Introduction
Non-small-cell lung cancer (NSCLC) is the most
frequent type of lung cancer and the most common cause of
cancer-associated mortality (1).
The poor outcome of NSCLC and patient survival are partly due to
the development of drug resistance. At present, cisplatin-based
chemotherapy is recommended as the first-line treatment for
advanced NSCLC. Despite extensive research on its resistance
mechanisms, pre-clinical data have not been incorporated into the
selection of NSCLC patients or tailored treatment regimens in
clinical trials. The current understanding of the molecular
mechanisms of NSCLC and its chemoresistance requires to be expanded
and applied for its treatment. It is important to identify novel
biomarkers and therapeutic targets for NSCLC and provide a
rationale to overcome the current therapeutic plateau.
Inhibitor of differentiation/DNA binding (Id)
proteins, which are negative regulators of basic helix-loop-helix
(bHLH) transcription factors, function as dominant-negative
inhibitors of E-proteins by inhibiting their ability to bind DNA
(2,3). Four members of the Id family, ID1-4,
occur in vertebrates. Id proteins have crucial roles in the
coordinated regulation of a variety of cellular process, including
cell growth, differentiation, apoptosis, tumorigenesis and
carcinogenesis (4–6). Numerous studies have shown that the
expressional regulation and functions of Ids are controlled by
complex mechanisms, which are distinct for various cancer cell
types and developmental stages (7–9).
The Id3 gene is likely to have similar biological
behaviors to those of other Ids, which have an important role in
cell apoptosis. In B-lymphocyte progenitors, Id3 was found to
induce cell growth arrest and caspase-3-dependent apoptosis
(10). In immortalized human
keratinocytes, Id3 as the apical gene in the mitochondrial pathway
of cell death, is able to induce caspase-3- and -9-dependent
apoptosis and mediate their UVB sensitization (11).
Id3 has been implicated in mediating apoptosis
induced by cisplatin, a DNA-damaging chemotherapeutic agent.
Cisplatin induced upregulation of Id3 mRNA, and ectopic expression
of Id3 sensitized MG-63 sarcoma cells to cisplatin-induced
caspase-3 activation and growth inhibition (12). However, the exact induction
mechanism was not described. Previous studies by our group showed
that Id3 was downregulated in A549 human lung adenocarcinoma
epithelial cells and that ectopic overexpression of Id3 in A549
cells inhibited their proliferation and induced apoptosis in
vitro, as well as reducing tumor growth in vivo
(13,14). These results suggested that Id3, as
an upstream gene of the apoptotic signaling cascade, can induce
cell apoptosis.
The present study was the first to perform
plasmid-mediated overexpression of Id3 in cisplatin-resistant A549
cells (A549/DDP) to assess its effect on the cells' proliferation
and apoptotic rate. The results suggested that ectopic expression
of Id3 may represent a promising approach for inhibiting
chemoresistant NSCLC cells.
Materials and methods
Cell lines and culture
The cisplatin-resistant A549/DDP cell line and
native A549 cells were purchased from the Cancer Institute of the
Chinese Academy of Medical Sciences (Beijing, China). Cells were
cultured in RPMI-1640 medium (HyClone Laboratories, Inc., Logan,
UT, USA) supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(HyClone Laboraties, Inc.) in an atmosphere containing 5%
CO2 at 37°C. In all experiments, exponentially growing
cells were used.
Transient transfection
Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used for transfection
following the manufacturer's instructions. In brief, A549/DDP cells
(0.5–2×105/well in 400 µl medium) were seeded
into 24-well plates and incubated for 24 h for attachment to reach
90–95% confluence. Enhanced green fluorescence protein-expressing
plasmid (pEGFP) or Id3/pEGFP (0.8 µg) and Lipofectamine 2000
(2 µl) were each diluted separately in 50 µl
serum-free Opti-MEM (Gibco BRL, Thermo Fisher Scientific, Inc.) and
incubated for 5 min at room temperature, followed by mixing of the
respective plasmid and Lipofectamine 2000 solutions and incubation
at room temperature for 20 min. The cells were then incubated with
this mixture (100 µl) at 37°C for 12–72 h depending on the
specific experiment and then subjected to further analysis.
Proliferation assay
The effects of DPP (Sigma-Aldrich, St. Louis, MO,
USA) on native A549 and A594/DPP cells as well as the effects of
Id3/pEGFP on A594/DPP cells were assessed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. In brief, cells were seeded into 96-well plates at
5×103 cells/well and allowed to attach overnight.
Subsequently DPP was added at various concentrations (0, 0.5, 1, 2,
5, 10, 15, 20, 30, 40 and 80 µg/ml), followed by incubation
for 24 h. In another experiment, A594/DPP cells were transfected
with pEGFP or Id3/pEGFP as described above for 12, 24, 48 or 72 h.
The cell viability was then assessed by addition of 0.5 mg/ml MTT
(Sigma-Aldrich), and cells were incubated at 37°C for 4 h. Then
culture medium was removed and 150 µl dimethyl sulfoxide
(Sigma-Aldrich) was added, followed by agitation for 10 min. The
absorbance at 570 nm (OD570) was measured by using a
Multiskan MS microplate reader (Labsystems Diagnostics Oy, Vantaa,
Finland) with a reference wavelength of 650 nm. The experiment was
repeated three times to generate a growth curve using the following
formula: Proliferation rate (%) = OD570 (experimental
group) / OD570 (control group) × 100%.
Reverse-transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen). Total RNA (1 µg)
was reverse-transcribed using the RevertAid First Strand cDNA
Synthesis kit (Fermentas, Vilnius, Lithuania). PCR was performed in
a total volume of 25 µl containing 12.5 µl Premix Ex
Tag loading dye mix (Takara Bio Inc., Otsu, Japan), 7.5 µl
double-distilled water, 1.5 µl Id3 forward primer
(5′-ATGAAGGCGCTGAGCCCGGT-3′), 1.5 µl Id3 reverse primer
(5′-TTTGCCACTCGGCCGT-3′) (both purchased from Invitrogen; Thermo
Fisher Scientific, Inc.) and 2 µl cDNA. Complementary DNA
was amplified under the following reaction conditions: 94°C for 5
min, followed by 35 amplification cycles of 94°C for 50 sec, 55°C
for 50 sec, 70°C for 50 sec and final elongation at 72°C for 5 min.
Three independent experiments were performed to confirm
reproducibility of the results.
Western blot analysis
A549/DDP cells were cultured in six-well plates,
transfected with pEGFP/Id3 for 24 h, washed twice with ice-cold
phosphate-buffered saline (PBS; pH 7.2), lysed in 200 µl
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Inc., Haimen, China) and recovered with a cell
scraper. Protein concentrations were determined using the Enhanced
BCA Protein Assay kit (Beyotime Institute of Biotechnology, Inc.).
Samples (20 µg) of the cellular lysate were denatured and
fractionated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis [SDS-PAGE; 12% (w/v) polyacrylamide gel] and
transferred onto a polyvinylidene difluoride membrane (Millipore,
Billerica, MA, USA) by semi-dry blotting (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were blocked with
Tris-buffered saline containing Tween 20 (TBST; Beyotime Institute
of Biotechnology, Inc.) with 5% (w/v) non-fat milk for 2 h and
incubated with mouse monoclonal anti-hId3 (1:1,000 dilution; cat.
no. ab55269; Abcam, Cambridge, MA, USA) or rabbit anti-β-actin
(1:800 dilution; cat. no. sc-10731; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 1 h at room temperature and overnight at 4°C.
After washing, the membranes were incubated with horseradish
peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin (Ig)G
(1:400 dilution; Santa Cruz Biotechnology, Inc.) or HRP-conjugated
goat anti-rabbit IgG (1:300 dilution; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. Antibody binding was detected
using an enhanced chemiluminescence detection system (Millipore).
The intensities of bands were measured using Quantity
One® software (version 170-9600; Bio-Rad Laboratories,
Inc.) with normalization to β-actin as the internal control.
Flow cytometric analysis
Apoptosis was quantified using AnnexinV-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) staining followed by
flow cytometry. A549/DDP cells (3.5×105 cells/well) were
cultured in six-well plates to 90% confluency, transfected for 24
h, collected by trypsinization, washed twice with PBS and suspended
in 100 µl binding buffer containing 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid/NaOH (pH 7.4),
140 mM NaCl and 2.5 mM CaCl2 (BD Biosciences, Franklin
Lakes, NJ, USA). 5 µl Annexin V-FITC and 5 µl PI were
then added to the wells, followed by incubation for 30 min at 37°C
in the dark. Following dilution with 400 µl binding buffer,
staining was analyzed within 1 h by flow cytometry. The
fluorescence intensity (green, FL1-H and red, FL2-H) was measured
using a FACSCalibur flow cytometer (BD Biosciences). CellQuest Pro
software (BD Biosciences) was used for acquisition and analysis of
data.
Hoechst 33258 staining
In addition to flow cytometric analysis, apoptosis
was also examined by nuclear staining with a Hoechst 33258 staining
kit (Beyotime Institute of Biotechnology, Inc.). In brief, A549/DPP
cells (1.0×105 cells/well) were grown on coverslips in
24-well plates and transfected with the respective plasmids for 48
h. Following two washes in PBS, cells were fixed in acetone at room
temperature for 2 h. Subsequent to rinsing with PBS, the cells were
stained with 0.5 ml Hoechst 33258 solution (167 µm) in the
dark for 5 min. Following washing with PBS, cells were observed
using a confocal fluorescence microscope (IX 71 Motorized Inverted
Microsope; Olympus Corporation, Tokyo, Japan).
Statistical analysis
All data were analyzed using SPSS version 13.0
software (SPSS, Inc., Chicago, IL, USA). Values are expressed as
the mean ± standard deviation. One-way analysis of variance was
used for statistical comparison. P≤0.05 was considered to indicate
a statistically significant difference between values.
Results
Determination of A549 and A549/DDP cell
drug sensitivity
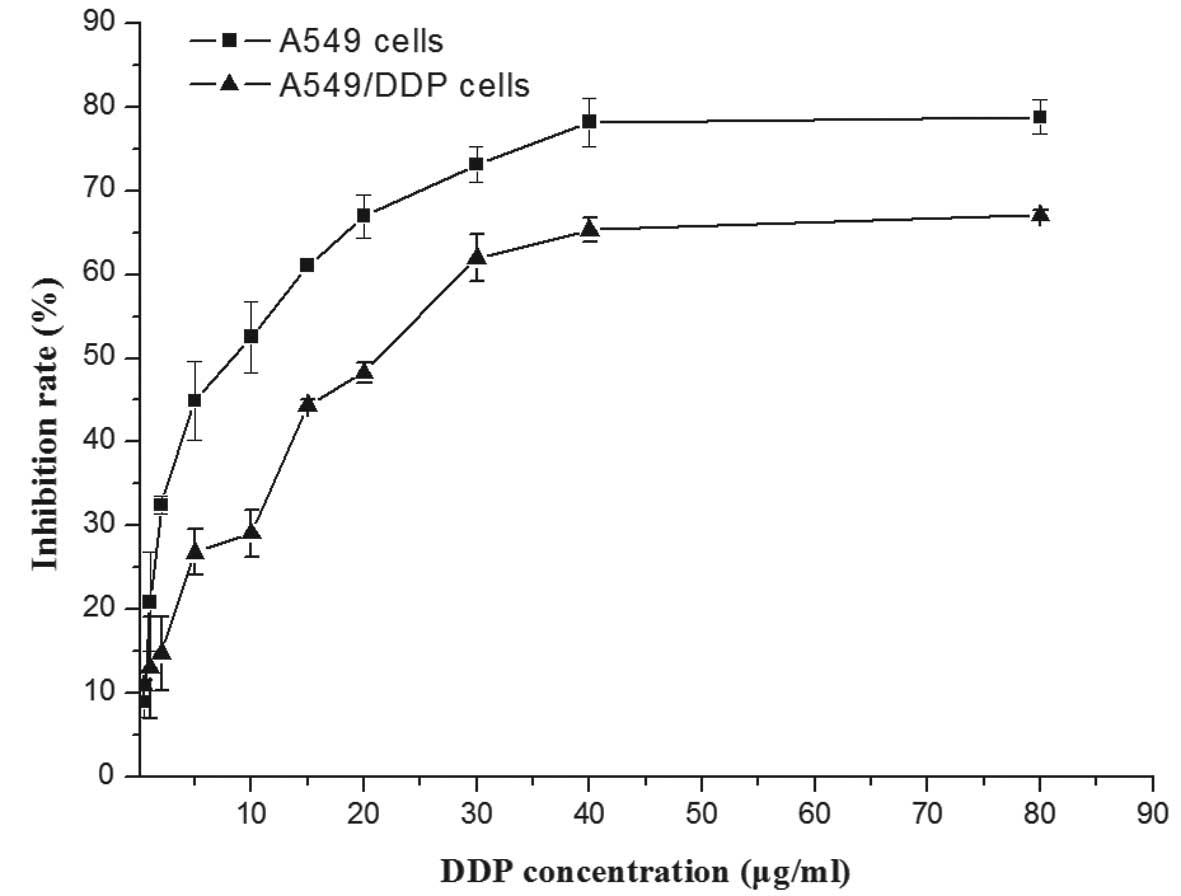
In order to assess the differential sensitivity of
A549 and A549/DDP cells to DDP, cells were incubated with various
concentrations of DDP for 24 h and subjected to an MTT assay. As
shown in Fig. 1 the viability of
A549 was reduced by DPP in a dose-dependent manner, while A549/DDP
cells showed partial resistance against the drug. The
IC50 value of A549/DPP cells (19.38±1.66 µg/ml)
was 3-4-fold increased compared to that of the native A549 cells
(5.32±3.11 µg/ml), confirming the drug resistance of the
A549/DDP cell line.
Overexpression of Id3 in A549/DDP
cells
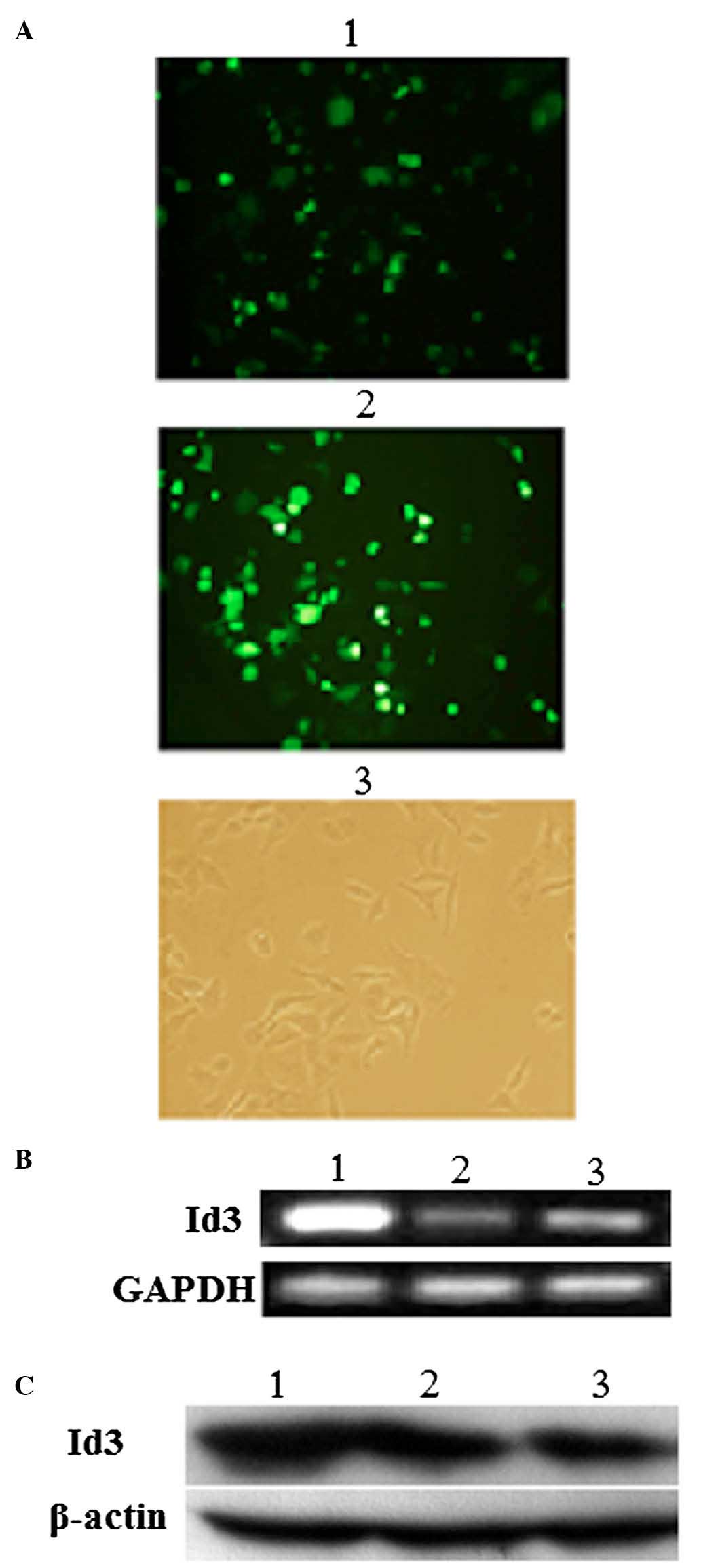
Transfection with the eukaryotic expression vectors
pEGFP or Id3/pEGFP for 24 h was successful, as indicated by
confocal fluorescence microscopy (Fig.
2A). Furthermore, the overexpression of Id3 in A549/DDP cells
transfected with Id3/pEGFP for 24 h was confirmed at the mRNA level
by RT-PCR (Fig. 2B) and at the
protein level by western blotting (Fig. 2C). There was significant difference
in Id3 transfected cells (P<0.05), but there was no significant
difference in the EGFP vector group and blank control group
(P>0.05).
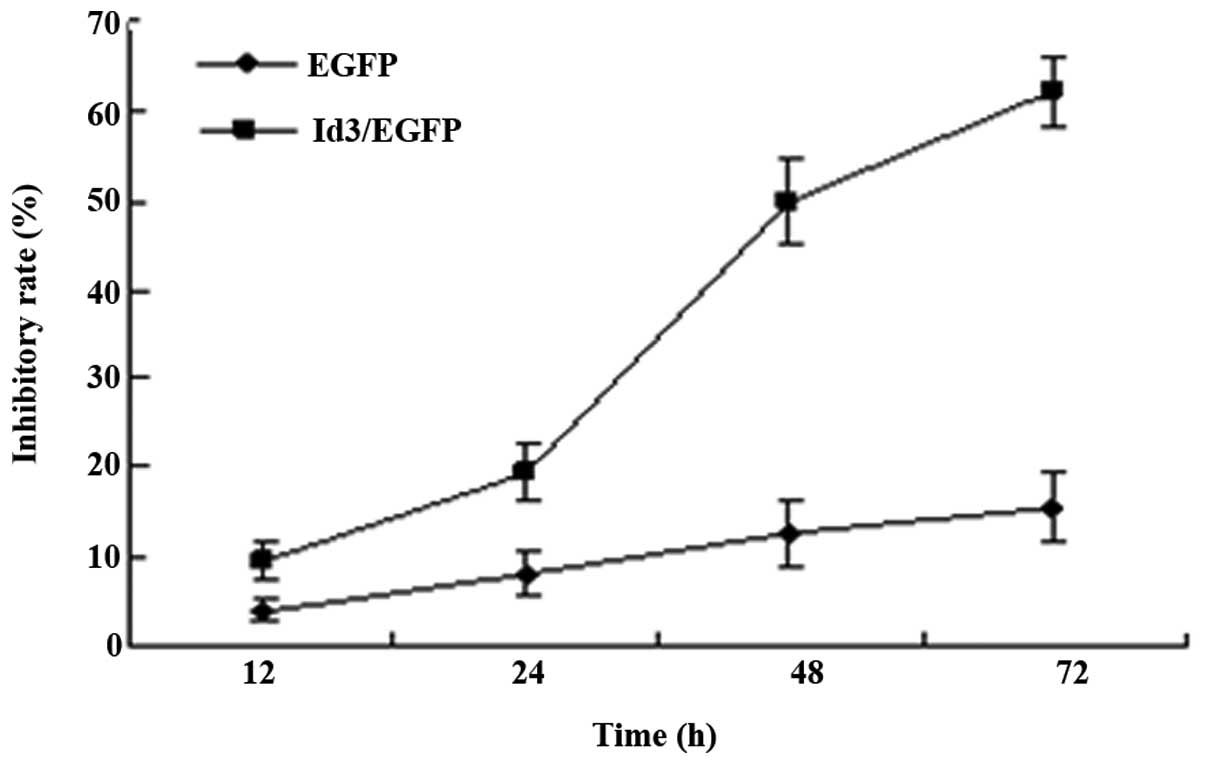
Id3 inhibits the proliferation of
A549/DDP cells
To investigate the effects of Id3 overexpression on
the proliferation of A549/DDP cells, an MTT assay was performed.
MTT analysis revealed that transfection with Id3/pEGFP for 12, 24,
48 or 72 h inhibited the proliferation of A549/DDP cells in a
time-dependent manner, but there was no trend in pEGFP-transfected
group (Fig. 3).
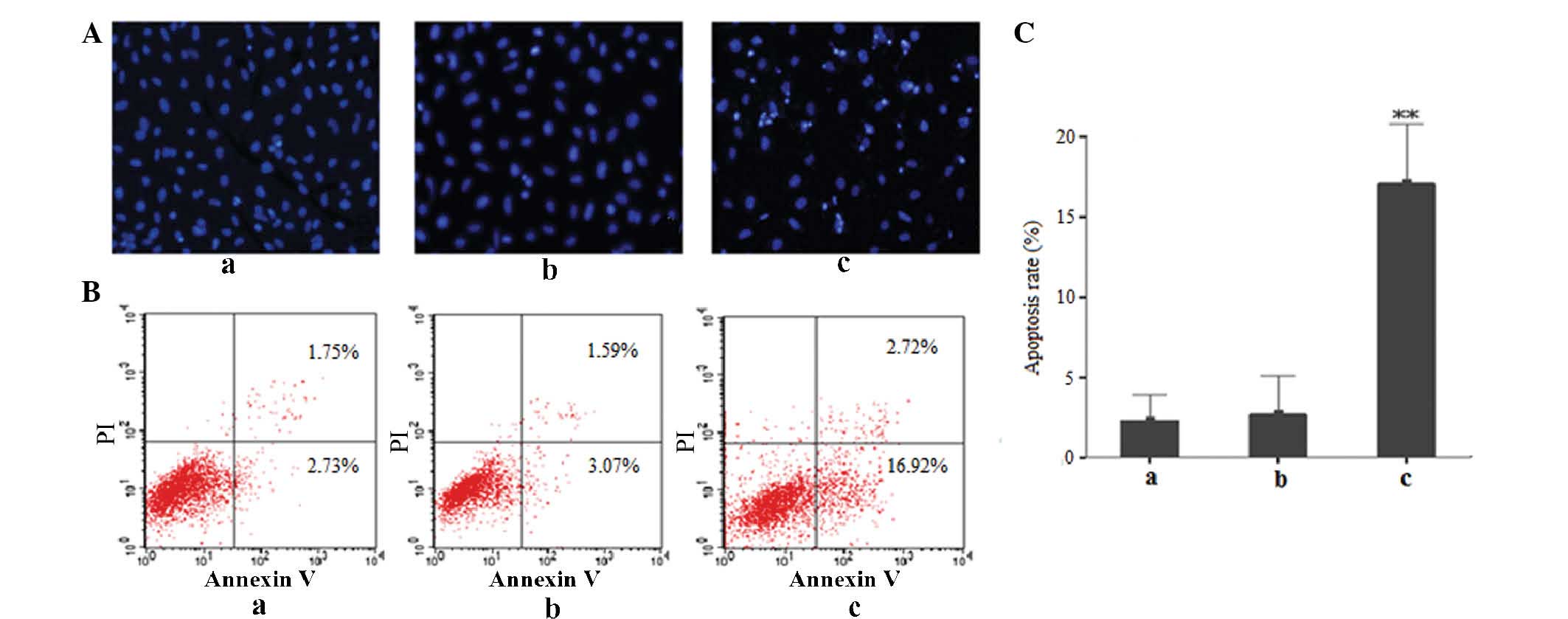
Id3 induces apoptosis in A549/DDP
cells
Fluorescence microscopy following Hoechst
33258 staining revealed that A549/DDP cells transfected with
Id3/pEGFP presented with apoptotic features, including partially
ruptured nuclei as well as cells of different sizes and with
shrunken or distorted nuclei, as indicated by conglomerated
fluorescence that presented the appearance of grains. In
comparison, only a very small proportion of cells in the
pEGFP-transfected and control groups showed these apoptotic
features (Fig. 4A). Flow
cytometric analysis further confirmed the above results: As shown
in Fig. 4B and C, increased levels
of early apoptotic cells (16.92±8.72%) were observed in the
Id3/pEGFP-transfected group, while the proportion of early
apoptotic cells in the untreated control or pEGFP-transfected
groups was markedly lower (2.73±2.54 and 3.07±5.03%, respectively).
All of these results demonstrated that ectopic expression of Id3
induced apoptosis in A549/DDP cells.
Discussion
Lung cancer is the most frequent cancer type
worldwide and its incidence increases by 0.5% per year (15). Despite major advances in disease
management, chemotherapy and radiotherapy, almost 80% of all
patients with lung cancer succumb to the disease within 1 year of
diagnosis and long-term survival is achieved in only 5–10% of all
cases (15,16). The major obstacle in lung cancer
chemotherapy is inherent and acquired drug resistance of the cancer
cells (17,18), which limits the efficacy of
chemotherapy. Therefore, it is important to identify novel
biomarkers for lung cancer which may be utilized as therapeutic
targets.
Id3 is a member of the Id family of proteins and is
a helix-loop-helix transcription factor. The tumor suppressor
function of Id3 has been reported in a variety of cancer types,
including hepatocellular carcinoma (19), prostate cancer (20) and colorectal adenocarcinoma
(21). Forced expression of Id3 in
head and neck squamous cell carcinoma cells reduced their
invasiveness interference with the transcription of matrix
metalloproteinase 2 (22). In
primary human colorectal adenocarcinomas, the expression of Id1,
Id2 and Id3 was found to be significantly increased compared with
that in normal mucosa and correlated with the presence of mutated
p53 (23,24). Numerous studies have assessed the
role of Id3 in various cancer types (25,26).
Previous studies by our group have shown that upregulation of Id3
inhibited the proliferation and induced apoptosis in A549 cells
in vitro and in vivo (13,14),
while further study is required to determine the underlying
mechanisms. Therefore, ectopic expression of Id3 may represent a
novel strategy for treating NSCLC. However, the effects of Id3 on
the drug resistant A549/DDP human lung cancer cell line have not
been previously reported, to the best of our knowledge.
Apoptosis is a form of programmed cell death, which
maintains the healthy survival/death balance in metazoan cells,
while it is generally circumvented by cancer cells (27). Apoptosis induction is an important
mechanism of action of anti-cancer agents. Numerous studies have
focused on the manipulation of specific genes to enhance the
sensitivity of cancer cells to drugs such as the DNA-damaging agent
cisplatin (28,29). High levels of Id3 have been
indicated to have a role in drug resistance and disease progression
and Id3 has been implicated in apoptosis in response to cisplatin.
Treatment with cisplatin increased the mRNA levels of Id3 in MG-63
sarcoma cells, while ectopic expression of Id3 sensitized them to
cisplatin-induced caspase-3 activation and growth inhibition
(12). The results of the present
study showed that overexpression of Id3 significantly inhibited the
growth of A549/DDP cells and induced apoptosis, indicating that
high levels of Id3 protein expression may be a potential target for
cisplatin resistance of lung adenocarinoma cells. The effects of
DDP on A549/DPP cells transfected with Id3/pEGFP will be
investigated in future studies.
The expression of Id3 is dependent on the cell type
and developmental stage. When different types of cell received
different types of stimulation, they regulated the expression of
Id3 through different mechanisms and signal transduction pathways.
Studies by Langenfeld et at (30,31)
showed that inhibition of bone morphogenetic protein signaling by
the selective antagonist DMH2 decreased the expression of Id1/Id3
and induced significant growth inhibition of lung cancer cells.
Furthermore, silencing of Id3 significantly decreased the
proliferation of lung cancer cells and induced cell death. However,
cells stably overexpressing Id3 were resistant to growth
suppression and induction of cell death induced by DMH2. By
contrast, Chen et al (32)
reported that suppression of Id3 expression in SCLC cells produced
a significant reduction in the proliferative rate and colony
formation. Another study demonstrated that co-expression of Id1 and
Id3 correlated with poor clinical outcome in patients with stage
III-N2 NSCLC treated with definitive chemoradiotherapy (33). The complexity of the regulatory
mechanism of Id3 expression determines the diversity of its
functions. These diverse effects of Id3 in tumor cells may depend
on the tumor type and stage.
The present study, for the first time, explored the
effects of Id3 on the cisplatin-resistant A549/DDP human lung
cancer cell line. Ectopic overexpression of Id3 in A549/DDP
significantly inhibited the proliferation was induced apoptosis
in vitro. Next, it will be explored whether Id3 gene
expression is associated with cisplatin resistance in
non-small-cell lung cancer, and whether Id3 overexpression can
enhance the sensitivity of lung adenocarinoma cells to DDP. Further
study is required to characterize the underlying mechanisms and the
apoptotic signaling pathways triggered by Id3; furthermore, the
effects of Id3 upregulation require verification in vivo. In
addition, the roles or association with other Id (Id1) genes may be
assessed in further studies. However, the results of the present
study indicated that Id3 may serve as a novel biomarker for NSCLC
and that its overexpression may represent a novel therapeutic
strategy for cisplatin-resistant NSCLC cells.
Acknowledgments
This work was supported by a grant from the National
Natural Science Foundation of China (no. 81171652). The authors
would like to thank International Science Editing (Shannon,
Ireland) for language editing of the manuscript.
References
|
1
|
Goldstraw P, Ball D, Jett JR, Le Chevalier
T, Lim E, Nicholson AG and Shepherd FA: Non-small-cell lung cancer.
Lancet. 378:1727–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benezra R, Davis RL, Lockshon D, Turner DL
and Weintraub H: The protein Id: A negative regulator of
helix-loop-helix DNA binding proteins. Cell. 61:49–59. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finkel T, Duc J, Fearon ER, Dang CV and
Tomaselli GF: Detection and modulation in vivo of helix-loop-helix
protein-protein interactions. J Biol Chem. 268:5–8. 1993.PubMed/NCBI
|
|
4
|
Lasorella A, Uo T and Iavarone A: Id
proteins at the cross-road of development and cancer. Oncogene.
20:8326–8333. 2001. View Article : Google Scholar
|
|
5
|
Ruzinova MB and Benezra R: Id proteins in
development, cell cycle and cancer. Trends Cell Biol. 13:410–418.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rotzer D, Krampert M, Sulyok S, Braun S,
Stark HJ, Boukamp P and Werner S: Id proteins: Novel targets of
activin action, which regulate epidermal homeostasis. Oncogene.
25:2070–2081. 2006. View Article : Google Scholar
|
|
7
|
Li XJ, Hata K and Mizuguchi J: Engagement
of membrane immunoglobulin enhances Id3 promoter activity in
WEHI-231 B lymphoma cells. Acta Pharmacol Sin. 26:486–491. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee KT, Lee YW, Lee JK, Choi SH, Rhee JC,
Paik SS and Kong G: Overexpression of Id-1 is significantly
associated with tumour angiogenesis in human pancreas cancers. Br J
Cancer. 90:1198–1203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu
BA, Luu HH, Park JK, Li X, Luo J, et al: Inhibitor of DNA
binding/differentiation helix-loop-helix proteins mediate bone
morphogenetic protein-induced osteoblast differentiation of
mesenchymal stem cells. J Biol Chem. 279:32941–32949. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kee BL, Rivera RR and Murre C: Id3
inhibits B lymphocyte progenitor growth and survival in response to
TGF-beta. Nat Immunol. 2:242–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simbulan-Rosenthal CM, Daher A, Trabosh V,
Chen WC, Gerstel D, Soeda E and Rosenthal DS: Id3 induces a
caspase-3- and -9-dependent apoptosis and mediates UVB
sensitization of HPV16 E6/7 immortalized human keratinocytes.
Oncogene. 25:3649–3660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koyama T, Suzuki H, Imakiire A, Yanase N,
Hata K and Mizuguchi J: Id3-mediated enhancement of
cisplatin-induced apoptosis in a sarcoma cell line MG-63.
Anticancer Res. 24:1519–1524. 2004.PubMed/NCBI
|
|
13
|
Li XJ, Zhu CD, Yu W, Wang P, Chen FF, Xia
XY and Luo B: Overexpression of Id3 induces apoptosis of A549 human
lung adenocarcinoma cells. Cell Prolif. 45:1–8. 2012. View Article : Google Scholar
|
|
14
|
Chen FF, Liu Y, Wang F, et al: Effects of
upregulation of Id3 in human lung adenocarcinoma cells on
proliferation, apoptosis, mobility and tumorigenicity. Cancer Gene
Therapy. 22:431–437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fridman E, Skarda J, Pinthus JH, Ramon J
and Mor Y: Expression of multidrug resistance-related protein
(MRP-1), lung resistance-related protein (LRP) and topoisomerase-II
(TOPO-II) in Wilms' tumor: Immunohistochemical study using TMA
methodology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
152:47–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asnaghi L, Calastretti A, Bevilacqua A,
D'Agnano I, Gatti G, Canti G, Delia D, Capaccioli S and Nicolin A:
Bcl-2 phosphorylation and apoptosis activated by damaged
microtubules require mTOR and are regulated by Akt. Oncogene.
23:5781–5791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scagliotti GV, Novello S and Selvaggi G:
Multidrug resistance in non-small-cell lung cancer. Ann Oncol.
10(Suppl 5): S83–S86. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takara K, Sakaeda T and Okumura K: An
update on overcoming MDR1-mediated multidrug resistance in cancer
chemotherapy. Curr Pharm Des. 12:273–286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Damdinsuren B, Nagano H, Kondo M, Yamamoto
H, Hiraoka N, Yamamoto T, Marubashi S, Miyamoto A, Umeshita K, Dono
K, et al: Expression of Id proteins in human hepatocellular
carcinoma: Relevance to tumor dedifferentiation. Int J Oncol.
26:319–321. 2005.PubMed/NCBI
|
|
20
|
Asirvatham AJ, Carey JP and Chaudhary J:
ID1-, ID2- and ID3-regulated gene expression in E2A positive or
negative prostate cancer cells. Prostate. 67:1411–1420. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arnold JM, Mok SC, Purdie D and
Chenevix-Trench G: Decreased expression of the Id3 gene at 1p36.1
in ovarian adenocarcinomas. Br J Cancer. 84:352–359. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon C, Oh Y and Roth JA: Current status
of gene therapy for lung cancer and head and neck cancer. Clin
Cancer Res. 9:5055–5067. 2003.PubMed/NCBI
|
|
23
|
Wilson JW, Deed RW, Inoue T, Balzi M,
Becciolini A, Faraoni P, Potten CS and Norton JD: Expression of Id
helix-loop-helix proteins in colorectal adenocarcinoma correlates
with p53 expression and mitotic index. Cancer Res. 61:8803–8810.
2001.PubMed/NCBI
|
|
24
|
Rockman SP, Currie SA, Ciavarella M,
Vincan E, Dow C, Thomas RJ and Phillips WA: Id2 is a target of the
beta-catenin/T cell factor pathway in colon carcinoma. J Biol Chem.
276:45113–45119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SH, Hao E, Kiselyuk A, Shapiro J,
Shields DJ, Lowy A, Levine F and Itkin-Ansari P: The Id3/E47 axis
mediates cell-cycle control in human pancreatic ducts and
adenocarcinoma. Mol Cancer Res. 9:782–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamalian L, Forootan SS, Bao ZZ, Zhang Y,
Gosney JR, Foster CS and Ke Y: Inhibition of tumourigenicity of
small cell lung cancer cells by suppressing Id3 expression. Int J
Oncol. 37:595–603. 2010.PubMed/NCBI
|
|
27
|
Pucci B, Kasten M and Giordano A: Cell
cycle and apoptosis. Neoplasia. 2:291–299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu MD, Xu JC, Fan Y, Xie QC, Li Q, Zhou
CX, Mao M and Yang Y: Hypoxia-inducible factor 1 promoter-induced
JAB1 overexpression enhances chemotherapeutic sensitivity of lung
cancer cell line A549 in an anoxic environment. Asian Pac J Cancer
Prev. 13:2115–2120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu HG, Wei W, Xia LH, Han WL, Zhao P, Wu
SJ, Li WD and Chen W: FBW7 upregulation enhances cisplatin
cytotoxicity in non-small cell lung cancer cells. Asian Pac J
Cancer Prev. 14:6321–6326. 2013. View Article : Google Scholar
|
|
30
|
Langenfeld E, Deen M, Zachariah E and
Langenfeld J: Small molecule antagonist of the bone morphogenetic
protein type I receptors suppresses growth and expression of Id1
and Id3 in lung cancer cells expressing Oct4 or nestin. Mol Cancer.
12:1292013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Langenfeld E, Hong CC, Lanke G and
Langenfeld J: Bone morphogenetic protein type I receptor
antagonists decrease growth and induce cell death of lung cancer
cell lines. PLoS One. 8:e612562013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen D, Forootan SS, Gosney JR, Forootan
FS and Ke Y: Increased expression of Id1 and Id3 promotes
tumorigenicity by enhancing angiogenesis and suppressing apoptosis
in small cell lung cancer. Genes Cancer. 5:212–225. 2014.PubMed/NCBI
|
|
33
|
Castañon E, Bosch-Barrera J, López I,
Collado V, Moreno M, López-Picazo JM, Arbea L, Lozano MD, Calvo A
and Gil-Bazo I: Id1 and Id3 co-expression correlates with clinical
outcome in stage III-N2 non-small cell lung cancer patients treated
with definitive chemoradiotherapy. J Transl Med. 11:132013.
View Article : Google Scholar : PubMed/NCBI
|