Introduction
Since the 2010 voluntary withdrawal of DePuy ASR Hip
Resurfacing System and ASR XL Acetabular System prompted by several
studies showing high failure rates of these hip implants (1–3),
careful attention has been given to metal-on-metal (MoM) hip
prostheses. The European community (4), in line with the international
scientific community (5) and the
Consensus Statement (6), has
decided to stop the use of MoM big head stemmed implants (diameter
≥36 mm).
The high failure rate of these devices is well
asserted by all national registers (7–10).
One of the factors considered to be responsible for this, was the
release and the systemic accumulation of surface released
microparticles, nanoparticles and ions (articular and trunnion)
(11). These prostheses were also
associated with local aseptic lymphocytic vasculitis, pseudotumours
and necrosis of surrounding tissues with consequent prosthetic
failure (12–14).
The MoM alloys are usually composed of chromium (Cr,
26–30%), molybdenum (Mo, 5–7%) and cobalt (Co) (for balancing ISO
5832-12:2007 High-Carbon-Alloy).
The accumulation of Co leads to a pathological
condition, defined as cobaltism, predominantly affecting the
nervous, cardiac and thyroid systems (15). The biological activity of Co is
dictated by the concentration of unbound ionic Co (II) (16–18).
Amongst the categories at risk of cobaltism are patients with big
head MoM prostheses, in addition to reported cases of occupational
or iatrogenic exposure investigated by toxicology experts (19).
While risk levels have already been established for
cases of occupational exposure (20), those for patients with prosthetics
have only been suggested by The Medicine and Healthcare products
Regulatory Agency (MHRA) (21) and
by the Consensus Statement (6).
They have been suggested to be 7 µg/l for Cr and Co
circulating ions, although certain authors have proposed 4
µg/l as a precaution (22).
In addition, the risk levels for urinary ions have not been
established yet.
Numerous studies have correlated the presence of
metal ions with the formation of reactive oxygen species (ROS)
(23), whose systemic and local
effects are well known in different tested models (24). The metal ions Cr (III) and Co (II)
catalyze the conversion of hydrogen peroxide into reactive hydroxyl
radicals by the Fenton reaction (25). In response to oxidative stress, the
organism protects itself by upregulating several enzymes, including
heme-oxygenase-1 (HMOX-1) (26).
HMOX-1 is a member of the oxidoreductase family and
catalyses the degradation of heme in carbon monoxide, divalent iron
and biliverdin. It is then converted in bilirubin, the most
abundant endogenous antioxidant in mammalian tissues, responsible
for a number of antioxidant activities (26).
HMOX-1 represents the inducible isoform of the
antioxidant system of heme-oxygenase and its induction is due to
the action of multiple oxidation factors, including certain heavy
metals (27), such as Co and
Cr.
As it is known that Co (II) can induce the
expression of HMOX-1 to counteract oxidative stress, the aim of the
present study was to verify whether mRNA and protein expression of
HMOX-1 was modulated by the presence of metal ions in patients with
a MoM prosthesis and whether patients without a prosthesis
exhibited a different expression pattern.
Materials and methods
Patient enrolment
This study was approved by the Institutional Review
Board of the Rizzoli Orthopaedic Institute (Bologna, Italy). All
investigations were conducted in conformity with ethical principles
of research, and informed consent for participation in the study
was obtained from all enrolled patients. This parallel cohort study
was designed in order to evaluate HMOX-1 expression in
patients with/without MoM prosthetics, in correlation with Co and
Cr levels in the blood and urine. It has been registered at
clinicaltrials.gov with the identification
number: NCT02427984.
Patients with primary coxarthrosis, on a waiting
list for primary hip prosthesis intervention, were enrolled in the
study as a control group (non-prosthetic group; n=22). These 22
patients were coupled with patients with aseptic loosening MoM hip
prostheses (prosthetic group; n=22), matched for gender, age and
smoking habits. The recruitment period was from March 2014 to
October 2014. The exclusion criteria were the presence of other
articular prostheses, sepsis or suspected sepsis, hematologic
pathologies and rheumatoid arthritis. Each group (prosthetic and
non-prosthetic) contained 17 women and 5 men, of which 4 were
smokers and 18 were non-smokers or ex-smokers (who had not smoked
for >10 years). The mean age ± standard error of the mean of the
patients in the prosthetic group was 64.9±1.9 years and of the
patients in the non-prosthetic group was 64.2±2.1 (Table I).
 | Table IPatients demographic characteristics
and metal ions distribution. |
Table I
Patients demographic characteristics
and metal ions distribution.
| Characteristic | Non-prosthetic
group | Prosthetic
group | P-value |
|---|
| Age, years
(mean±SEM) | 64.2±2.1 | 64.9±1.9 | – |
| Gender |
| Male | 5 | 5 | – |
| Female | 17 | 17 | – |
| Smoking habit |
| Non-smokers
(n) | 18 | 18 | – |
| Smokers (n) | 4 | 4 | – |
| Time from implant
(years) range | 3.5–15 | – | – |
| Co-blood
(µg/l) range | 0.09–0.65 | 0.40–35.70 | 0.0001 |
| Cr-blood
(µg/l) range | 0.03–2.03 | 0.05–12.50 | 0.0001 |
| Co-urine
(µg/l) range | 0.20–1.50 | 2.00–867.00 | 0.0001 |
| Cr-urine
(µg/l) range | 0.08–0.90 | 1.00–138.20 | 0.0001 |
Sample collection
Peripheral blood samples (total, 18 ml) were
obtained using a disposable intravenous cannula, the first 3 ml
were discarded to eliminate possible contamination by metals caused
by the sampling system, then 10 ml of blood were withdrawn and
transferred into two separate trace element vacutainer tubes (5
ml/tube) containing ethylenediaminetetraacetic acid (BD
Biosciences, Franklin Lakes, NJ, USA) for whole blood. An
additional 5 ml of blood aliquot was transferred into a trace
element serum vacutainer tube and centrifuged at 800 × g for 7 min
at 4°C to obtain blood serum. Next, 1 ml samples of whole blood and
serum were immediately frozen and stored at −80°C for the ion
analysis. The remaining 4 ml aliquot of blood was collected to
isolate white cells using a density gradient separation medium
Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA), following the
manufacturer's protocol. The blood sample was diluted 1:1 in PBS
and was layered on 4 ml of the Histopaque-1077 medium and
centrifuged at 400 × g for 30 min at room temperature. The ring of
white cells was collected and washed with 10 ml of PBS centrifuging
at 250 × g for 10 min at room temperature. The cell pellet was
resuspended in 1 ml of TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) to preserve the white cell
lysates, which were stored at −80°C until RNA extraction.
Clean-catch urine samples (10 ml) were collected in
universal sample pots. These samples were frozen and stored at
−20°C until the analysis was conducted.
Determination of ionic circulating and
urinary levels of Co and Cr
Inductively coupled plasma mass spectrometry
(ICP-MS; Perkin Elmer Inc., Waltham, MA, USA) equipped with dynamic
cell reaction (ELAN DRC II, Perkin Elmer Inc.) was used for the
measurements. A reaction system with ammonia gas was used for the
elimination of spectral interferences.
Blood samples were diluted (1:20) with 0.05% Triton
X-100 while urine samples were diluted with bi-distilled water, for
inorganic trace analysis (Merck KgaA, Darmstadt, Germany).
The calibration curve and the sample solutions were
pumped in the spray chamber using a peristaltic pump. Blank samples
were used to correct for any contamination in each batch. The
concentration of metal ions was expressed as µg/l. The
calibration curve was prepared by dilution of a standard solution
ranging from 0.5 to 1,000 mg/l (cobalt in HNO3 2% mono
elemental standard solution, Carlo Erba Reagenti, Milano, Italy;
chromium in HCl atomic absorption standard solution,
Sigma-Aldrich). The procedure followed was previously described
(28,29).
The accuracy of the method was verified by
comparison with certified reference materials for blood obtained
from the German External Quality Assessment Scheme (Institute for
Occupational, Social and Environmental Medicine, Erlangen,
Germany). The coefficients of variation ranged from 4 to 8% and the
limit of detection, calculated as three standard deviations of the
background signal obtained on 10 blinded samples, was 0.05
µg/l in all matrices (whole blood and urine).
The exclusion criteria of the American Conference of
Governmental Industrial Hygienists recommendation for very diluted
(creatinine concentrations less than 0.3 g/l) or very concentrated
(creatinine concentration greater than 3.0 g/l) urine samples were
adopted (30). Urinary creatinine
was determined by a modified Jaffè reaction (ILab 350 Clinical
Chemistry System, Instrumentation Laboratories SpA, Bedford, MA,
USA).
RNA extraction and reverse
transcription
From the white cell lysates, the aqueous phase
containing RNA was isolated using TRIzol and total RNA was purified
following the clean-up protocol of the RNeasy Mini kit (Qiagen,
Valencia, CA, USA). RNA quantity and quality was analysed using a
spectophotometer (Nanodrop ND 1000; Thermo Fisher Scientific, Inc.)
and genomic DNA contamination was excluded by RNA gel
electrophoresis in 1% agarose gel in 1X TAE (Merck & Co.,
Whitehouse Station, NJ, USA) stained with 0.5 µg/ml ethidium
bromide (Sigma-Aldrich) and visualized with UV-light.
RNA was subjected to reverse transcription using the
following: 1 µg total RNA, 200 units Moloney murine
leukaemia virus reverse-transcriptase (Promega Corporation,
Madison, WI, USA; used with companion buffer), 2.5 µM oligo
dT-15 (Sigma-Aldrich), 2 µM random hexamers (Sigma-Aldrich)
and 500 µM dNTPs (Takara Biotechnology Co., Ltd., Shiga,
Japan). RT reaction was performed in a final volume of 25 µl
for 60 min at 37°C. In order to verify that the RT reaction was
successful, amplification of the human glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) gene was performed, using specific
primers (GAPDH forward: 5′-GAAATCCCATCACCATCTTCCAG-3′ and
reverse: 5′-AGGAGACCACCTGGTGCTCAGTGTAGC-3′). GAPDH amplification
was performed in a final volume of 25 µl, containing 1
µl cDNA, 0.2 µM each primer, 12.5 µl BioMix
Red (Bioline, Taunton, MA, USA) under the following conditions:
Initial denaturation for 2 min at 94°C; 25 cycles of 30 sec at
94°C, 30 sec at 61°C (annealing temperature of GAPDH
primers), 30 sec at 72°C followed by a final extension for 7 min at
72°C. Amplicon detection was performed by gel electrophoresis in
1.5% agarose gel as aforementioned.
Quantitative -polymerase chain reaction
(qPCR)
qPCR was performed using the CFX-96 system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Amplification of 5
µl diluted cDNA (i.e. 25 ng) were amplified in 20-µl
reactions using Sso Advanced SYBR Green Supermix (Bio-Rad
Laboratories, Inc.) according to the manufacturer's instructions.
Following an initial denaturation step at 95°C for 2 min,
temperature cycling was initiated. Each cycle consisted of 95°C for
5 sec, and 60°C for 30 sec repeated 40 times with the fluorescence
being read at the end of this step. The primers were obtained from
the PrimePCR SYBR Green Assay (Bio-Rad Laboratories, Inc.) and were
specific for human HMOX-1, GAPDH, hypoxanthine
phosphoribosyltransferase 1 (HPRT1) and TATA-box binding
protein (TBP). Every sample was amplified as a technical
duplicate and its specificity was evaluated with the melting
curves, performed from 65 to 95°C for 2 sec every 0.5°C.
The quality of technical duplicates was established
setting a Cq value of 0.3 as the limit for the standard deviation.
The quality of the reference genes was evaluated based on their M
value (<0.5), calculated by the CFX Manager software (version
3.1, Bio-Rad Laboratories, Inc.).
HMOX-1 relative expression was determined
using the 2−ΔΔCq method (31) with GAPDH, HPRT1 and
TBP as reference genes.
Analysis of HMOX-1 protein
expression
The concentration of HMOX-1 in the serum was
measured using an anti-human HMOX-1 enzyme-linked immunosorbent
assay. kit (Enzo Life Sciences, Inc. Farmingdale, NY, USA), whose
detection range for HMOX-1 concentration was 0.78–25 ng/ml,
according the manufacturer's instructions for undiluted samples.
This analysis was conducted on 39 out of 44 total samples due to of
lack of samples or reagents.
Statistical analysis
In order to evaluate the differences between the
prosthetic and non-prosthetic groups in circulating and urinary Co
and Cr values, the Mann-Whitney test was used. The same test was
used to analyze the difference in serum protein levels of HMOX-1
between patients with circulating values >7 µg/l (high)
and <7 µg/l (low), this threshold was selected in
agreement with previous studies (6,21).
The same test was used to analyze difference of expression levels
of HMOX-1, between prosthetic and non-prosthetic patients, or
between those with high and low ion levels. For the correlation
between Co and Cr levels in the blood and urine and the gene and
protein levels of HMOX-1 the Pearson's correlation test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Statistical analysis and graphs were conducted using
SPSS software (version 14.0; SPSS Inc., Chicago, IL, USA).
Results
Difference in circulating and urinary Co
and Cr levels in the prosthetic and non-prosthetic groups
Circulating blood Co levels ranged between 0.09 and
0.65 µg/l and urine levels ranged between 0.2 and 1.5
µg/l in controls, while in patients from the prosthetic
group these values ranged between 0.4 and 35.7 µg/l in blood
and between 2 and 867.1 µg/l in urine, in this group 15 out
of 22 patients had Co <7 µg/l; the difference between
controls and prosthetic patients was significant (P<0.0001) as
determined using the Mann-Whitney test. Circulating blood Cr levels
ranged between 0.03 and 2.03 µg/l in controls, while in the
prosthetic group these values ranged between 0.05 and 12.50
µg/l. In urine samples the Cr values ranged between 0.08 and
0.90 µg/l in controls and between 1.00 and 138.20
µg/l in the prosthetic group; in this group 17 out of 22
patients had Cr <7 µg/l. The difference between controls
and patients in the prosthetic group was significant (P<0.0001)
using the Mann-Whitney test. These results are summarized in
Table I.
Difference in gene expression of HMOX-1
between the prosthetic and non-prosthetic groups
Gene expression of HMOX-1 in patients in the
prosthetic group compared with controls, regardless of Co and Cr
levels, did not differ significantly using the Mann-Whitney test
(P=0.581). Even when samples were stratified by Co levels, no
statistically significant differences were observed (P=0.837) using
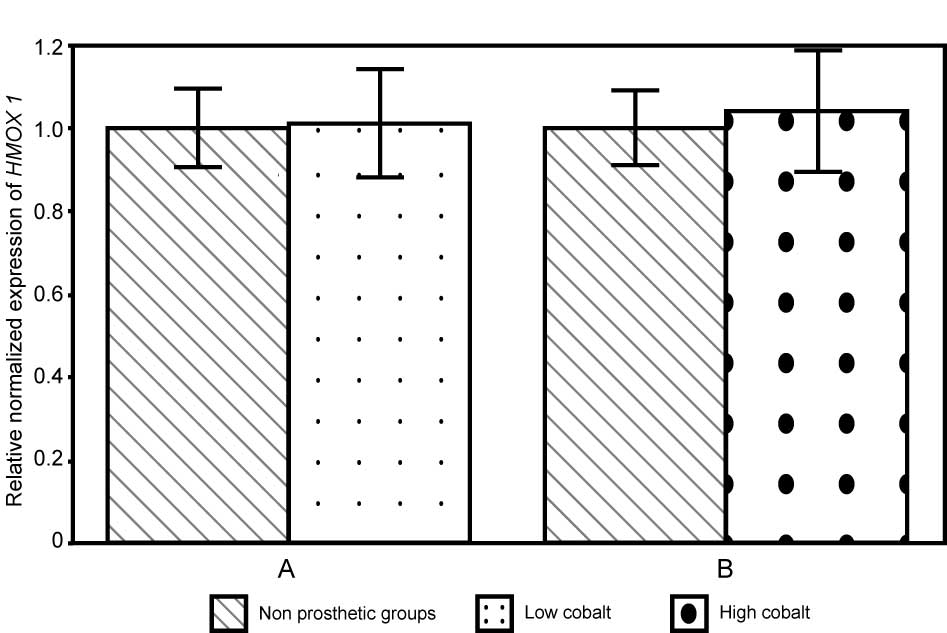
the Mann-Whitney test. In subjects with high levels of Co,
HMOX-1 expression was 1.05±0.15 folds the paired controls
value, while in subjects with low levels of Co HMOX-1
expression was 1.02±0.13 folds the paired controls value (Fig. 1).
The same analysis was conducted based on circulating
Cr values. HMOX-1 expression in prosthetic patients with
high levels of Cr compared to those with low levels of Cr was not
identified to be statistically different (P=0.802) using the
Mann-Whitney test. The relative mRNA levels in patients with low
levels of Cr was 1.00±0.04 fold compared with controls, and
1.10±0.20 fold compared with controls in patients with high levels
of Cr (Fig. 2). In summary, for
high Cr and Co groups and for low Cr and Co groups, the HMOX1 gene
expression was increased, compared with the respective coupled
control groups.
In addition, HMOX-1 expression was also
evaluated in the samples stratified by gender (P=0.901), age
(P=0.413) and smoking habits (P=0.598), but no significant
differences were observed.
Difference in protein expression of
HMOX-1 between the prosthetic and non-prosthetic groups
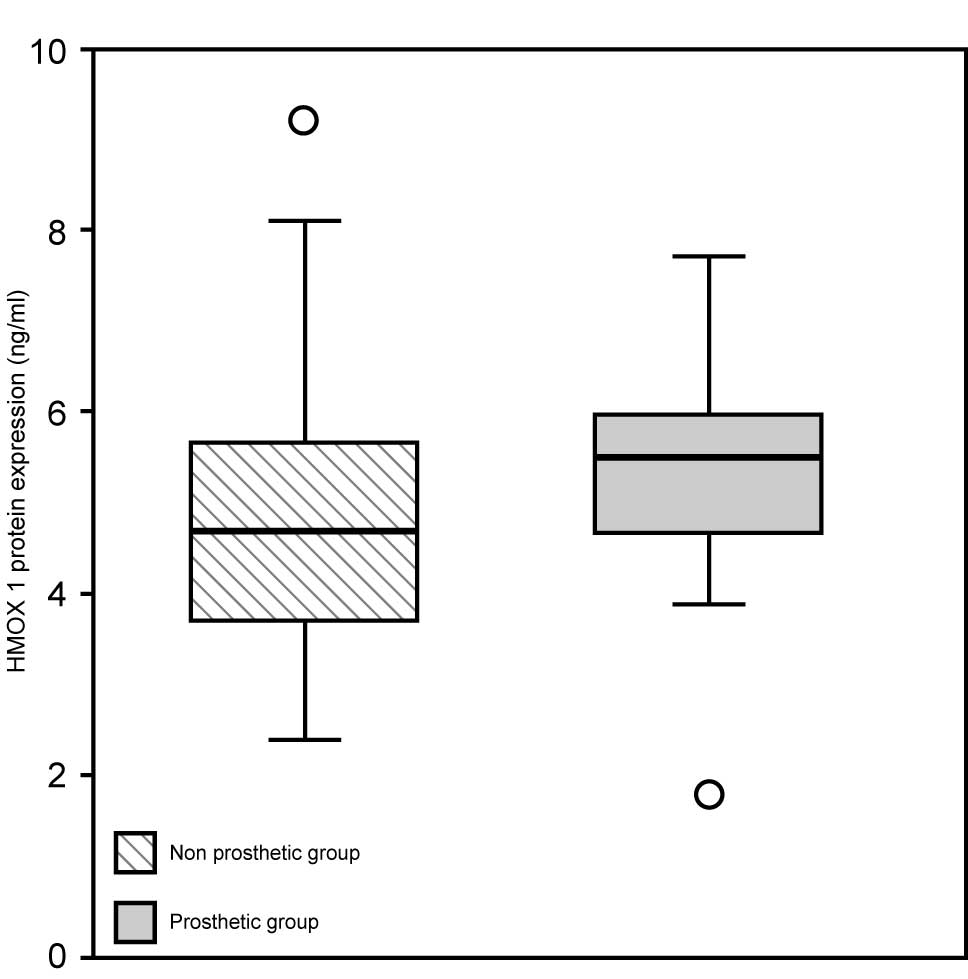
Protein expression of HMOX-1 in serum ranged
from 1.8 to 7.7 ng/ml in patients in the prosthetic group, while it
ranged from 2.4 to 9.2 ng/ml in controls with median values of 5.5
and 4.7 ng/ml, respectively (Fig.
3). Protein expression of HMOX-1 was not statistically
different among prosthetic patients and controls (P=0.143), as well
as among patients with high circulating metal ions and low
circulating metal ions (P=0.494) using the Mann-Whitney test.
Correlation between Co and Cr levels in
the blood and urine, and the gene and protein levels of HMOX-1
Finally, the Pearson test did not identify any
correlation between gene and protein expression of HMOX-1 (r=−0.06;
P=0.74), nor between gene and protein HMOX-1 expression and Co
blood (r=0.11; P=0.48 and r=0.01; P=0.93) and urinary (r=−0.1;
P=0.52 and r=−0.06; P=0.74) levels in the studied sample.
There was no significant correlation between gene
and protein expression of HMOX-1 and the Cr blood (r=0.22; P=0.16
and r=0.09; P=0.59) and urine (r=0.02; P=0.92 and r=0.02; P=0.90)
values.
Discussion
The accumulation of metal ions is considered,
together with other factors, responsible for the high failure rates
of MoM big head hip devices. In a number of studies, the presence
of these ions was associated with the induction of oxidative stress
(32–40).
Since HMOX-1 is one of the most important
antioxidant enzymes to be induced by the presence of metal ions,
the aim of the present study was to verify whether, in patients
with MoM hip prosthesis, mRNA and protein expression of HMOX-1 was
correlated with the level of released metal ions. This was
investigated by comparing with patients without prostheses and
intentionally not considering implant manufacturers, diameters and
performances of the devices, but only the level of metal
released.
mRNA and protein expression of HMOX-1 was not
identified to be statistically different between patients in the
prosthetic and non-prosthetic groups, as well as between patients
with high and low ion levels. Moreover, no correlation was
identified between the expression of the HMOX-1 gene and its
relative protein. This may be due to the use of white blood cells
to determine gene expression and the use of the serum alone for the
protein assays. Despite the significant differences identified in
the ion values between patients in the prosthetic and
non-prosthetic groups, there was no correlation between Co and Cr
levels and HMOX-1 gene expression.
HMOX-1 production (the predicted physiological
response) is induced by the increase of metallic ions; however, it
is limited in the high ions group. This production is often not
enough to avoid circulating ions contributing to the formation of
ROS, which may lead to cellular damage and later, the symptoms
reported by patients with prosthetic hips.
The levels of HMOX-1 identified in the present study
were lower than expected in high Co patients, this may be due to
the fact that in the current study, the exposure to Co was from an
internal source, whereas in other studies where HMOX-1 was
overexpressed, the source of Co was external (36,38,41).
In the present study conditions, the stimulus that should induce
oxidative stress, is the internal continuous chronic release of
ions as the patients have had the prosthesis for at least 3.5
years, However, in a previous study subjects ingested a bolus or
have received injection/drugs with high concentrations of Co
(42).
HMOX-1 was selected as an enzyme involved in
oxidative stress response, as there are numerous studies in the
literature that support the correlation between HMOX-1 and metal
ion concentration. In vitro studies demonstrated that Co
(II) dose- and time-dependently induces HMOX-1 expression in
different cell lines (33,40). In addition, in vivo studies
that demonstrated HMOX-1 induction by Co, were conducted
predominantly in the seventies and eighties (36–38),
while the most recent studies were conducted in animal models
(32,34,35,39).
In these studies Cr appears to exhibit a different role on HMOX-1,
depending on whether it is in the Cr (III) or Cr (VI) form. Indeed,
it has been demonstrated that Cr (III) can be reduced to Cr (II) by
biological reductants (i.e. l-cysteine and NADPH), which in turn
react with hydrogen peroxide via the Fenton reaction to produce
hydroxyl radicals. However, Cr (VI)-induced cytotoxicity and
overexpression of HMOX-1 were shown to be dependent on the
glutathione level (43).
Therefore, it cannot be excluded that the molecular
mechanisms involved in the present study could be different or
differently regulated from those observed in other studies. For
that reason it would be noteworthy in future studies to measure
HMOX-1 levels present in the synovial fluid, where a regulation of
the expression similar to that found in this study cannot be ruled
out. The discrepancy between the results in the present study and
previous literature is possibly due to the small sample size, which
had a few uncommon cases, that may have influenced the results.
In the current study, the expression level of HMOX-1
was not affected by the presence of Co, this may be due to the
species of Co that was investigated here, the majority of the
evidence of interactions between HMOX-1 and Co is in relation to
the Co (II) species; however, it is possible that in the present
study the Co metallic form (Co0) may also be involved.
Occupational exposure to hard metal dust (WC-Co) induced effects
similar to those of exposure to Co (II) via a different molecular
mechanism which does not involve HMOX-1 (44,45).
Metallic Co is able to produce ROS; however, the kinetics of this
process is slower due to the reduced capacity of oxygen to bind to
the surface of the metallic particles (46). In addition, Co0 does not
react with H2O2 via the Fenton reaction
(43) and for this reason, if
Co0 was the predominant species circulating, this could
explain the results of the present study.
Conversely, as far as the lack of effect of
circulating Cr on HMOX-1 induction is concerned, this is probably
due to the fact that only Cr (III) was circulating and does not
appear to exert any direct effect on HMOX-1 (43). Previous studies (47,48),
have demonstrated that the Cr released by MoM prostheses and
present in circulation is in the Cr (III) form. This was confirmed
by preliminary evaluations of a small group of samples, in which
the chemical speciation was determined by hyphenated techniques
(HPLC-ICP-MS), investigating the concentration of Cr (III) and Cr
(VI) in the synovial fluid of patients with prostheses, confirming
that the only species present is Cr (III) (unpublished data from
Laboratory of Toxicology and Industrial Hygiene, University of
Brescia, Italy). Therefore, the results of this study confirm the
requirement for greater comprehension of the following for Co and
Cr: Ion transport within the organism once released by MoM
prosthesis, the identity of the species involved, movement of the
ions and the mechanisms of elimination. This has also be suggested
by Paustenbach et al (49)
who hypothesized the existence of a subjective susceptibility to Co
(possibly correlated with low albumin levels), which may explain
its varied response and transport within the organism. In this
case, the identification of individual susceptibility markers,
detectable in the peripheral blood, would be an innovative element
for investigation of the mechanism by which a patient with a Co-Cr
prosthesis may react to Co ions.
Despite the limitations highlighted, the methodology
in the present study was robust and accurate. The preliminary
results obtained here may be extrapolated to a wider context and
suggest that Co and Cr ions, released by articular prostheses, do
not induce an increase in HMOX-1 gene and protein expression at
least 3.5 years following the insertion of the implant. However,
the involvement of other metal-induced oxidative stress enzymes
cannot be excluded and will be the subject of future studies.
Acknowledgments
The present study was supported by the Italian
Ministry of Health for 'Early diagnosis of pending failure in hard
bearings' (grant no. RF-2009-1472961) and by the Fondazione Del
Monte di Bologna e Ravenna. The authors would like to thank the
Orthopaedic surgeons and nursing staff of the Prosthetic Surgery
and Revisions of Hip and Knee Implants Division (Rizzoli
Orthopaedic Institute, Bologna, Italy) as well as Dr Marilina
Amabile for their contribution to sample collection; Dr Lucia
Mancini for her support in revising the manuscript; and Dr Marco
Bianchi, (Bio-Rad Laboratories, Inc.), for his technical
support.
References
|
1
|
De Steiger RN, Hang JR, Miller LN, Graves
SE and Davidson DC: Five-year results of the ASR XL acetabular
system and the ASR hip resurfacing system: An analysis from the
Australian orthopaedic association national joint replacement
registry. J Bone Joint Surg Am. 93:2287–2293. 2011. View Article : Google Scholar
|
|
2
|
Wienroth M, McCormack P and Joyce TJ:
Precaution, governance and the failure of medical implants: The
ASR((TM)) hip in the UK. Life Sci Soc Policy. 10:192014. View Article : Google Scholar
|
|
3
|
Wong JM, Liu YL, Graves S and de Steiger
R: What is the rerevision rate after revising a hip resurfacing
arthroplasty? Analysis from the AOANJRR. Clin Orthop Relat Res.
473:3458–3464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
SCENIHR: Scientific Committee on Emerging
and Newly Identified Health Risks: Final opinion on the safety of
metal-on-metal joint replacements with a particular focus on hip
implants. 2014, Downloadable at: http://ec.europa.eu/health/scientific_committees/consultations/public_consultations/scenihr_consultation_20_en.htm.
Accessed: 27/02/2015.
|
|
5
|
FDA: Food and Drug Administration: Meeting
materials of the orthopaedic and rehabilitation devices panel.
2012, Downloadable at: http:www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/OrthopaedicandRehabilitationDevicesPanel/ucm309184.htmhttps://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/OrthopaedicandRehabilitationDevicesPanel/ucm309184.htm.
Accessed: 27/02/2015.
|
|
6
|
Günther KP, Schmitt J, Campbell P,
Delaunay CP, Drexler H, Ettema HB, García-Cimbrelo E, Hannemann F,
Hartmann A, Huberti H, et al: Consensus statement 'Current evidence
on the management of metal-on-metal bearings'. Hip Int. 23:2–5.
2013. View Article : Google Scholar
|
|
7
|
AOANJRR: Australian orthopaedic
association national joint replacement registry: Annual report
2014. Downloadable at: https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2014.
Accessed: 03/02/2015.
|
|
8
|
NJR: National joint registry for England,
Wales and Northern Ireland: 11th annual report 2014 and
supplementary report metal on metal bearing surface total
conventional hip arthroplasty. Downloadable at: http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/11th_annual_report/NJR%2011th%20Annual%20Report%20.
Accessed: 03/02/2015.
|
|
9
|
RIPO: Register of the orthopaedic
prosthetic implants (Emilia-Romagna, Italy): Annual report 2013.
Downloadable at: https://ripo.cineca.it.
Accessed: 03/02/2015.
|
|
10
|
The New Zealand joint registry: 15th
Annual report 2013. Downloadable at: http://www.nzoa.org.nz/nz-joint-registry.
Accessed: 03/02/2015.
|
|
11
|
Pastides PS, Dodd M, Sarraf KM and
Willis-Owen CA: Trunnionosis: A pain in the neck. World J Orthop.
4:161–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Haan R, Pattyn C, Gill HS, Murray DW,
Campbell PA and De Smet K: Correlation between inclination of the
acetabular component and metal ion levels in metal-on-metal hip
resurfacing replacement. J Bone Joint Surg Br. 90:1291–1297. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Langton DJ, Jameson SS, Joyce TJ, Hallab
NJ, Natu S and Nargo AV: Early failure of metal-on-metal bearings
in hip resurfacing and large-diameter total hip replacement: A
consequence of excess wear. J Bone Joint Surg Br. 92:38–46. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morlock MM, Bishop N, Zustin J, Hahn M,
Rüther W and Amling M: Modes of implant failure after hip
resurfacing: Morphological and wear analysis of 267 retrieval
specimens. J Bone Joint Surg Am. 90(Suppl 3): 89–95. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bradberry SM, Wilkinson JM and Ferner RE:
Systemic toxicity related to metal hip prostheses. Clin Toxicol
(Phila). 52:837–847. 2014. View Article : Google Scholar
|
|
16
|
Konttinen YT and Pajarinen J: Adverse
reactions to metal-on-metal implants. Nat Rev Rheumatol. 9:5–6.
2013. View Article : Google Scholar
|
|
17
|
Tvermoes BE, Paustenbach DJ, Kerger BD,
Finley BL and Unice KM: Review of cobalt toxicokinetics following
oral dosing: Implications for health risk assessments and
metal-on-metal hip implant patients. Crit Rev Toxicol. 45:367–387.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tyson-Capper AJ, Lawrence H, Holland JP,
Deehan DJ and Kirby JA: Metal-on-metal hips: Cobalt can induce an
endotoxin-like response. Ann Rheum Dis. 72:460–461. 2013.
View Article : Google Scholar
|
|
19
|
Catalani S, Rizzetti MC, Padovani A and
Apostoli P: Neurotoxicity of cobalt. Hum Exp Toxicol. 31:421–437.
2012. View Article : Google Scholar
|
|
20
|
ACGIH: American conference of industrial
hygienists: TLVs® and BEIs®: Threshold limit values for chemical
and physical agents and biological exposure indices. Cincinnati,
USA: 2014, Downloadable at: http://www.acgih.org.
Accessed: 27/02/2015.
|
|
21
|
MHRA: The medicine and Health care
products regulatory agency: Medical device alert. Device: All
metal-on-metal (MoM) hip replacement. 2012, Downloadable at:
https://assets.digital.cabinet-office.gov.uk/media/5485abf6ed915d4c10000273/con155767.pdf.
Accessed: 27/02/2015.
|
|
22
|
Estey MP, Diamandis EP, Van Der Straeten
C, Tower SS, Hart AJ and Moyer TP: Cobalt and chromium measurement
in patients with metal hip prostheses. Clin Chem. 59:880–886. 2013.
View Article : Google Scholar
|
|
23
|
Angelé-Martínez C, Goodman C and Brumaghim
J: Metal-mediated DNA damage and cell death: Mechanisms, detection
methods and cellular consequences. Metallomics. 6:1358–1381. 2014.
View Article : Google Scholar
|
|
24
|
Srivastava KK and Kumar R: Stress,
oxidative injury and disease. Indian J Clin Biochem. 30:3–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beyersmann D and Hartwig A: Carcinogenic
metal compounds: Recent insight into molecular and cellular
mechanisms. Arch Toxicol. 82:493–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maines MD: Heme oxygenase: Function,
multiplicity, regulatory mechanisms and clinical applications.
FASEB J. 2:2557–2568. 1988.PubMed/NCBI
|
|
27
|
Choi AM and Alam J: Heme oxygenase-1:
Function, regulation and implication of a novel stress-inducible
protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol.
15:9–19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pazzaglia UE, Apostoli P, Congiu T,
Catalani S, Marchese M and Zarattini G: Cobalt, chromium and
molybdenum ions kinetics in the human body: Data gained from a
total hip replacement with massive third body wear of the head and
neuropathy by cobalt intoxication. Arch Orthop Trauma Surg.
131:1299–1308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Catalani S, Fostinelli J, Gilberti ME and
Apostoli P: Application of a metal free high performance liquid
chromatography with inductively coupled plasma mass spectrometry
(HPLC-ICP-MS) for the determination of chromium species in drinking
and tap water. Inter J Mass Spect. 387:31–37. 2015. View Article : Google Scholar
|
|
30
|
World Health Organization WHO: Biological
Monitoring of chemical exposure in the workplace. Guidelines.
Geneva: World Health Organization; 1996, 1.
|
|
31
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai Y, Li W, Zhong M, Chen J, Liu Y, Cheng
Q and Li T: Preconditioning and post-treatment with cobalt chloride
in rat model of perinatal hypoxic-ischemic encephalopathy. Brain
Dev. 36:228–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fleury C, Petit A, Mwale F, Antoniou J,
Zukor DJ, Tabrizian M and Huk OL: Effect of cobalt and chromium
ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity
and oxidative stress. Biomaterials. 27:3351–3360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Issan Y, Kornowski R, Aravot D, Shainberg
A, Laniado-Schwartzman M, Sodhi K, Abraham NG and Hochhauser E:
Heme oxygenase-1 induction improves cardiac function following
myocardial ischemia by reducing oxidative stress. PLoS One.
9:e922462014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim S, Lee JC, Cho ES and Kwon J:
COMP-Ang1 accelerates chondrocyte maturation by decreasing HO-1
expression. J Cell Biochem. 114:2513–2521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maines MD and Kappas A: Cobalt induction
of hepatic heme oxygenase; with evidence that cytochrome P450 is
not essential for this enzyme activity. Proc Natl Acad Sci USA.
71:4293–4297. 1974. View Article : Google Scholar
|
|
37
|
Maines MD and Kappas A: Regulation of heme
pathway enzymes and cellular glutathione content by metals that do
not chelate with tetrapyrroles: Blockade of metal effects by
thiols. Proc Natl Acad Sci USA. 74:1875–1878. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maines MD, Trakshel GM and Kutty RK:
Characterization of two constitutive forms of rat liver microsomal
heme oxygenase: Only one molecular species of the enzyme is
inducible. J Biol Chem. 261:411–419. 1986.PubMed/NCBI
|
|
39
|
Stec DE, Vera T, McLemore GR Jr, Kelsen S,
Rimoldi JM, Gadepalli RS and Ryan MJ: Heme oxygenase-1 induction
does not improve vascular relaxation in angiotensin II hypertensive
mice. Am J Hypertens. 21:189–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tkaczyk C, Huk OL, Mwale F, Antoniou J,
Zukor DJ, Petit A and Tabrizian M: Effect of chromium and cobalt
ions on the expression of antioxidant enzymes in human U937
macrophage-like cells. J Biomed Mater Res A. 94:419–425.
2010.PubMed/NCBI
|
|
41
|
Piotrowski J, Jedrzejewski T and Kozak W:
Heme oxygenase-1 induction by cobalt protoporphyrin enhances fever
and inhibits pyrogenic tolerance to lipopolysaccharide. J Therm
Biol. 45:69–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Finley BL, Unice KM, Kerger BD, Otani JM,
Paustenbach DJ, Galbraith DA and Tvermoes BE: 31-day study of
cobalt(II) chloride ingestion in humans: Pharmacokinetics and
clinical effects. J Toxicol Environ Health A. 76:1210–1224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicol.
283:65–87. 2011. View Article : Google Scholar
|
|
44
|
De Boeck M, Kirsch-Volders M and Lison D:
Cobalt and antimony: Genotoxicity and carcinogenicity. Mutat Res.
533:135–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stefaniak AB, Harvey CJ, Bukowski VC and
Leonard SS: Comparison of free radical generation by pre- and
post-sintered cemented carbide particles. J Occup Environ Hyg.
7:23–34. 2010. View Article : Google Scholar
|
|
46
|
Lison D, De Boeck M, Verougstraete V and
Kirsch-Volders M: Update on the genotoxicity and carcinogenicity of
cobalt compounds. Occup Environ Med. 58:619–625. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Walter LR, Marel E, Harbury R and Wearne
J: Distribution of chromium and cobalt ions in various blood
fractions after resurfacing hip arthroplasty. J Arthroplasty.
23:814–821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beraudi A, Stea S, De Pasquale D, Bordini
B, Catalani S, Apostoli P and Toni A: Metal ion release: Also a
concern for ceramic-on-ceramic couplings? Hip Int. 24:321–326.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Paustenbach DJ, Galbraith DA and Finley
BL: Interpreting cobalt blood concentrations in hip implant
patients. Clin Toxicol (Phila). 52:98–112. 2014. View Article : Google Scholar
|