Introduction
Atherosclerosis (AS) is a leading contributor to
mortality rates in developed countries; it is a complex and
multifactorial disease and is affected by genetic and environment
factors (1). Due to the methods of
diagnosing AS being limited and the poor reversibility of the
disease, accurate and timely detection of atherosclerotic lesions
is particularly important. Several techniques have been used for
the analysis of AS clinically, including X-ray angiography,
computed tomography, magnetic resonance imaging and intravascular
ultrasound. However, the majority of these methods are unable to
detect AS until the onset of symptoms of AS (2), and several of the biological changes
occur in one event with no prior symptoms. Therefore, the
identification of an suitable method of imaging AS prior to an
advanced stage is likely to be of significance for the prevention
and treatment of AS.
Several retrospective and prospective studies in
humans and animals have shown that hyperhomocysteinemia (HHcy) is
an independent risk factor for AS, which contributes to
atherosclerotic plaque formation and development (3), and the toxicity in AS is associated
with the metabolism of homocysteine (Hcy). Hcy functions as an
intermediate in methionine metabolism and is converted to
methionine, the precursor of S-adenosylmethionine (SAM), which is
the primary methyl group donor in the majority of biological
methylation events, particularly DNA. Following transfer of the
methyl group, SAM is converted to S-adenosylhomocysteine (SAH), a
potent inhibitor of several SAM-dependent methyltransferases
(4). Previous investigations have
suggested that DNA methylation may cause disease either by
silencing genes through hyper-methylation or activating genes
through hypomethylation under HHcy (5,6).
Aberrant DNA methylation serves as an important mechanism, which
controls gene expression in a variety of chronic diseases,
particularly AS (7). In addition,
associated studies have confirmed the clinical value of certain DNA
methylation markers for the early detection of cancer, risk
assessment and prediction of therapeutic responses, as altered DNA
methylation occurs prior to the onset of symptoms of diseases,
including AS (8). Kerins et
al (9) found that SAH was a
more sensitive indicator of risk in a group of patients with a
diagnosis of cardiovascular disease, compared with Hcy, however,
other evidence has suggested that the appearance of SAH in the
plasma following an oral load of SAM and following an oral load of
methionine (10,11). In addition, patients with end-stage
renal disease had higher plasma levels of SAM and SAH, as well as
Hcy (12). As there are
differences among findings, the identification of novel and more
sensitive biomarkers for imaging AS prior to the appearance of
symptoms is important.
Increasing attention has focussed on the mechanisms
of AS, and several hypotheses have been accepted, including
oxidative stress, inflammation and altered methylation levels of
certain typical AS-associated genes (13), including extracellular superoxide
dismutase (EC-SOD), adipocyte fatty acid binding protein (FABP4)
and monocyte chemoattractant protein-1 (MCP-1) (14–16),
which are involved in the mechanisms of HHcy-induced AS. There have
been several attempts to elucidate the mechanisms of AS and DNA
methylation. Lakshmi et al (17) found that alterations in the
methylation level of EC-SOD contributed to increased oxidative
stress and increased the susceptibility of cardiovascular disease,
particularly AS. Liu et al (18) found that MCP-1 is a potent
chemokine and important in AS, in addition to reporting that the
hypomethylation of CpG sites in the MCP-1 promoter region may be
important in the vascular complications of type 2 diabetes. In our
previous studies, it was demonstrated that B1 repetitive element
hypomethylation is involved in the pathogenesis of AS (19), in which EC-SOD, FABP4, MCP-1 and B1
repetitive elements were analyzed to determine their DNA
methylation levels to confirm the association between the
methylation status of these genes and the development of AS.
The present study was designed to examine the
correlation between the methylation levels of typical AS-associated
genes and the ratio of SAM/SAH in atherosclerotic-based
apolipoprotein E (ApoE)−/− mice, and to determine
whether the SAM/SAH ratio is a superior biomarker for AS. In
addition, the present study aimed to investigate whether the
SAM/SAH ratio may be used as a rational factor in diagnosing AS in
the future.
Materials and methods
Animals and diets
A total of 48 male ApoE−/− mice (6 weeks
old; 18–22 g in weight) were provided by the Animal Center of
Peking University (Beijing, China) and were bred in the Animal
Center of Beijing University (Beijing China). The mice were housed
(6 mice per cage) in a climate-controlled room (22±2°C) with a 12 h
light/dark cycle, and provided with free access to water and a
pelleted diet (Keaoxieli, Beijing, China) for 15 weeks. The animals
were divided into the following groups: Normal control group
(n=12), wild-type mice with a regular mouse diet;
ApoE−/− control group (n=12), ApoE−/− mice
with a regular mouse diet; high-methionine group (Meth group;
n=12), ApoE−/− mice with a regular mouse diet+1.7%
methionine; high-methionine+folic acid and vitamin B12
group (Meth-F group; n=12), ApoE−/− mice with a regular
mouse diet+1.7% methionine+0.006% folic acid+0.0004% vitamin
B12. The experimental procedures used on the mice were
approved by the Ningxia Medical University Animal Care and Use
Committee and were conducted according to the Ningxia Medical
University Animal Care Committee guidelines.
Determination of serum levels of Hcy, SAM
and SAH
After 15-week experimental diets, mice were
euthanized by abdominal injection with 20% ethylcarbamate (5
ml/kg), and blood was immediately collected (~0.8–1.2 ml) to
measure serum Hcy, SAM, SAH levels. Blood was collected by cardiac
puncture and serum was separated by centrifugation (1,000 × g for
10 min at 4°C). The concentrations of Hcy were measured using an
ADVIA 2400 Chemistry System (Siemens AG, Munich, Germany). Serum
concentrations of SAM and SAH were measured using high-performance
liquid chromatography (HPLC). The supernatant of each sample was
filtered through a 0.22 µm filter (EMD Millipore, Billerica,
MA, USA) and was then loaded into a C18 column (250×4.6 mm ID; 5
µm particles; Shimadzu Corporation, Kyoto, Japan), run by a
water HPLC system (D-2000 Elite HPLC system; Hitachi
High-Technologies Corporation, Tokyo, Japan) and connected to an
ultraviolet detector. The absorption values of the eluted compounds
were monitored at (λ) ex=254 nm. Chromatograms were recorded using
an HPLC integrator, and quantification was performed by automatic
peak area integration. SAM and SAH standards were used to identify
the elution peaks. All analyses were performed in triplicate.
Tissue preparation and evaluation of
atherosclerotic lesions in the aorta
The aorta were excised and placed in plastic
processing cassettes to avoid warping of tissues. The aortas were
embedded in glycolmethacrylate (Technovit 7100; Kulzer, Wehrheim,
Germany). To quantify plaques by stereo-logical methods, ~10 evenly
spaced sections of each aortic segment were sampled. The blocks
were exhaustively sectioned at 10 µM and stained with
hematoxylin and eosin (H&E; Boster Biological Technology, Ltd.,
Wuhan, China) (19,20) as follows: The aortas were separated
carefully and then put into 4% (w/v) formalin for 24 h. The samples
subsequently underwent washing with water, dehydration with a
Citadel tissue processor (Thermo Fisher Scientific, Inc.), clearing
of surplus liquid from tissues, embedding using a Histostar tissue
embed ding system (Thermo Fisher Scientific, Inc.), coating with
paraffin wax, slicing (thickness, 4 µM) on a HM340E
micro-tome (Thermo Fisher Scientific, Inc.) and H&E staining.
The sampled sections of the aorta were projected onto a table top
at 40× magnification using a BX50F4 microscope (Olympus
Corporation, Tokyo, Japan). An orthogonal grid (2×2 cm) was
superimposed over the projected image, and the number of grid
points overlying the intima and media of the aorta were counted for
relative atherosclerotic plaque areas (21).
Nested touchdown-methylation specific
polymerase chain reaction (nt-MSP) analysis
Blood used for separation of mononuclear cells was
fresh and free of clots, in preservative-free anticoagulant.
Histopaque 1083 (Sigma-Aldrich, St. Louis, MO, USA) was added to
centrifuge tubes, then whole blood was carefully layered onto the
Histopaque 1083 surface and centrifuged at 400 × g for 30 min at
room temperature. After centrifugation, the upper layer was
aspirated carefully with a plastic pipette leaving 3 mm of the
opaque interface containing the mononuclear cells. Isotonic
phosphate-buffered saline (PBS) was then added, and this was
centrifuged at 250 × g for 10 min for three times to remove any
remaining Histopaque 1083 from the mononuclear cells. The
peripheral blood mononuclear cells (PBMCs) suspended in isotonic
PBS were used for the following assays. Genomic DNA was isolated
from the PBMCs using a Wizard® Genomic DNA purification
kit (Promega, Madison, WI, USA). DNA from each sample (4 µg)
was treated with sodium bisulfite (Sigma-Aldrich). nt-MSP analysis
was used for the detection of DNA methylation patterns in the
FABP4, EC-SOD, MCP-1 and B1 repetitive elements. The first step of
nt-MSP used a pair of outer primers, and the second polymerase
chain reaction (PCR) step was performed using conventional PCR
primers. The primer sequences are listed in Table I.
 | Table IPrimer sequences of FABP4, EC-SOD,
MCP-1 and B1 repetitive elements. |
Table I
Primer sequences of FABP4, EC-SOD,
MCP-1 and B1 repetitive elements.
| Primer set | Primer sequence
(5′→3′) | Size (bp) | Amplification
temperature (°C) |
|---|
| FABP4-O | F:
ATTTTATTAGGGAGAGAAGGAAAAA
R: TCACATCTCAAAATCTAAAACTAAC | 134 | 60.7 |
| FABP4-M | F:
AAGTTGGAAGTTTTTTTTGTTAACG
R: CCTTTACCTATATTTAGTCTTTCGAA | 150 | 60.9 |
| FABP4-U | F:
AGTTGGAAGTTTTTTTTGTTAATGG
R: TTTACCTATATTTACTTCTTTCAAA | 140 | 60.9 |
| EC-SOD-O | F:
TTTTTGAATAGAATGAAGAGGGTGTA
R: AACCAAATCAAAATTTCAATCATAAA | 543 | 55.4 |
| EC-SOD-M | F:
AGTAATGATGGAGAGGTTAGGTTTC
R: AAATAAAACAAAAAAAACACTCGTA | 110 | 64.0 |
| EC-SOD-U | F:
GTAATGATGGAGAGGTTAGGTTTTG
R: AAAATAAAACAAAAAAAACACTCATA | 110 | 64.0 |
| MCP-1-O | F:
TTGTTGAAATGAATTTTAAGGGTTT
R: CCCAAATAACTCCAACCTAACTATC | 140 | 50.0 |
| MCP-1-M | F:
TTTAAGGGTTTTTAGATTTTATCGT
R: AACTCTCTACCCTATTTCCTTCGTA | 137 | 61.6 |
| MCP-1-U | F:
TTTAAGGGTTTTTAGATTTTATTGT
R: AACTCTCTACCCTATTTCCTTCATA | 145 | 61.6 |
| B1-O | F:
ATAGAAGTGGATGTTTATAGTTAGTTATTG
R: CACTCCAACTTTTTAACCCTAAC | 269 | 64.7 |
| B1-M | F:
GTTAGTTATTGGATGGGTTATACGG
R: TACAACTAAAAACAAAAACTCCGAA | 139 | 62.1 |
| B1-U | F:
GTTAGTTATTGGATGGGTTATATGG
R: TACAACTAAAAACAAAAACTCCAAA | 139 | 62.1 |
To reduce misprinting and increase efficiency,
nt-MSP was used for amplification. Quantitative (q)PCR analysis was
performed with the ABI Prism 7000 Sequence Detection System (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Nested-MSP consisted of
two-step PCR amplification after a standard sodium bisulfite DNA
modification. The first step used an outer primer pair set that did
not contain any CpG. The second PCR step was performed with the
conventional PCR primers (Table
I). To reduce mispriming and to increase efficiency, touchdown
(TD) PCR was used in the amplification, whereby the temperatures
are decreased sequentially for each cycle. Samples were subjected
to 30 cycles in a TD program (0.5°C reduction in temperatures each
cycle). After completion of the TD program, 20 cycles were run,
ending with a 5-min extension at 72°C. The PCR cycling conditions
were as follows: 30 TD cycles (0.5°C decrease in all temperatures
at each cycle) of 94°C for 45 sec, amplification temperature
(Table I) for 45 sec, and 72°C for
45 sec; then 20 cycles of 94°C for 45 sec, amplification
temperature for 45 sec and 72°C for 45 sec, with a final extension
step of 5 min at 72°C. The PCR products were separated by
electrophoresis in 2% agarose gel containing ethidium bromide at
120 V for 20 min. DNA bands were visualized using a ChemiDoc XRS
system with Image Lab software, version 4.1 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and calculated usingthe following
formula: Methylation (%) = methylation/(methylation +
unmethylation) x 100.
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. Data were analyzed using one-way analysis of
variance, and additional analyses were performed using the Student
Newman-Keuls test for multiple comparisons within treatment groups
or a t-test for between two groups. The correlation between
the SAM/SAH ratio and the methylation levels of the AS-associated
genes in the arteries of the ApoE−/− mice were analyzed
using Pearson correlation and linear regression analyses. P<0.05
was considered to indicate a statistical significant
difference.
Results
Serum levels of Hcy and atherosclerotic
plaque areas in ApoE−/− mice
To confirm whether the atherosclerotic model was
successfully established, the serum levels of Hcy and
atherosclerotic plaque areas were measured, as shown in Table II. After 15 weeks, the serum
concentrations of Hcy were significantly increased by 1.57-, 3.41-
and 1.97-fold in the ApoE−/− control group, Meth group
and Meth-F group, respectively, compared with the normal control
group (P<0.05 or P<0.01). These data suggested that a
high-methionine diet induced HHcy in the ApoE−/− mice.
However, the serum concentration of Hcy was decreased by 42.3% in
the Meth-F group, compared with the Meth group (P<0.05), which
indicated that folate and vitamin B12 modulated the
effect caused by the high methionine diet.
 | Table IISerum levels of Hcy and
atherosclerotic lesion area. |
Table II
Serum levels of Hcy and
atherosclerotic lesion area.
| Group | Hcy
(µmol/l) | Atherosclerotic
lesion area (105/µm2) |
|---|
| Normal control | 6.51±0.21 | 0 |
| ApoE−/−
control | 10.25±0.86a | 3.99±0.45b |
| Meth | 22.22±1.54b | 6.22±0.49b |
| Meth-F | 12.82±1.80c | 4.41±0.57c |
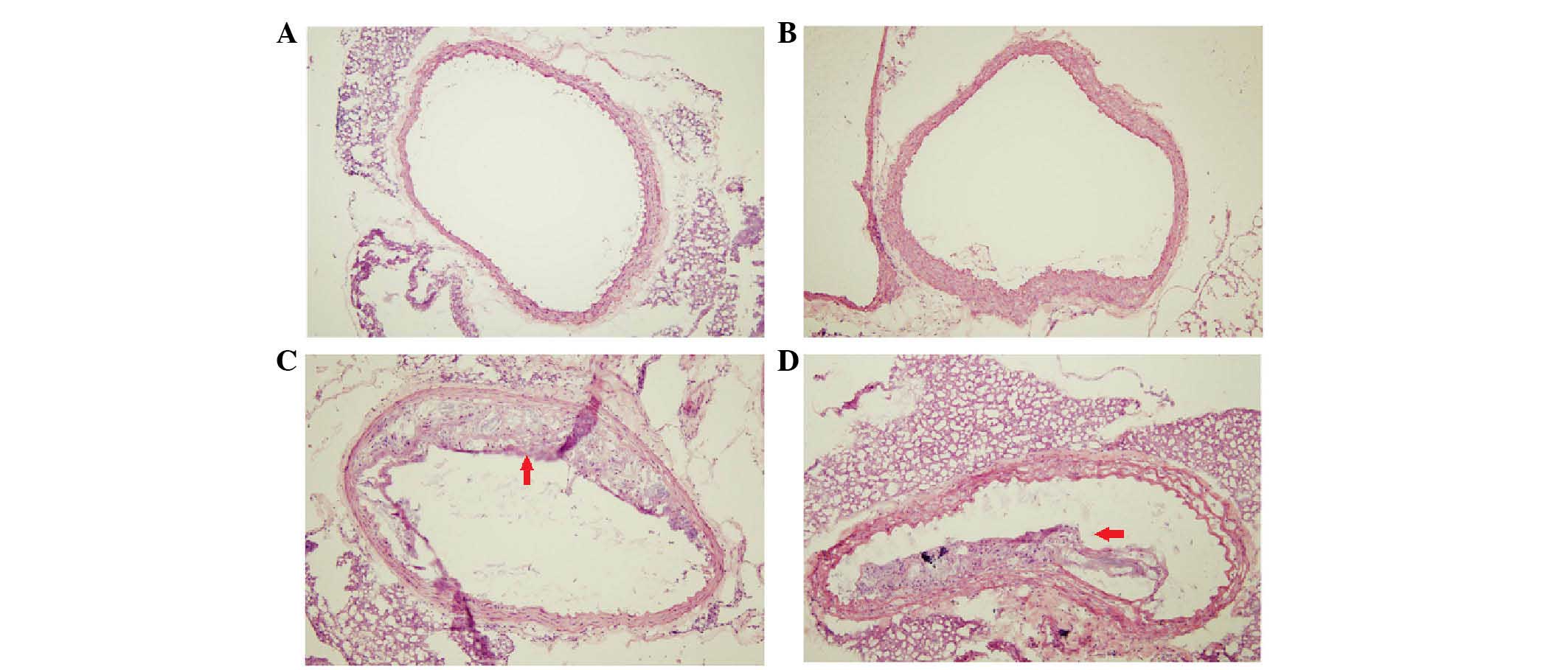
The atherosclerotic lesions were examined and
analyzed using a cross-sectional stereological method for the
aortic root, as shown in Fig. 1
and Table II. Compared with the
normal control group, the sizes of the atherosclerotic lesions were
significantly increased in the n ApoE−/− control group,
Meth group and Meth-F group (P<0.05 or P<0.01). However, the
area of the atherosclerotic lesions was decreased by 39.4% in the
Meth-F group, compared with the Meth group, (P<0.05). The
atherosclerotic lesions in the Meth group were the most severe and
widespread, which suggested HHcy may have had an effect in
accelerating the development of AS, whereas folate and vitamin
B12 modulated the effect of HHcy on the formation of
atherosclerotic plaques (19,20).
Serum concentrations of SAM and SAH,
measured using HPLC, in mice
SAM and SAH are intermediates of methionine
metabolism and, for the majority of cellular methyltransferase
reactions, SAM is a priority for one-carbon metabolism as the
methyl donor. SAM is converted to SAH within the active site of the
methyltransferase by transfer of the methyl group (22). As shown in the diagram in Fig. 2, the serum concentrations of SAM
and SAH were detected, and the ratio of SAM/SAH was calculated.
After 15 weeks on the experimental diets, the concentrations of SAM
were increased by 3.02-, 3.42- and 2.46-fold in the
ApoE−/− control group, Meth group and Meth-F group,
respectively, compared with the normal control group (P<0.05 or
P<0.01). In addition, the serum concentrations of SAH were
1.08-fold higher in the Meth group, compared with the normal
control group (P<0.05). By contrast, the ratio of SAM/SAH,
compared with the normal control group, was increased by 1.67- and
2.75-fold in the ApoE−/− control group and Meth group,
respectively (P<0.05 or P<0.01). These data indicated that
the atherogenic diets caused a parallel increase in the levels of
SAM and SAH, and the SAM/SAH ratio.
DNA methylation levels of B1 repetitive
elements, FABP4, EC-SOD and MCP-1, and their correlation with SAM,
SAH and SAM/SAH
To confirm whether the methylation levels of the
AS-associated genes were correlated with the ratio of SAM/SAH, the
present study measured the methylation levels of B1 repetitive
elements, FABP4, EC-SOD and MCP-1. As shown in Fig. 3A, the methylation levels of the B1
repetitive elements were decreased by 40% in the Meth group,
compared with the normal control group (P<0.05), however, the
methylation levels of the B1 repetitive elements were increased by
1.54-fold in the Meth-F group. These data indicated that the B1
repetitive elements were hypomethylated, which accounted for the
majority of gene transcription activity in the pathogenesis of AS,
induced by HHcy. The levels of B1 methylation were negatively
correlated with levels of SAH (P=0.0212; γ=−0.3634); However, as
shown in Fig. 3D, there was a
positive correlation between the methylation levels of the B1
repetitive elements and the ratio of SAM/SAH (P=0.0210; γ=−0.3638).
The B1 methylation levels were correlated with the concentration of
serum SAM; however, the results showed that the correlation between
the B1 methylation levels and the SAM/SAH ratio was more
marked.
FABP4 is important in metabolic deterioration and
the development of AS (23). As
shown in Fig. 4A, the methylation
levels of FABP4 detected by nt-MSP decreased by 5.02% in the Meth
group, compared with the normal control group (P<0.05). In
addition, FABP4 methylation levels were negatively correlated with
the ratio of SAM/SAH (P= 0.00158; γ=−0.3792). These findings
indicated that FABP4, a crucial gene in lipid metabolism and
inflammatory reactions exhibited with hypomethylation in the AS
model induced by HHcy.
EC-SOD regulates baseline cardiac morphology and
protects the heart from fibrosis, apoptosis and loss of function
following oxidative injury caused by HHcy (24). As shown in Fig. 5A the methylation status of EC-SOD
showed marked hypermethylation. Compared with the normal control
group, the methylation of EC-SOD in the Meth group was increased by
1.08-fold (P<0.05). Additionally, as shown in Fig. 5D, the methylation levels of EC-SOD
were significantly negatively correlated with the ratio of SAM/SAH
(P=0.0117; γ=−0.3949).
MCP-1 is important in inflammation and is a basic
mediator in a biological system, which is valuable in the formation
of AS (25). Following feeding
with the respective experimental diets for 15 weeks, the status of
MCP-1 exhibited hypomethylation in the PBMCs. In the Meth group,
the methylation levels were decreased by 6.18%, compared with the
normal control group (P<0.05), as shown in Fig. 6A. In investigating the association
between MCP-1 methylation and SAM/SAH ratio, a negative correlation
was found (P=0.0496; γ=−0.3125).
Taken together, the data of the present study
indicated that there was a marked correlation between the SAM/SAH
ratio and the methylation levels of atherosclerotic-associated
genes, which were considered a biomarker of AS. These findings
provided evidence that the ratio of SAM/SAH may be a more sensitive
indictor for AS.
Discussion
Numerous epidemiological investigations and
case-control studies have demonstrated that elevated plasma Hcy is
associated with an increased risk of vascular disease (26–28).
However, other case-control studies have not demonstrated such a
causal association, and the mechanism of Hcy-induced AS remains to
be fully elucidated (29,30). In addition, whether Hcy itself is a
major factor or an indirect metabolic marker of certain biochemical
processes, which occur in AS is unclear. The present study aimed to
identify an effective and reliable biomarker of Hcy-induced AS.
In the processes of methionine metabolism, there are
several byproducts, including Hcy, SAM and SAH, and previous
studies have demonstrated that alterations in the SAM/SAH ratio may
be more directly associated with vascular damage, compared with Hcy
or the other intermediates (31–33).
A number of studies have described that increased levels of Hcy are
always followed by higher concentrations of SAH, and it may be an
important mediator of toxicity in the body. In addition, the
SAM/SAH ratio is considered to be an indicator of the transfer of
methyl groups from SAM to the numerous methyl acceptors in cells,
and thereby reflects its methylation potential (34). The significance of this observation
is associated with the hypothesis that SAM and SAH may be key
components in the pathophysiology of the Hcy-vascular disease axis.
Although the hypothesis that Hcy is directly linked to damage in
the vascular endothelium has not been validated, studies have
suggested that there is an association between the SAM/SAH ratio
and vascular injury, and a decrease in the SAM/SAH ratio is often
regarded as an indicator of reduced cellular methylation capacity
(35). In the present study,
ApoE−/− mice were fed a high methionine diet, and it was
found that the serum concentrations of Hcy were increased,
particularly in the Meth group. Compared with the normal group, the
serum concentrations of Hcy were increased by 1.15-, 2.54- and
1.17-fold in the ApoE−/− control group, Meth group and
Meth-F group, respectively. In addition, as the concentration of
Hcy increased, the atherosclerotic lesion was more severely
widespread. The present study also found that folate and vitamin
B12 suppressed the effect of Hcy-induced AS, and the
sizes of the atherosclerotic lesions were significantly increased
in the ApoE−/− control group, Meth group and Meth-F
group, by up to 1.44-, 2.40- and 1.45-fold, respectively, compared
with those of the normal control group. Taken together, these data
suggested that establishment of the AS model induced by HHcy was
successful. As the intermediates of methionine metabolism, the
concentrations of SAM and SAH also increased, as did the ratio of
SAM/SAH.
Several studies have confirmed that alterations in
the methylation levels of specific genes are usually associated
with the occurrence and development of cancer (36). AS is similar to cancer in certain
aspects and has been referred to as a cancer-like disease (37,38);
AS and cancer are chronic and progressive, and the inducing factors
are very similar. In addition, the pathological mechanisms are
similar, involving genetic and environmental elements. In the
present study, the methylation statuses of specific genes
associated with AS were examined. Cash et al (39) first suggested that DNA
hypo-methylation is involved in the development of cardiovascular
disease, and evidence from animal studies has suggested that global
DNA hypomethylation is associated with atherosclerotic lesions. A
number of previous reports have focused on alterations in the DNA
methylation of specific genes associated with AS (40,41).
Global hypomethylation affects CpG dinucleotides in repetitive
elements and in certain gene-specific promoter CpG islands
(42). In the present study, it
was found that the methylation levels of pivotal AS-associated
genes were altered. B1 repetitive elements are the sequences of
short interspersed nucleotide elements in mice, corresponding to
the Alu sequence in humans (19).
Increasing evidence suggests that B1 repetitive element
hypomethylation is associated with gene transcriptional activity in
the pathogenesis of cardiovascular diseases, particularly AS
(43). In the present study, it
was also found that B1 repetitive elements exhibited
hypomethylation in the blood cells of the AS model. FABP4 has been
identified as a potential circulation marker for metabolic
syndrome, and is increased in individuals with diabetes and
metabolic syndrome due to the development of subclinical
inflammation, which leads to AS (44). The present study is the first, to
the best of our knowledge to report that the methylation levels of
FABP4 were closely associated with AS. EC-SOD protects arteries
against the deleterious effects of superoxide anions and the
development of AS. Laukkanen et al (45) reported that the altered methylation
of EC-SOD is associated with the development of AS, and suggested
that it may affect the structures and functions of EC-SOD and other
genes, which may be involved in the development of atherosclerotic
lesions in mice. The present study found that EC-SOD was
hypermethylated, particularly in the Meth group; the methylation of
EC-SOD was increased by 1.08-fold, compared with that in the normal
control group. It has been reported that CpG site hypomethylation
in the MCP-1 promoter region may be important in the AS of type 2
diabetes (18). The present study
also found that, in the Meth group, MCP-1 was hypomethylated, and
the methylation levels were decreased by 6.18%, compared with the
normal control group. Therefore it was hypothesized that the DNA
methylation levels of FABP4, EC-SOD, MCP-1 and B1 repetitive
elements may be an indicator of AS.
Several studies investigating biomarkers of AS have
been performed, and have reported that Hcy may be a sensitive
biomarker for AS (46–48). It has also been reported that
plasma SAH is a more sensitive marker of clinical cardiovascular
disease than plasma Hcy (49).
However, the data of the present study suggested that the SAM/SAH
ratio may be a more sensitive biomarker of AS. By causing feedback
inhibition of SAM-dependent methyltransferases, the accumulation of
SAH can affect the DNA methylation pattern and lead to the
promotion of chronic diseases, and the SAM/SAH ratio is the prime
regulator of the activities of the majority of methyltransferases
in the cell (50). SAH is the
metabolic precursor of Hcy in a reaction which catalyzes SAM by SAH
hydrolase. Due to this reaction, changes in the concentrations of
SAM and SAH are always equal, but the ratio usually does not change
significantly. A study by Loehrer et al (10) described the levels of SAH in the
plasma following an oral load of SAM and following an oral load of
methionine. In the present study, SAM and SAH concentrations were
determined by HPLC. Serum Hcy concentrations were measured by an
automatic biochemical analyzer. HPLC has high sensitivity and
accuracy, and so the ratio of S-adenosylmethionine to
S-adenosylhomocysteine can be used as early diagnostic indicators.
Therefore, the present study performed correlation analyses of
FABP4, EC-SOD, MCP-1 and B1 repetitive elements, respectively, with
serum levels of SAM and SAH, and the SAM/SAH ratio, to confirm
which is the optimal biomarker of AS. The results of the present
study showed that the methylation levels of these AS-associated
genes were significantly correlated with the ratio of SAM/SAH in
the ApoE−/− mice.
In conclusion, FABP4, EC-SOD and MCP-1 are important
in the formation and/or development of AS, and previous studies
have confirmed that DNA methylation is a diagnostic biomarker of AS
(51–53). On this basis, the present study
analyzed the correlation between DNA methylation of FABP4, EC-SOD,
MCP-1, and B1 repetitive elements and the serum SAM/SAH ratio to
confirm the hypothesis that the serum SAM/SAH ratio may be a more
sensitive biomarker of AS, and may be used for the clinical
diagnosis of AS. Further investigations are required to support and
confirm this conclusion, to determine its potential for clinical
usage.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81160044, 81260105
and 81260063), the Colleges and Universities Focus on Science and
Technology research projects (grant no. NGY2010039) and the Ning
Xia Science and Technique project (grant no. 20100820).
References
|
1
|
Segers D, Lipton JA, Leenen PJ, Cheng C,
Tempel D, Pasterkamp G, Moll FL, de Crom R and Krams R:
Atherosclerotic plaque stability is affected by the chemokine
CXCL10 in both mice and humans. Int J Inflam. 2011:9361092011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orbay H, Hong H, Zhang Y and Cai W:
Positron emission tomography imaging of atherosclerosis.
Theranostics. 3:894–902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma S, Zhang H, Sun W, Gong H, Wang Y, Ma
C, Wang J, Cao C, Yang X, Tian J and Jiang Y: Hyperhomocysteinemia
induces cardiac injury by up-regulation of p53-dependent Noxa and
Bax expression through the p53 DNA methylation in
ApoE−/− mice. Acta Biochim Biophys Sin (Shanghai).
45:391–400. 2013. View Article : Google Scholar
|
|
4
|
Green TJ, Skeaff CM, McMahon JA, Venn BJ,
Williams SM, Devlin AM and Innis SM: Homocysteine-lowering vitamins
do not lower plasma S-adenosylhomocysteine in older people with
elevated homocysteine concentrations. Br J Nutr. 103:1629–1634.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pizzolo F, Blom HJ, Choi SW, Girelli D,
Guarini P, Martinelli N, Stanzial AM, Corrocher R, Olivieri O and
Friso S: Folic acid effects on s-adenosylmethionine,
s-adenosylhomocysteine, and DNA methylation in patients with
intermediate hyperhomocysteinemia. J Am Coll Nutr. 30:11–18. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma SL, Tang NL and Lam LC: Association of
gene expression and methylation of UQCRC1 to the predisposition of
Alzheimer's disease in a Chinese population. J Psychiatr Res.
76:143–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sipkens JA, Hahn NE, Blom HJ, Lougheed SM,
Stehouwer CD, Rauwerda JA, Krijnen PA, van Hinsbergh VW and Niessen
HW: S-Adenosylhomocysteine induces apoptosis and
phosphatidyl-serine exposure in endothelial cells independent of
homocysteine. Atherosclerosis. 221:48–54. 2012. View Article : Google Scholar
|
|
8
|
Tehlivets O: Homocysteine as a risk factor
for atherosclerosis: Is its conversion to s-adenosyl-L-homocysteine
the key to deregulated lipid metabolism? J Lipids. 2011:7028532011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kerins DM, Koury MJ, Capdevila A, Rana S
and Wagner C: Plasma S-adenosylhomocysteine is a more sensitive
indicator of cardiovascular disease than plasma homocysteine. Am J
Clin Nutr. 74:723–729. 2001.PubMed/NCBI
|
|
10
|
Loehrer FM, Schwab R, Angst CP, Haefeli WE
and Fowler B: Influence of oral S-adenosyl-methionine on plasma
5-methyl-tetrahydrofolate, S-adenosylhomocysteine, homocysteine and
methionine in healthy humans. J Pharmacol Exp Ther. 282:845–850.
1997.PubMed/NCBI
|
|
11
|
Loehrer FM, Haefeli WE, Angst CP, Browne
G, Frick G and Fowler B: Effect of methionine loading on
5-methyltetrahydro-folate, 5-adenosyl-methionine and
S-adenosylhomocysteine in plasma of healthy humans. Clin Sci.
91:79–86. 1996. View Article : Google Scholar
|
|
12
|
Loehrer FM, Angst CP, Brunner FP, Haefeli
WE and Fowler B: Evidence for disturbed S-adenosylmethionine:
S-adenosylhomocysteine ratio in patients with end-stage renal
failure: A cause for disturbed methylation reactions? Nephrol Dial
Transplant. 13:656–661. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meehan RR and Stancheva I: DNA methylation
and control of gene expression in vertebrate development. Essays
Biochem. 37:59–70. 2001. View Article : Google Scholar
|
|
14
|
Chen NC, Yang F, Capecci LM, Gu Z, Schafer
AI, Durante W, Yang XF and Wang H: Regulation of homocysteine
metabolism and methylation in human and mouse tissues. FASEB J.
24:2804–2817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bird A: DNA methylation patterns and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukushige S and Horii A: DNA methylation
in cancer: A gene silencing mechanism and the clinical potential of
its biomarkers. Tohoku J Exp Med. 229:173–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lakshmi SV, Naushad SM, Reddy CA, Saumya
K, Rao DS, Kotamraju S and Kutala VK: Oxidative stress in coronary
artery disease: Epigenetic perspective. Mol Cell Biochem.
374:203–211. 2013. View Article : Google Scholar
|
|
18
|
Liu ZH, Chen LL, Deng XL, Song HJ, Liao
YF, Zeng TS, Zheng J and Li HQ: Methylation status of CpG sites in
the MCP-1 promoter is correlated to serum MCP-1 in Type 2 diabetes.
J Endocrinol Invest. 35:585–589. 2012.
|
|
19
|
Jiang Y, Zhang H, Sun T, Wang J, Sun W,
Gong H, Yang B, Shi Y and Wei J: The comprehensive effects of
hyperlipidemia and hyperhomocy-steinemia on pathogenesis of
atherosclerosis and DNA hypomethylation in ApoE−/− mice.
Acta Biochim Biophys Sin (Shanghai). 44:866–875. 2012. View Article : Google Scholar
|
|
20
|
Zulli A, Widdop RE, Hare DL, Buxton BF and
Black MJ: High methionine and cholesterol diet abolishes
endothelial relaxation. Arterioscler Thromb Vasc Biol.
23:1358–1363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Sun T, Xiong J, Cao J, Li G and
Wang S: Hyperhomo cysteinemia-mediated DNA hypomethylation and its
potential epigenetic role in rats. Acta Biochim Biophys Sin
(Shanghai). 39:657–667. 2007. View Article : Google Scholar
|
|
22
|
Hagebeuk EE, Duran M, Abeling NG, Vyth A
and Poll-The BT: S-adenosylmethionine and S-adenosylhomocysteine in
plasma and cerebrospinal fluid in Rett syndrome and the effect of
folinic acid supplementation. J Inherit Metab Dis. 36:967–972.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agardh HE, Gertow K, Salvado DM,
Hermansson A, van Puijvelde GH, Hansson GK, N-Berne GP and
Gabrielsen A: Fatty acid binding protein 4 in circulating
leucocytes reflects atherosclerotic lesion progression in
Apoe(−/−) mice. J Cell Mol Med. 17:303–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maksimenko AV and Vavaev AV: Antioxidant
enzymes as potential targets in cardioprotection and treatment of
cardiovascular diseases. Enzyme antioxidants: The next stage of
pharmacological counterwork to the oxidative stress. Heart Int.
7:e32012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Linic IS, Sosa I, Kovacevic M, Ivancic A,
Trobonjaca Z, Ledic D, Grubesic A, Dvornik S and Stifter S:
Predicting carotid restenosis by comparison of plaque MCP-1 mRNA
expression and serum levels. Med Hypotheses. 80:26–28. 2013.
View Article : Google Scholar
|
|
26
|
Ikkruthi S, Rajappa M, Nandeesha H,
Satheesh S, Sundar I, Ananthanarayanan PH and Harichandrakumar KT:
Hyperhomocysteinemia and hyperlipoproteinemia (a) in obese south
indian men: An indication for increased cardiovascular risk. Acta
Physiol Hung. 101:13–20. 2014. View Article : Google Scholar
|
|
27
|
Ajith TA and Ranimenon: Homocysteine in
ocular diseases. Clin Chim Acta. 450:316–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Chen S, Yao T, Li D, Wang Y, Li Y,
Wu S and Cai J: Homocysteine as a risk factor for hypertension: A
2-year follow-up study. PLoS One. 9:e1082232014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang D, Wen X, Wu W, Xu E, Zhang Y and
Cui W: Homocysteine-related hTERT DNA demethylation contributes to
shortened leukocyte telomere length in atherosclerosis.
Atherosclerosis. 231:173–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Griffiths HR, Aldred S, Dale C, Nakano E,
Kitas GD, Grant MG, Nugent D, Taiwo FA, Li L and Powers HJ:
Homocysteine from endothelial cells promotes LDL nitration and
scavenger receptor uptake. Free Radic Biol Med. 40:488–500. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hagebeuk EE, Duran M, Abeling NG, Vyth A
and Poll-The BT: S-adenosylmethionine and S-adenosylhomocysteine in
plasma and cerebrospinal fluid in Rett syndrome and the effect of
folinic acid supplementation. J Inherit Metab Dis. 36:967–972.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yideng J, Jianzhong Z, Ying H, Juan S,
Jinge Z, Shenglan W, Xiaoqun H and Shuren W: Homocysteine-mediated
expression of SAHH, DNMTs, MBD2, and DNA hypomethylation potential
pathogenic mechanism in VSMCs. DNA Cell Biol. 26:603–611. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang XL, Tian J, Liang Y, Ma CJ, Yang AN,
Wang J, Ma SC, Cheng Y, Hua X and Jiang YD: Homocysteine induces
blood vessel global hypomethylation mediated by LOX-1. Genet Mol
Res. 13:3787–3799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Enneman AW, van der Velde N, de Jonge R,
Heil SG, Stolk L, Hofman A, Rivadeneira F, Zillikens MC,
Uitterlinden AG and van Meurs JB: The association between plasma
homocysteine levels, methylation capacity and incident osteoporotic
fractures. Bone. 50:1401–1405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maegawa S, Hinkal G, Kim HS, Shen L, Zhang
L, Zhang J, Zhang N, Liang S, Donehower LA and Issa JP: Widespread
and tissue specific age-related DNA methylation changes in mice.
Genome Res. 20:332–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holm S, Ueland T, Dahl TB, Michelsen AE,
Skjelland M, Russell D, Nymo SH, Krohg-Sørensen K, Clausen OP, Atar
D, et al: Fatty acid binding protein 4 is associated with carotid
atherosclerosis and outcome in patients with acute ischemic stroke.
PLoS One. 6:e287852011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orekhov AN, Sobenin IA, Gavrilin MA,
Gratchev A, Kotyashova SY, Nikiforov NG and Kzhyshkowska J:
Macrophages in immunopathology of atherosclerosis: A target for
diagnostics and therapy. Curr Pharm Des. 21:1172–1179. 2015.
View Article : Google Scholar :
|
|
38
|
Shepard CW and Steinberger J: Premature
atherosclerotic cardiovascular disease in childhood cancer
survivors. Prog Pediatr Cardiol. 39:59–66. 2015. View Article : Google Scholar
|
|
39
|
Cash HL, McGarvey ST, Houseman EA, Marsit
CJ, Hawley NL, Lambert-Messerlian GM, Viali S, Tuitele J and Kelsey
KT: Cardiovascular disease risk factors and DNA methylation at the
LINE-1 repeat region in peripheral blood from Samoan Islanders.
Epigenetics. 6:1257–1264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Connelly JJ, Cherepanova OA, Doss JF,
Karaoli T, Lillard TS, Markunas CA, Nelson S, Wang T, Ellis PD,
Langford CF, et al: Epigenetic regulation of COL15A1 in smooth
muscle cell replicative aging and atherosclerosis. Hum Mol Genet.
22:5107–5120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Terra X, Quintero Y, Auguet T, Porras JA,
Hernández M, Sabench F, Aguilar C, Luna AM, Del Castillo D and
Richart C: FABP 4 is associated with inflammatory markers and
metabolic syndrome in morbidly obese women. Eur J Endocrinol.
164:539–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lisanti S, Omar WA, Tomaszewski B, De
Prins S, Jacobs G, Koppen G, Mathers JC and Langie SA: Comparison
of methods for quantification of global DNA methylation in human
cells and tissues. PLoS One. 8:e790442013. View Article : Google Scholar : PubMed/NCBI
Wagner C and Koury MJ:
S-Adenosylhomocysteine: A better indicator of vascular disease than
homocysteine? Am J Clin Nutr. 86:1581–1595. 2007.PubMed/NCBI
|
|
43
|
Girona J, Rosales R, Plana N, Saavedra P,
Masana L and Vallvé JC: FABP4 induces vascular smooth muscle cell
proliferation and migration through a MAPK-dependent pathway. PLoS
One. 8:e819142013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang AN, Zhang HP, Sun Y, Yang XL, Wang N,
Zhu G, Zhang H, Xu H, Ma SC, Zhang Y, et al: High-methionine diets
accelerate atherosclerosis by HHcy-mediated FABP4 gene
demethylation pathway via DNMT1 in ApoE(−/−) mice. FEBS Lett.
589:3998–4009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Laukkanen MO, Kivelä A, Rissanen T,
Rutanen J, Karkkainen MK, Leppanen O, Bräsen JH and Yla-Herttuala
S: Adenovirus-mediated extracellular superoxide dismutase gene
therapy reduces neointima formation in balloon-denuded rabbit
aorta. Circulation. 106:1999–2003. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Giovannone R, Busetto GM, Antonini G, De
Cobelli O, Ferro M, Tricarico S, Del Giudice F, Ragonesi G, Conti
SL, Lucarelli G, et al: Hyperhomocysteinemia as an early predictor
of erectile dysfunction: International Index of Erectile Function
(IIEF) and penile doppler ultrasound correlation with plasma levels
of homocysteine. Medicine (Baltimore). 94:e15562015. View Article : Google Scholar
|
|
47
|
Kim SJ, Choe YH and Bang OY;
Chaos-Biomarker Collaborators: Are stroke biomarkers seeing brain
vessels in patients with ischemic stroke?: A C-reactive protein and
homocysteine study. Stroke. 42:1464–1468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cummings DM, King DE, Mainous AG and
Geesey ME: Combining serum biomarkers: The association of
C-reactive protein, insulin sensitivity, and homocysteine with
cardiovascular disease history in the general US population. Eur J
Cardiovasc Prev Rehabil. 13:180–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu C, Wang Q, Guo H, Xia M, Yuan Q, Hu Y,
Zhu H, Hou M, Ma J, Tang Z and Ling W: Plasma
S-adenosylhomocysteine is a better biomarker of atherosclerosis
than homocysteine in apolipoprotein E-deficient mice fed high
dietary methionine. J Nutr. 138:311–315. 2008.PubMed/NCBI
|
|
50
|
Han XB, Zhang HP, Cao CJ, Wang YH, Tian J,
Yang XL, Yang AN, Wang J, Jiang YD and Xu H: Aberrant DNA
methylation of the PDGF gene in homocysteine-mediated VSMC
proliferation and its underlying mechanism. Mol Med Rep.
10:947–954. 2014.PubMed/NCBI
|
|
51
|
Perng W, Villamor E, Shroff MR, Nettleton
JA, Pilsner JR, Liu Y and Diez-Roux AV: Dietary intake, plasma
homocysteine, and repetitive element DNA methylation in the
Multi-Ethnic Study of Atherosclerosis (MESA). Nutr Metab Cardiovasc
Dis. 24:614–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao J, Forsberg CW, Goldberg J, Smith NL
and Vaccarino V: MAOA promoter methylation and susceptibility to
carotid atherosclerosis: Role of familial factors in a monozygotic
twin sample. BMC Med Genet. 13:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wilson AS, Power BE and Molloy PL: DNA
hypomethylation and human diseases. Biochim Biophys Acta.
1775:138–162. 2007.
|