells by RT-PCR, and
flow cytometry also indicated the presence of MICA/B and ULBP
protein. These results indicate that signals for the activation of
NK cells may be stimulated by binding of MICA/B and ULBP in U251
cells, and NKG2D receptors in NK cells, leading to the killing of
U251 cells by NK cells. To support this hypothesis, monoclonal
antibodies for MICA/B and ULBP were used to block NKG2D receptors
in U251 cells, and changes to the cytotoxic activity of NK cells
were observed. When the E:T ratio was 20:1 and the relevant NKG2D
receptors on U251 cells were blocked by the administration of
antibodies (AMO-1, BAMO-1, M295, M310 and M551) the cytotoxic
effect of NK cells on K562 and U251 cells was significantly reduced
(P<0.05). The findings of the current study demonstrated that
the NKG2D-MICA/B interaction is important for the cytotoxic
activity of NK cells on K562 and U251 cells, which also supports
the hypothesis of the current study. However, the antibody blocking
assay indicated that the cytotoxic activity was not completely
inhibited, suggesting that other signaling pathways may contribute
to the cytotoxic effect of NK cells on tumors, in addition to the
activity mediated by NKG2D (15).
As the expression rate of MICA/B and ULBP increased, the cytotoxic
activity of NK cells also increased. Thus, expression levels of
NKG2D ligands influences the effect of NK cells in the immune
response.
Previous studies have demonstrated that if the
expression of transforming growth factor-β (TGF-β) increases as the
degree of malignant glioma increases, the expression levels of
MICA, ULBP2 and 4 are reduced, whereas, the expression levels of
MICB, ULBP1 and 3 are not affected. The expression levels of MICA
and ULBP2 are upregulated following reduction of TGF-β and
inhibitory metalloproteinases in vitro, and therefore the
NKG2D-mediated cytotoxic effect can be restored. Thus, the major
mechanism by which glioma escapes immune surveillance may be via
the autocrine expression of TGF-β by neuroglioma cells, and the
damage of MICA and ULBP2 by metalloproteinases (16,17).
In a previous study, when LN-229 human glioma transfected with the
gene encoding MICA were inoculated into nude mice, tumor growth was
significantly delayed compared with wild-type LN-229 cells, and
tumor size was significantly decreased (18). When rat glioma cells transfected
with the genes encoding MICA/B and ULBP were inoculated into
homologous mice following radiographic exposure, an
immunoprotective effect was generated when SMA560 cells were
re-vaccinated. This previous study suggests that upregulation of
MICA/B and ULBP expression in tumor cells overcomes the inhibitory
signal transduced by human leukocyte antigen-I molecules, and
enhances the cytotoxic activity of NK cells. Wu et al
(19) demonstrated that MHC I and
the activating receptors of NK cells were not expressed in
CD133+ neuroglioma cells, but the expression of NKG2D
receptors was upregulated following incubation with interferon
(IFN)-γ. Neuroglioma cells were subsequently killed by NK cells.
Thus, increased expression levels of the NKG2D receptor enhances
the cytotoxic activity of NK cells and may provide a novel method
for the development of immunotherapies.
Autologous NK cells have been applied as a treatment
for neurogliosis in Japan (20).
Autologous peripheral blood was obtained from patients with glioma
then transfused to nine patients exhibiting glioma recurrence.
Amplified NK cells possess 82.2±10.5% leukomonocytes (mean ±
standard deviation) and the therapeutic effects of NK cells are
enhanced by simultaneous venous injection with IFN-β. Clinical
results demonstrate that autologous NK cells are safe and somewhat
effective for the treatment of glioma (19–23).
The present study indicates that allogeneic NK cells exhibit
specific cytotoxic activity on glioma cells. Based on the theory of
immune escape, glioma cells evade the cytotoxic effect of NK cells
resulting in the development of tumors. The results of the current
study suggest that treatment with allogeneic NK cells will present
as a superior method to treatment with autologous NK cells in
glioma cases.
In conclusion, the current study indicates that
allogeneic NK cells exhibit a therapeutic effect against glioma
cells and that NK cell cytotoxic activity is associated with the
expression of NKG2D ligands. Further research is required to
address the mechanism by which the expression of NKG2D ligands may
be increased to further enhance the anti-cancer activity of NK
cells.
Acknowledgments
The present study was supported by Nanyang Municipal
Science and Technology Research Projects (grant nos. 2011GG014 and
2012GG088).
References
|
1
|
Fecci PE, Heimberger AB and Sampson JH:
Immunotherapy for primary brain tumors: No longer a matter of
privilege. Clin Cancer Res. 20:5620–5629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart LA: Chemotherapy in adult
high-grade glioma: A systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Claus EB, Walsh KM, Wiencke JK, Molinaro
AM, Wiemels JL, Schildkraut JM, Bondy ML, Berger M, Jenkins R and
Wrensch M: Survival and low-grade glioma: The emergence of genetic
information. Neurosurg Focus. 38:E62015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boiardi A, Bartolomei M, Silvani A, Eoli
M, Salmaggi A, Lamperti E, Milanesi I, Botturi A, Rocca P, Bodei L,
et al: Intratumoral delivery of mitoxantrone in association with
90-Y radioimmunotherapy (RIT) in recurrent glioblastoma. J
Neurooncol. 72:125–131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moretta L, Locatelli F, Pende D, Marcenaro
E, Mingari MC and Moretta A: Killer Ig-like receptor-mediated
control of natural killer cell alloreactivity in haploidentical
hematopoietic stem cell transplantation. Blood. 117:764–771. 2011.
View Article : Google Scholar
|
|
6
|
Leung W, Iyengar R, Turner V, Lang P,
Bader P, Conn P, Niethammer D and Handgretinger R: Determinants of
antileukemia effects of allogeneic NK cells. J Immunol.
172:644–650. 2004. View Article : Google Scholar
|
|
7
|
Igarashi T, Wynberg J, Srinivasan R,
Becknell B, McCoy JP Jr, Takahashi Y, Suffredini DA, Linehan WM,
Caligiuri MA and Childs RW: Enhanced cytotoxicity of allogeneic NK
cells with killer immunoglobulin-like receptor ligand
incompatibility against melanoma and renal cell carcinoma cells.
Blood. 104:170–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu T, Zhang C, Tian Z, Wang J, Zhang J and
Sun R: Preliminary study in purification, proliferation and cloning
of human NK cells. Shanghai Mian Yi Xue Za Zhi. 20:148–151. 2000.In
Chinese.
|
|
9
|
Rossjohn J, Pellicci DG, Patel O, Gapin L
and Godfrey DI: Recognition of CD1d-restricted antigens by natural
killer T cells. Nat Rev Immunol. 12:845–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Zhang JH, Xu XQ, Feng JB, Sun N
and Tiang ZG: The characterization of NK-92 cell line transfected
with human stem cell factor gene. Zhong Guo Zhong Liu Sheng Wu Zhi
Liao Za Zhi Bian Ji Bu. 10:21–24. 2003.In Chinese.
|
|
11
|
Wilson JL, Charo J, Martín-Fontecha A,
Dellabona P, Casorati G, Chambers BJ, Kiessling R, Bejarano MT and
Ljunggren HG: NK cell triggering by the human costimulatory
molecules CD80 and CD86. J Immunol. 163:4207–4212. 1999.PubMed/NCBI
|
|
12
|
Farag SS, Fehniger TA, Ruggeri L, Velardi
A and Caligiuri MA: Natural killer cell receptors: New biology and
insights into the graft-versus-leukemia effect. Blood.
100:1935–1947. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chalupny NJ, Sutherland CL, Lawrence WA,
Rein-Weston A and Cosman D: ULBP4 is a novel ligand for human
NKG2D. Biochem Biophys Res Commun. 305:129–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vitale M, Cantoni C, Pietra G, Mingari MC
and Moretta L: Effect of tumor cells and tumor microenvironment on
NK-cell function. Eur J Immunol. 44:1582–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kazimer RM, Whisler RL, Stephens RE, Pearl
DK and Yates AJ: Sensitivity of glioma and fetal brain cell lines
to natural killer cytolysis in a monolayer assay. J Neurooncol.
7:145–150. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sivori S, Parolini S, Marcenaro E,
Castriconi R, Pende D, Millo R and Moretta A: Involvement of
natural cytotoxicity receptors in human natural killer
cell-mediated lysis of neuroblastoma and glioblastoma cell lines. J
Neurooncol. 107:220–225. 2000.
|
|
17
|
Ghadially H, Ohana M, Elboim M, Gazit R,
Gur C, Nagler A and Mandelboim O: NK cell receptor NKp46 regulates
graft-versus-Host disease. Cell Rep. 7:1809–1814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eisele G, Wischhusen J, Mittelbronn M,
Meyermann R, Waldhauer I, Steinle A, Weller M and Friese MA:
TGF-beta and metalloproteinases differentially suppress NKG2D
ligand surface expression on malignant glioma cells. Brain.
129:2416–2425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu A, Wiesner S, Xiao J, Ericson K, Chen
W, Hall WA, Low WC and Ohlfest JR: Expression of MHC I and NK
ligands on human CD133(+) glioma cells: Possible targets of
immunotherapy. J Neurooncol. 83:121–131. 2007. View Article : Google Scholar
|
|
20
|
Ishikawa E, Tsuboi K, Saijo K, Harada H,
Takano S, Nose T and Ohno T: Autologous natural killer cell therapy
for human recurrent malignant glioma. Anticancer Res. 24:1861–1871.
2004.PubMed/NCBI
|
|
21
|
Avril T, Vauleon E, Hamlat A, Saikali S,
Etcheverry A, Delmas C, Diabira S, Mosser J and Quillien V: Human
glioblastoma stem-like cells are more sensitive to allogeneic NK
and T cell-mediated killing compared with serum-cultured
glioblastoma cells. Brain Pathol. 22:159–174. 2012. View Article : Google Scholar
|
|
22
|
Iovino F, Meraviglia S, Spina M, Orlando
V, Saladino V, Dieli F, Stassi G and Todaro M: Immunotherapy
targeting colon cancer stem cells. Immunotherapy. 3:97–106. 2011.
View Article : Google Scholar
|
|
23
|
Qi Y, Li RM, Kong FM, Li H, Yu JP and Ren
XB: How do tumor stem cells actively escape from host
immunosurveillance? Biochem Biophys Res Commun. 420:699–703. 2012.
View Article : Google Scholar : PubMed/NCBI
|