Introduction
Bone marrow stem cells (BMSCs) are considered as the
best 'seeds' in cell replacement therapy (CRT) and tissue
engineering due to their strong differentiation potential, easy
availability and amplification, and absence of rejection in
autotransfusion. BMSCs have wide applications in bone and cartilage
reconstruction (1,2) and the repair and therapy of bone
marrow injury (3),
hypoxic-ischemic nerve cells (4)
and myocardial cells (5,6). Previous studies have demonstrated
that the growth and differentiation potential may be affected
either due to the accumulation of reactive oxygen species (ROS) in
the region of BMSCs transplantation caused by ischemic injury
(7) or due to the increased level
of ROS resulting from natural aging or estrogen deficiency
(8,9). Therefore, the effect of BMSCs
transplantation in CRT and tissue engineering is greatly
impaired.
Elevation of ROS levels is the major reason for
mitochondrial swelling, decline of mitochondrial membrane
potential, calcium overload and the release of precursor proteins
in mitochondrial death pathway (10,11).
Oxidative stress leads to apoptotic injury in BMSCs. Therefore, the
improvement of the survival of BMSCs in the transplantation region
by anti-oxidative and anti-apoptotic therapies is key to the
success of CRT and tissue engineering.
Previous studies indicated that estrogen antagonizes
the apoptosis of BMSCs under oxidative stress by protecting the
mitochondrial membrane integrity (12–14).
Phytoestrogen possesses estrogen-like activity and exhibits the
function of clearing free radicals and anti-oxidative effects in
cellular experiments (15,16). Ginsenoside Rg1 is a representative
monomer in panaxatriol saponins and the primary active compound in
ginseng. As a type of phytoestrogen, ginsenoside Rg1 demonstrates
anti-aging, anti-oxidative and anti-apoptotic abilities in nerve
and cardiovascular cells (17–20).
The present study hypothesized that ginsenoside Rg1 may antagonize
the apoptosis of BMSCs under oxidative stress by protecting
mitochondrial membrane integrity.
The phosphatidylinositol-3 kinase/protein kinase B
(PI3K/Akt) pathway is one of the primary signal transduction
pathways inhibiting cell apoptosis and promoting cell survival
(21). The PI3K/Akt pathway has an
anti-apoptotic effect via the phosphorylation of downstream protein
Bad and the activation of B-cell lymphoma 2 (Bcl-2) (22). Previous studies demonstrated that
activation of the PI3K/Akt pathway regulates the
H2O2-induced apoptosis of rat BMSCs (rBMSCs)
(23–27). The current study hypothesized that
the antagonistic effect of ginsenoside Rg1 against the apoptosis of
BMSCs induced by oxidative stress may be associated with the
activation of the PI3K/Akt pathway.
A model of H2O2-induced
oxidative injury in rBMSCs was developed in order to verify the
above hypothesis. The protective effect of ginsenoside Rg1 against
H2O2-induced oxidative injury in rBMSCs was
observed. In addition, the possible associations with the PI3K/Akt
pathway were investigated to understand the application of BMSCs in
CRT and tissue engineering.
Materials and methods
Materials
Healthy female Sprague-Dawley rats (n=10, weight,
180±20 g; age, 4 weeks) were provided by Nanjing Medical University
(Jiangsu, China). Ginsenoside Rg1 was purchased from Shanghai
Oriental Pharmaceutical Co., Ltd. (Shanghai, China). Gibco
low-glucose Dulbecco's modified Eagle's medium (DMEM), fetal bovine
serum (FBS) and trypsin were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The Cell Counting Kit-8
(CCK-8) assay kit was supplied by Dojindo Laboratories (Kumamoto,
Japan) and polyvinylidene fluoride membrane by Roche Diagnostics
(Basel, Switzerland). Bax, Bcl-2, phosphorylated (p)-Akt and Akt
primary antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). This study was approved by the ethics
committee of Nanjing Medical University.
Isolation, culture and identification of
rBMSCs
The rats were sacrificed by cervical dislocation and
soaked in 70% alcohol for 20 min. The tibia and femur were
harvested under aseptic conditions, and the diaphysis was severed
using sterilized ophthalmic scissors. The marrow cavity was flushed
repeatedly with phosphate-buffered saline (PBS; Hyclone, Logan, UT,
USA) solution, and then transferred to a centrifuge tube for
centrifugation for 5 min at 120 × g for 5 min. The supernatant was
discarded, and the precipitate was resuspended in 85% low-glucose
DMEM [15% FBS + penicillin/streptomycin (Hangzhou Sijiqing
Biological Engineering Materials, Co., Ltd., Hangzhou, China)]. The
cells were then seeded at 1×106 cells/cm2 and
cultured in a 37°C, 5% CO2 incubator. Half a volume of
the medium was replaced 24 h later, and the full volume was
replaced 2–3 days later. When the cells reached 80% confluency,
they were passaged by digestion using 0.25% trypsin. The cells of
the third generation were collected to detect purity and were free
from the non-adherence spherocytes, thus the purified rBMSCs were
obtained.
Determination of final concentrations of
H2O2 and ginsenoside Rg1 using the CCK-8
assay
rBMSCs of the third generation were prepared into
1×105 cells/ml single-cell suspension and seeded to
96-well plates (104 cells/well). Following cell
adherence to the wall of the plate, the cells were starved for 24 h
by adding 100 µl serum-free culture medium. Five wells were
randomly selected, and culture media containing different
concentrations of H2O2 (0, 200, 400, 600 and
800 µM; Beijing Haiderun (Sea Derun) Pharmaceutical Co.,
Ltd., Beijing, China) was added to treat the cells for 6 h. Cells
were then incubated at 37°C for 30 min with 10 µl CCK-8
solution. The absorbance was measured at 450 nm (Model 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
To determine the final concentration of
H2O2, cells were starved for 24 h using the
method described above and 4 wells were randomly selected.
Ginsenoside Rg1 of different concentrations (0, 0.1, 1 and 10
µM) was added into each well to treat the cells for 24 h,
followed by 800 µM H2O2 treatment for
6 h. One group was randomly selected as the control group, for
which no treatment was performed. The absorbance was measured using
the CCK-8 solution as described above.
Grouping
Cells of third generation reaching the logarithmic
growth phase were divided into 4 groups as follows: Control,
untreated; H2O2-treated, addition of 800
µM H2O2 to induce oxidative injury;
ginsenoside Rg1-treated, 10 µM ginsenoside Rg1 for 24 h,
followed by 800 µM H2O2 for 6h; and
Akt pathway blockage group, blockage achieved by addition of 25
µM LY294002 (Cell Signaling Technologies, Inc., Boston, MA,
USA) for 1 h, followed by ginsenoside Rg1 and
H2O2 treatments.
Detection of cellular apoptotic rate by
TUNEL staining
The rBMSCs of the third generation were seeded into
a 24-well plate. The cells in sub-aggregation state were starved
for 24 h using the serum-free culture medium. When the cells in
each treatment were dried, they were fixed in 4% paraformaldehyde
(Wuhan GoodBio Technology, Co., Wuhan, China) for 1 h, and sealed
for 10 min using the confining liquid (3%
H2O2, dissolved in methanol). Subsequent to
transparentization for 2 min using 0.1% Triton X-100 (Biosharp,
Hefei, China), the cells were sealed for 1 h in the TUNEL reaction
mixture at 37°C in a dark box. The cells were incubated with
4′,6-diamidino-2-phenylindole (DAPI; Beyotime Institute of
Biotechnology, Haimen, China) for 5 min, and the fluorescence was
detected Nikon eclipse Ti (Tokyo, Japan). TUNEL (Roche,
Indianapolis, IN, USA) staining is an intuitive method to detect
apoptosis. With DAPI-TUNEL double staining, the cells are counted
directly, which enables the comparison of cellular apoptotic rate
between the samples. The blue fluorescence indicates that the cells
were stained by DAPI and the green fluorescence indicates that the
cells were stained by TUNEL.
Detection of cellular apoptosis using
flow cytometry and Annexin V-fluorescein isothiocyanate/propidium
iodide (FITC/PI) double staining
rBMSCs of the third generation were inoculated into
a 6-well plate. Following cell adherence to the walls of the plate,
cells were divided into the 4 treatment groups. Following
treatment, cells were collected and centrifuged for 5 min at 270 ×
g. The supernatant was discarded and cells were washed twice with
PBS. Cells were then resuspended in 500 µl of binding
buffer, and 5 µl FITC-labeled Annexin V (20 µg/ml)
and 5 µl PI (50 µg/ml) (all from; BD PharMingen,San
Diego, CA, USA) were added to the solution. The reaction was
conducted in the dark for 15 min, and cell apoptosis was detected
by BD FACS ARIA II flow cytometry (BD Biosciences, San Jose, CA,
USA).
Detection of p-Akt, Bcl-2, Bax and
cleaved caspase-3 protein expression levels by western
blotting
Western blotting was conducted to detect
apoptosis-associated proteins and p-Akt levels in
H2O2-treated rBMSCs with Rg1 pretreatment.
Subsequent to each treatment, cells were washed twice with PBS, and
the pre-cooled cell lysis buffer was added. The reaction proceeded
on ice for 5 min and the cells were then scraped off. The products
of lysis were centrifuged for 15 min at 4°C at 14,000 × g. The
supernatant was collected to determine the protein concentration
using the Bicinchoninic Acid assay kit (Beyotime Institute of
Biotechnology). Equal quantities of protein sample were subjected
to 10% sodium dodecyl sulfate-polyacrylimide gel electrophoresis 80
mA for first 30 min then 120 mA for last 90 min (Beyotime Institute
of Biotechnology). Following electrophoresis, proteins were
transferred to a nitrocellulose membrane (Biosharp), which was
sealed with 5% nonfat milk powder containing 0.05% Tween-20 with
Tris-buffered saline (TBST; Biosharp) at room temperature for 1 h.
The membranes were then incubated with the following antibodies:
Rabbit anti-p-Akt (1:1,000; cat. no. #9271), polyclonal anti-Akt
(1:1,000; cat. no. #9272), polyclonal anti-Bcl-2 (1:1,000; cat. no.
#2876), polyclonal anti-Bax (1:1,000; cat. no. #2772) and
monoclonal anti-caspase-3, (1:1,000; cat. no. #9665) (all from Cell
Signaling Technologies, Inc.) primary antibodies at 4°C overnight.
Membranes were washed 3 times with TBST for 15 min, incubated with
goat anti-rabbit horseradish peroxidase-labeled secondary
antibodies (1:2,000; cat. no. #7074; Cell Signaling Technologies,
Inc.) for 2 h, followed by 3 washes with TBST for 10 min. Enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.) was utilized to
visualize the proteins. Images were captured using an imaging
system (UVP Inc., Upland, CA, USA). The images were analyzed using
Image Lab software v2.0.1 (Bio-Rad Laboratories, Inc.). The ratio
of absorbance of the target band to that of the glyceraldehyde
3-phosphate dehydrogenase band was used to measure the expression
level of the proteins.
Statistical analysis
All statistical processes were performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). The
measurement data were expressed as the mean ± standard deviation.
One-way analysis of variance was adopted for intergroup comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
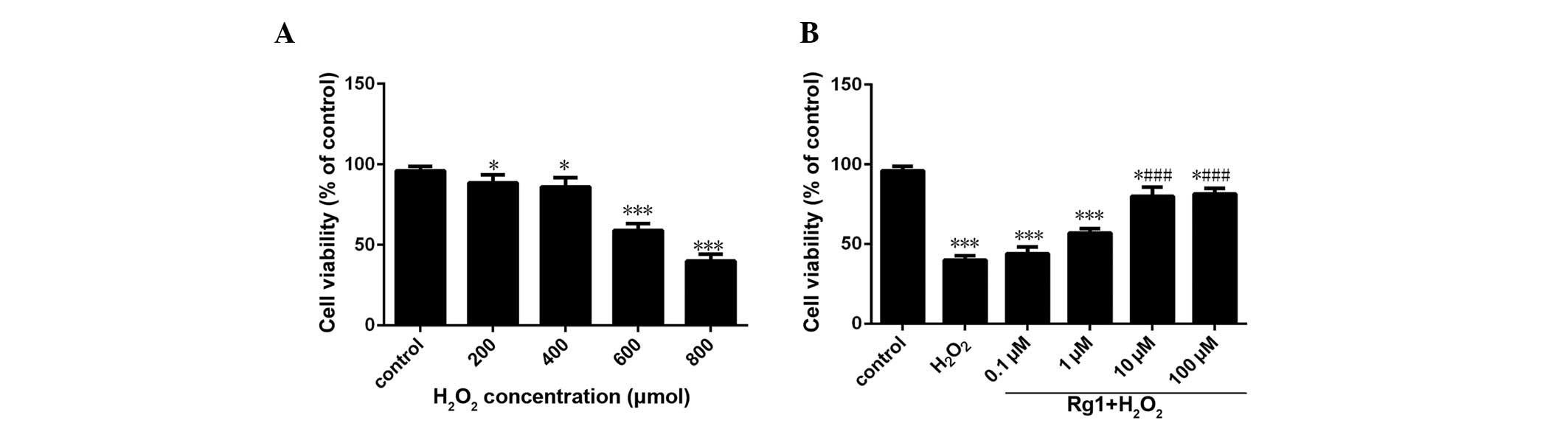
Effect of various concentrations of
H2O2 on the cell viability of rBMSCs
As demonstrated in Fig.
1, subsequent to 6 h of H2O2 treatment,
the cell viability was significantly reduced compared with the
control group in a dose-dependent manner. Except 200 and 400
µM group, the reduction of cell viability in all groups had
a statistical significance compared with the control group
(P<0.05). In the 600 µM group, the cell survival rate
approached 50% (Fig. 1A).
Effect of various concentrations of
ginsenoside Rg1 on the rBMSC viability under oxidative stress
Compared with the control group, the
H2O2 model group demonstrated a significant
reduction in cell viability (P<0.05). The cell viability of 10
and 100 µM ginsenoside Rg1 groups increased considerably
compared with the H2O2 model group with
statistical significance (P<0.05). There was no significant
difference in cell viability between 10 µM group and 100
µM group (P>0.05) (Fig.
1B).
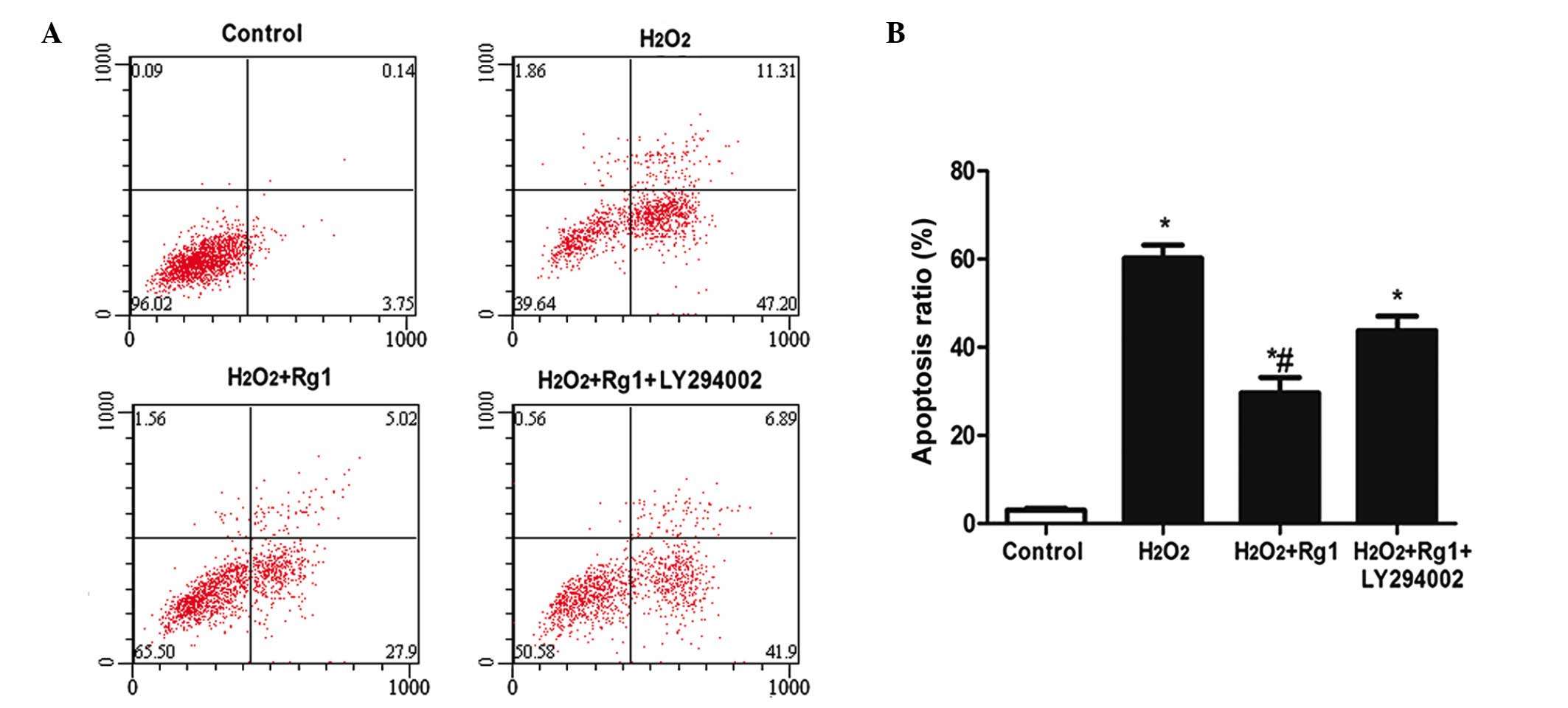
Detection of apoptotic rate of rBMSCs by
flow cytometry
The results of flow cytometry for each treatment
indicated that the apoptosis rate of the H2O2
model group increased significantly compared with the control group
(from 3.92±0.128 in the control to 59.44±3.21%; Fig. 2; P<0.05). The apoptotic ratio
following H2O2 + Rg1 treatment was
significantly reduced to 33.41±4.88% compared with the
H2O2 model group (59.44±3.21%) and
H2O2 + Rg1 +LY294002 group (49.64±2.33%;
Fig. 2; P<0.05).
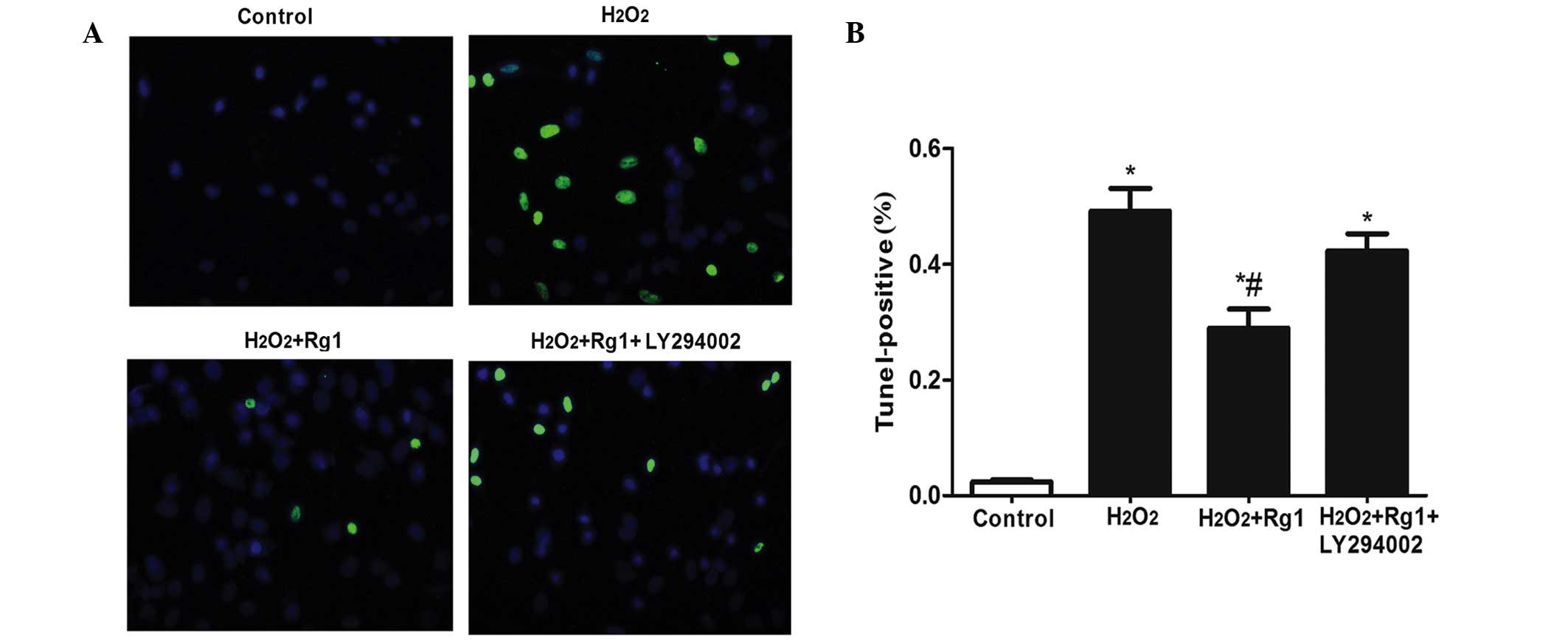
Detection of apoptosis of rBMSCs by TUNEL
staining
Following TUNEL staining, the positive percentage of
the H2O2 model group demonstrated a
significant increase compared with the control group
(1.88±0.133–48±4.65%; Fig. 3;
P<0.05). The H2O2 + Rg1 group demonstrated
a significantly decreased TUNEL-positive percentage compared with
the H2O2 model group (25.29±4.33%; Fig. 3; P<0.05). The difference was
also significant (P<0.05). The percentage of TUNEL-positive
cells in the Akt pathway the blockage group (38.42±2.46%) increased
compared with the control group (P<0.05) (Fig. 3).
Effect of ginsenoside Rg1 on the
expression levels of Bax, Bcl-2, cleaved caspase-3 and p-Akt
The results of the western blot assay indicated that
the protein expression levels of cleaved caspase-3 were
significantly upregulated in the H2O2 model
group compared with the control group (Fig. 4; P<0.05). Increased expression
of Bax and reduced expression of Bcl-2 were observed in the
H2O2-treated cells compared with the control
group (Fig. 4; P<0.05). Further
treatment with ginsenoside Rg1 significantly prevented the
upregulation of Bax and the downregulation of Bcl-2, while LY294002
treatment increased the ratio of Bax/Bcl-2, compared with the
H2O2-treated group (Fig. 4; P<0.05). The expression levels
of Bax and cleaved caspase-3 were significantly reduced in the
ginsenoside Rg1 treatment groups compared with the
H2O2 model group, while the expression levels
of Bcl-2 and p-Akt were significantly upregulated (Fig. 4; P<0.05). No statistical
significance was observed between the LY294002-treated and
H2O2-treated groups in terms of protein
expression (Fig. 4;
P>0.05).
 | Figure 4Detection of Bcl-2, Bax, caspase-3,
p-Akt and Akt protein expression levels in
H2O2-, Rg1- and LY294002-treated rat bone
marrow stem cells. Western blotting was utilized to analyze (A)
Bcl-2 and Bax, (B) total/cleaved caspase-3, and (C) p-Akt and Akt
protein expression levels. GAPDH was used as a loading control.
Results are presented as the ratio of (A) Bax to Bcl-2, (B)
caspase-3 to GAPDH and (C) p-Akt to Akt. Data are presented as the
mean ± standard error of the mean (n=3). *P<0.05 vs.
the control group and #P<0.05 vs. the
H2O2 model group and the
H2O2+Rg1+LY294002 group. p-, phosphorylated;
Akt, protein kinase B; H2O2, hydrogen
peroxide; Rg1, ginsenoside Rg1; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Discussion
BMSCs are known as 'seeds' in cell replacement
therapy and tissue engineering due to their strong proliferation
and multi-directional differentiation potential. BMSCs
transplantation therapy has attracted increasing attention. A
previous study demonstrated that the hypoxic-ischemic tissues and
high oxidative stress resulting from ischemia-reperfusion injury
have an adverse impact on the survival of BMSCs in the
transplantation area (23). As a
result, the effect of BMSC transplantation in CRT is reduced. The
marked accumulation of ROS may alter the redox state of the cells.
Cells, tissues and organs undergo various injuries through the
oxidation of DNA, proteins, lipids and other biomacromolecules.
Excess ROS increase the permeability of the mitochondrial outer
membrane, inducing the leakage of cytochrome c and
apoptosis-inducing factors, thus this results in cell apoptosis.
Therefore, it is necessary to reduce the oxidative stress in the
transplantation region and to inhibit the apoptosis of BMSCs under
oxidative stress.
Ginsenoside Rg1 is a phytoestrogen (28), exhibiting anti-oxidative and
anti-apoptotic potential in myocardial cells and nerve cells
(29,30). However, it remains to be reported
whether ginsenoside Rg1 has an antagonist effect against the
apoptosis of BMSCs. To verify the anti-oxidative effect of
ginsenoside Rg1 in BMSCs, the cells were pretreated with different
concentrations of ginsenoside Rg1 (1–100 µM) for 24 h prior
to H2O2 treatment. The results of the CCK-8
assay indicated that ginsenoside Rg1 pretreatment significantly
improved the survival of rBMSCs under oxidative stress. To further
confirm the anti-apoptotic effect of ginsenoside Rg1 in BMSCs, the
apoptosis of rBMSCs was determined using flow cytometry and TUNEL
staining under H2O2-induced oxidative stress.
The results indicated that under high oxidative stress, the
pretreatment of 10 µM ginsenoside Rg1 effectively reversed
the H2O2-induced apoptosis of BMSCs.
Bcl-2 is an anti-apoptotic protein that acts to
prevent apoptosis by inhibiting mitochondrial depolarization
(31). Bax belongs to the same
family as Bcl-2 and is a pro-apoptotic protein that induces
apoptosis by promoting mitochondrial depolarization (32). The initiation of apoptosis is
associated with the activation of promoters and the protease
cascade reaction. During the protease cascade process, caspase-3 is
the primary executor of apoptosis (33,34)
and the downstream effector protein of several apoptotic pathways.
The present study aimed to reveal the protective mechanism of
ginsenoside Rg1 against the apoptosis of BMSCs under oxidative
stress, thus the expression levels of apoptosis-associated proteins
were detected using western blot analysis. The results indicated
that H2O2-induced oxidative stress increased
the intracellular expression levels of Bax and cleaved caspase-3,
while reducing the expression of Bcl-2. In addition, ginsenoside
Rg1 pretreatment significantly reversed this phenomenon. This
further indicates that the anti-apoptotic mechanism of ginsenoside
Rg1 may be associated with the inhibition of apoptotic proteins
involved in the mitochondrial pathways.
The PI3K/Akt signaling pathway is one of the most
important pathways discovered to be associated with cell survival
(21,35). Certain stimuli activate the pathway
and promote cell survival, the activated Akt is then directly
involved in the regulation of cell growth, proliferation and the
cell cycle (36). It exhibits an
anti-apoptotic effect by enhancing the expression levels of
anti-apoptotic proteins and by inhibiting the expression levels of
pro-apoptotic proteins (37).
Preliminary experiments of a previous study demonstrated that
ginsenoside Rg1 inhibited the apoptosis of rat chondrocytes by
activating the Akt signaling pathway (30). However, it remains unclear whether
BMSCs apoptosis may be inhibited by ginsenoside Rg1 activating the
PI3K/Akt pathway. The results of the current study demonstrated
that ginsenoside Rg1 pretreatment effectively reversed the
H2O2-induced downregulation of p-Akt,
significantly increased the expression of Bcl-2 and inhibited the
expression of Bax and cleaved caspase-3. In order to verify the
role of the PI3K/Akt pathway in the anti-apoptotic effect of
ginsenoside Rg1 in BMSCs, LY294002, the specific PI3K inhibitor was
administered. The results indicated that the antagonistic capacity
of ginsenoside Rg1 against H2O2-induced
apoptosis was inhibited. This confirmed the importance of the
PI3K/Akt pathway in the protective effect of ginsenoside Rg1
against the apoptosis of BMSCs.
In addition, only the proteins associated with the
mitochondrial apoptosis pathway were detected, and cell apoptosis
may be additionally associated with the death receptor (36) and endoplasmic reticulum pathways
(37). In conclusion, ginsenoside
Rg1 was demonstrated to possess an antagonistic effect against the
oxidative stress-induced apoptosis of BMSCs, and the specific
mechanism may be associated with the activation of the PI3K/Akt
pathway.
References
|
1
|
Song IH, Caplan AI and Dennis JE: In vitro
dexamethasone pretreatment enhances bone formation of human
mesenchymal stem cells in vivo. J Orthop Res. 27:916–921. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Granero-Moltó F, Weis JA, Miga MI, Landis
B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP and
Spagnoli A: Regenerative effects of transplanted mesenchymal stem
cells in fracture healing. Stem Cells. 27:1887–1898. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen WJ, Huang JW, Niu CC, Chen LH, Yuan
LJ, Lai PL, Yang CY and Lin SS: Use of fluorescence labeled
mesenchymal stem cells in pluronic F127 and porous hydroxyapatite
as a bone substitute for posterolateral spinal fusion. J Orthop
Res. 27:1631–1636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacigaluppi M, Pluchino S, Martino G,
Kilic E and Hermann DM: Neural stem/precursor cells for the
treatment of ischemic stroke. J Neurol Sci. 265:73–77. 2008.
View Article : Google Scholar
|
|
5
|
Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M,
Shi J, Yang YZ, Pan C, Ge J and Phillips MI: Autologous mesenchymal
stem cell transplantation induce VEGF and neovascularization in
ischemic myocardium. Regul Pept. 117:3–10. 2004. View Article : Google Scholar
|
|
6
|
Kaminski A and Steinhoff G: Current status
of intramyocardial bone marrow stem cell transplantation. Semin
Thorac Cardiovasc Surg. 20:119–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei H, Li Z, Hu S, Chen X and Cong X:
Apoptosis of mesenchymal stem cells induced by hydrogen peroxide
concerns both endoplasmic reticulum stress and mitochondrial death
pathway through regulation of caspases, p38 and JNK. J Cell
Biochem. 111:967–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muthusami S, Ramachandran I, Muthusamy B,
Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A and Narasimhan
S: Ovariectomy induces oxidative stress and impairs bone
antioxidant system in adult rats. J Clin Chim Acta. 360:81–86.
2005. View Article : Google Scholar
|
|
9
|
Kadenbach B, Ramzan R and Vogt S:
Degenerative diseases, oxidative stress and cytochrome c oxidase
function. Trends Mol Med. 15:139–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee GJ, Chae SJ, Jeong JH, Lee SR, Ha SJ,
Pak YK, Kim W and Park HK: Characterization of mitochondria
isolated from normal and ischemic hearts in rats utilizing atomic
force microscopy. Micron. 42:299–304. 2011. View Article : Google Scholar
|
|
11
|
Schaller S, Paradis S, Ngoh GA, Assaly R,
Buisson B, Drouot C, Ostuni MA, Lacapere JJ, Bassissi F, Bordet T,
et al: TRO40303, a new cardioprotective compound, inhibits
mitochondrial permeability transition. J Pharmacol Exp Ther.
333:696–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H-Y, Zhang X, Chen S-F, Zhang Y-X,
Liu Y-H, Ma LL and Wang LX: The protective effect of 17β-estradiol
against hydrogen peroxide-induced apoptosis on mesenchymal stem
cell. Biomed Pharmacother. 66:57–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Zhang P, Dai QG, Ouyang NJ, Jiang
LY and Fang B: The effect of estrogen on proliferation and
osteogenic differentiation of rat bone marrow mesenchymal stem
cells. Shanghai Kou Qiang Yi Xue. 23:654–660. 2014.In Chinese.
|
|
14
|
Zhou S, Zilberman Y, Wassermann K, Bain
SD, Sadovsky Y and Gazit D: Estrogen modulates estrogen receptor
alpha and beta expression, osteogenic activity, and apoptosis in
mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem.
Suppl(Suppl 36): 144–155. 2001. View
Article : Google Scholar
|
|
15
|
Hamden K, Carreau S, Ayadi F, Masmoudi H
and El Feki A: Inhibitory effect of estrogens, phytoestrogens, and
caloric restriction on oxidative stress and hepatotoxicity in aged
rats. Biomed Environ Sci. 22:381–387. 2009. View Article : Google Scholar
|
|
16
|
Aneja R, Upadhyaya G, Prakash S, Dass SK
and Chandra R: Ameliorating effect of phytoestrogens on
CCl4-induced oxidative stress in the livers of male Wistar rats.
Artif Cells Blood Substit Immobil Biotechnol. 33:201–213. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen XC, Zhou YC, Chen Y, Zhu YG, Fang F
and Chen LM: Ginsenoside Rg1 reduces MPTP-induced substantia nigra
neuron loss by suppressing oxidative stress. Acta Pharmacol Sin.
26:56–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma J, Liu J, Wang Q, Yu H, Chen Y and
Xiang L: The beneficial effect of ginsenoside Rg1 on Schwann cells
subjected to hydrogen peroxide induced oxidative injury. Int J Biol
Sci. 9:624–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu D, Wu L, Li CR, Wang XW, Ma YJ, Zhong
ZY, Zhao HB, Cui J, Xun SF, Huang XL, et al: Ginsenoside Rg1
protects rat cardiomyocyte from hypoxia/reoxygenation oxidative
injury via antioxidant and intracellular calcium homeostasis. J
Cell Biochem. 108:117–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong L, Li SL, Li H and Zhang L:
Ginsenoside Rg1 protects primary cultured rat hippocampal neurons
from cell apoptosis induced by β-amyloid protein. Pharm Biol.
49:501–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sen P, Mukherjee S, Ray D and Raha S:
Involvement of the Akt/PKB signaling pathway with disease
processes. Mol Cell Biochem. 253:241–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khor TO, Gul A, Ithnin H and Seow HF:
Positive correlation between overexpression of phospho-BAD with
phosphorylated Akt at serine 473 but not threonine 308 in
colorectal carcinoma. Cancer Lett. 210:139–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun B, Feng M, Tian X, Lu X, Zhang Y, Ke
X, Huang S, Cao J and Ding X: DL-3-n-Butylphthalide protects rat
bone marrow stem cells against hydrogen peroxide-induced cell death
through antioxidation and activation of PI3K-Akt pathway. Neurosci
Lett. 516:247–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu WY and Zhao MF: Effect of oxidative
stress on bone marrow mesenchymal stem cells. (Article in Chinese)
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 34:90–94. 2012.
|
|
25
|
Lv C, Hao Y, Han Y, Zhang W, Cong L, Shi Y
and Tu G: Role and mechanism of microRNA-21 in H2O2-induced
apoptosis in bone marrow mesenchymal stem cells. J Clin Neurosci.
Jan 22–2016.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang XY, Fan XS, Cai L, Liu S, Cong XF and
Chen X: Lysophosphatidic acid rescues bone mesenchymal stem cells
from hydrogen peroxide-induced apoptosis. Apoptosis. 20:273–284.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng X, Yu SP, Taylor T, Ogle M and Wei L:
Protective effect of apelin on cultured rat bone marrow mesenchymal
stem cells against apoptosis. Stem Cell Res. 8:357–367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao QG, Chan HY, Man CW and Wong MS:
Differential ERα-mediated rapid estrogenic actions of ginsenoside
Rg1 and estren in human breast cancer MCF-7 cells. J Steroid
Biochem Mol Biol. 141:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
González-Burgos, Fernandez-Moriano C and
Gómez-Serranillos MP: Potential neuroprotective activity of Ginseng
in Parkinson's Disease: A review. J Neuroimmune Pharmacol.
102015.
|
|
30
|
Huang Y, Wu D and Fan W: Protection of
ginsenoside Rg1 on chondrocyte from IL-1β-induced
mitochondria-activated apoptosis through PI3K/Akt signaling. Mol
Cell Biochem. 392:249–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi Y: A structural view of
mitochondria-mediated apoptosis. Nat Struct Biol. 8:394–401. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Narita M, Shimizu S, Ito T, Chittenden T,
Lutz RJ, Matsuda H and Tsujimoto Y: Bax interacts with the
permeability transition pore to induce permeability transition and
cytochrome c release in isolated mitochondria. Proc Natl Acad Sci
USA. 95:14681–14686. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Odonkor CA and Achilefu S: Modulation of
effector caspase cleavage determines response of breast and lung
tumor cell lines to chemotherapy. Cancer Invest. 27:417–429. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin HH, Chen JH, Huang CC and Wang CJ:
Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric
carcinoma cells involving JNK/p38 MAPK signaling activation. Int J
Cancer. 120:2306–2316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsui T, Li L, Wu JC, Cook SA, Nagoshi T,
Picard MH, Liao R and Rosenzweig A: Phenotypic spectrum caused by
transgenic overexpression of activated Akt in the heart. J Biol
Chem. 277:22896–22901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sussman M: 'AKT'ing lessons for stem
cells: Regulation of cardiac myocyte and progenitor cell
proliferation. Trends Cardiovasc Med. 17:235–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Downward J: PI 3-kinase, Akt and cell
survival. Semin Cell Dev Biol. 15:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|