Introduction
The female pelvic floor is subjected to a constantly
changing mechanical load due to intra-abdominal pressure and
gravity. Clinical studies have identified that vaginal labor and
multi-gravidity are key risk factors for pelvic organ prolapse
(POP) (1–3). External tensile load is associated
with POP via its mechanical stretch and compression on pelvic
support (4,5). Pelvic supports are collagen-rich,
dense connective tissues predominantly composed of fibroblasts,
these allow the tissue to respond directly to the surrounding
environment and they are particularly mechanoresponsive (6). Fibroblasts secrete extracellular
matrix (ECM), maintain tissue homeostasis, and are involved in
repair and remodeling. Previous studies have demonstrated that the
biomechanical properties of pelvic supports are abnormally altered
in POP patients (7–9), thus, it is hypothesized that
mechanical force is associated with POP (5). Based on these findings, the present
study hypothesizes that mechanical loading is a potential mechanism
involved in POP. An increased understanding of the specific
molecular signaling pathways associating physical force with POP
may be beneficial in defining the underlying etiologies of the
development of POP and aid in the development of novel treatment
options for women with this disorder.
Mechanotransduction, including mechanical sensing
molecules (for example, integrins) and formation of stress fibers
and focal adhesions convert extracellular mechanical stimulations
into intracellular signals, and result in a series of cellular
responses. Transmembrane integrins transmit extracellular
mechanical signals via intracellular kinase networks, and Akt is
involved in the mechanotransduction as a component of these kinase
networks (6,10–12).
Akt, a serine/threonine kinase, is a downstream effector of
integrin linked kinase, it is activated by mechanical strain and
regulates a broad range of cellular functions (13,14).
When unstimulated, Akt is present in the cytoplasm, and two
regulatory phosphorylated sites (Thr308 and Ser473) are
unphosphorylated. Following biochemical or mechanical stimulation,
Akt is recruited to the plasma membrane. Activated Akt
phosphorylates its downstream targets, which translocate to
subcellular locations (15).
Mechanical strain activates the
phosphati-dylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt
signaling pathway in order to regulate cellular apoptosis,
proliferation, ageing and metabolism. It has been demonstrated in
fibroblasts from the skin, osteoblasts, airway smooth muscle cells
and periodontal ligament fibroblasts (6,13,14,16),
however, to the best of our knowledge, it has not yet been
demonstrated in pelvic support fibroblasts (PSF).
It has also been demonstrated that the applied
mechanical strain results in intracellular reactive oxygen species
(ROS) accumulation and oxidative stress (OS) in pelvic support
uterosacral ligament (USL) fibroblasts (17). OS influences proliferation,
differentiation, apoptosis and senescence in fibroblasts (18). In addition, OS in pelvic supports
increases the development of POP. Kim et al (19) identified that OS markers,
8-hydroxy-2′-deoxyguanosine (8-OHdG) and 4-hydroxy-2-nonenal
(4-HNE) were increased in the USLs of patients with POP. The
expression of glutathione peroxidase 1 (GPX1) in USLs of POP
patients has been demonstrated to be markedly suppressed (9). Thus, the present study hypothesized
that mechanical force may induce OS in pelvic supports and may be
involved in the pathogenesis of POP.
Activated Akt directly phosphorylates its downstream
transcription factors, which regulate expression of genes via
binding with DNA. The forkhead box O (FOXO) family is an important
downstream target of Akt and the phosphorylation of FOXO1 may be
controlled by activated Akt, which results in nuclear exclusion and
degradation, as well as inhibition of transcriptional activation.
FOXO1 is involved in the control of gene transcription, for
example, it decreases the expression of antioxidase (20–23),
which decreases the ability of ROS detoxification and results in
OS.
The present study aimed to determine the effects of
mechanical loading on human USL fibroblast (hUSLF) apoptosis,
senescence and production of collagen. Based on our previous
studies (17,24), the present study focused on the
involvement of the PI3K/Akt signaling pathway and OS. The results
of the present study demonstrate that mechanical strain activates
Akt signaling-induced OS and affects apoptosis, senescence and
collagen production in hUSLF. The present study demonstrates the
importance of mechanical strain in the pathogenesis of POP, in
addition to the underlying molecular mechanisms.
Materials and methods
Patients and sample collection
The present study was approved by the ethics
committee of Renmin Hospital of Wuhan University was obtained prior
to the commencement of the study, and written informed consent was
obtained from all donors prior to sample collection. All donors
underwent hysterectomy for benign indications. One year of
amenorrhea in women aged >45 years was defined as menopause.
Prior to surgery, a pelvic examination was performed to evaluate
for the presence of POP. Uterovaginal prolapse was graded according
to the POP quantification system advocated by the International
Continence Society. Of the 56 women who underwent hysterectomy, the
20 who were diagnosed with stage II POP or greater were assigned to
the POP group and the 36 without POP were assigned to the control
group. Of the control group, 16 patients without POP were used to
develop primary cultures of hUSLFs. Donors who had pelvic
operations, pelvic inflammation, serious systemic diseases,
reproductive system cancer, pelvic radiation exposure or were
taking hormone replacement therapy were excluded.
Cell culture
Specimens were taken from uterosacral ligaments and
fibroblasts were cultured and purified as described previously
(25). Briefly, the USL tissues
were cut into pieces, placed in culture bottles and digested with
modified collagenase type I (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and trypsinase (Sigma-Aldrich, St. Louis,
MO, USA). The fibroblasts were grown in serum-free Dulbecco′s
modified Eagle′s medium (DMEM; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences), 100 U/ml
penicillin/streptomycin (Beyotime Institute of Biotechnology,
Haimen, China) at 37°C in a humidified incubator (Heal Force
Development, Ltd., Hong Kong, China) with 5% CO2. Cells
were passaged at 85% confluency. The cells were characterized by
their spindle-like morphology, and identified by hematoxylin and
eosin staining and immunohistochemistry, which indicated positive
staining for vimentin and negative staining for keratin, as
previously described (17). Cells
from passage 3–6 were used in the current study. Cells from 20
non-POP donors were used in the present study and each experiment
was repeated in cells from at least three donors. The PI3K/Akt
specific inhibitor LY294002 (20 µM) dissolved in dimethyl
sulfoxide (Selleck Chemicals, Houston, TX, USA) was added to cell
culture 30 min prior to the application of mechanical strain.
Mechanical strain application
hUSLFs were seeded on flexible and transparent
plates, which were pre-coated with rat tail collagen type I (25
µg/ml in 0.02 N acetic acid; Sigma-Aldrich, St. Louis, MO,
USA). Following reaching confluence, cells were rendered quiescent
by incubation in serum-free DMEM for 24 h and prepared for
mechanical strain. To exert mechanical strain on the cells, a
four-point bending device (SXG4201; Chengdu Miracle Chemicals Co.,
Ltd., Chengdu, China) was used (17). In brief, the device was composed of
a drive-control unit, loading unit and strain plates and dishes.
Mechanical strain was set at 0.3 Hz of 5,333 με (loading
displacement is 4 mm) for the indicated time periods. When the
control group cells reached confluency, the serum-free DMEM was
replaced.
Western blotting
Total protein was extracted from patient tissues
using radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology) and quantified using the BCA Protein assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocols. Protein samples (40 µg) were
separated by 10% SDS-PAGE for 90 min at 100 V, and transferred onto
a polyvinylidene difluoride membrane, which was blocked in 5 g/l
skimmed milk for 1 h. The membrane was incubated with appropriate
monoclonal antibodies at 4°C overnight. Following washing in
Tris-buffered saline with Tween 20 (TBST; Wuhan Goodbio Technology
Co., Ltd., Wuhan, China), the membrane was incubated with
horseradish peroxidase-conjugated goat anti-mouse (1:500; A0216)
and goat anti-rabbit (1:500; A0208) secondary antibodies (Beyotime
Institute of Biotechnology) at 37°C for 1 h, and the target
proteins were visualized using an ECL detection kit (Beyotime
Institute of Biotechnology). The following primary antibodies were
used: Rabbit polyclonal Akt (1:500; cat. no. 9272), rabbit
monoclonal phosphorylated (p)-Akt (1:500; cat. no. 4058), mouse
monoclonal FOXO1 (1:1,000; cat. no. 97635) and rabbit polyclonal
p-FOXO1 (1:1,000; cat. no. 9461) all obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA); mouse monoclonal Mn-superoxide
dismutase (Mn-SOD; 1:500; cat. no. sc-130345) and rabbit polyclonal
procollagen type I α1 (COL1A1; 1:1,000; cat. no. sc-28657) all
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA); and
rabbit polyclonal GPX1 (1:1,000; cat. no. ab22604) and rabbit
polyclonal GAPDH (1:1,000; cat. no. ab9485) obtained from Abcam,
Cambridge, UK). GAPDH served as a control in the immunoblot
analysis to verify the specificity of the antibody. Images of the
blots were captured using a Gel-Doc XR imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and they were analyzed using
Quantity One 4.62 software (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression levels of GPX1, Mn-SOD, COL1A1 and
GAPDH was evaluated by RT-qPCR. hUSLF RNA was extracted by using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). DNase I
(Sigma-Aldrich) was used and RNA was reverse transcribed to cDNA
using a RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). The temperature protocol was as follows: 95°C
for 30 sec; 40 cycles of 95°C for 5 sec, 60°C for 34 sec, and 95°C
for 15 sec; 60°C for 1 min, 95°C for 15 sec and 60°C for 15 sec.
SYBR Green I labeled probes (Takara Bio, Inc., Otsu, Japan) were
used for the qPCR conducted in an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with
Platinum® Taq DNase (Invitrogen; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 34 sec;
95°C for 15 sec; 60°C for 1 min; 95°C for 15 sec; and 60°C for 1
min. Standard curves were generated to determine the copy number of
mRNA in the experimental samples, and all measurements were
normalized to the expression of the GAPDH gene (data not shown).
The primer sequences purchased from Beijing SBS Genetech Co., Ltd.
(Beijing, China) were as follows: Forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′
for GAPDH; forward, 5′-CAAGACGAAGACATCCCACCAATC-3′ and reverse,
5′-ACAGATCACGTCATCGCACAACA-3′ for COL1A1; and forward,
5′-CGCTTCCAGAGCATTGACATC-3′ and reverse
5′-CGAGGTGGTATTTTCTGTAAGATCA-3′ for GPX1. Gene expression was
normalized to the expression of GAPDH, a housekeeping gene, and
mRNA levels were quantified using the 2−ΔΔCq method
(26).
Intracellular ROS assay
Cells (1×105/ml) were seeded into the
plates with or without exposure to strain, with 10 µM
serum-free DMEM containing 2′,7′-dihydrodichlorofluorescein
diacetate (H2DCF-DA; Applygen Technologies, Inc.,
Beijing, China) and cultured at 37°C. Following incubation for 30
min the cells were washed twice with phosphate-buffered saline
(PBS; Wuhan Goodbio Technology Co., Ltd.). The plates were observed
by fluorescence microscopy (IX51; Olympus Corporation, Tokyo,
Japan) at a wavelength of 450 nm.
Intracellular 8-OHdG assay
Cells (1×105/ml) were plated with or
without exposure to strain and fixed with formaldehyde (Wuhan
Goodbio Technology Co., Ltd.). and incubated overnight at 4°C with
mouse monoclonal antibodies against 8-OHdG (1:200; Abcam; cat. no.
ab62623). The plates were washed in TBST and incubated with goat
anti-mouse secondary antibodies labeled with FITC (1:1,000; Abcam;
ab7064). The nuclei were stained with 4′,6-diamidino
2-phenylindole. The fluorescence-stained cells were observed under
fluorescence microscopy (IX51).
Flow cytometry
Annexin V (Beyotime Institute of
Biotechnology)/propidium iodide (PI; Beyotime Institute of
Biotechnology) double staining was conducted to detect apoptosis.
Suspended cells were collected in 15 ml centrifuge tubes
(5×106 cells/tube), and centrifuged at 200 × g for 5
min. The supernatant was discarded and the cells were resuspended
with PBS. Annexin V labeled with fluorescein isothiocyanate was
added, which is indicated by green fluorescence, and PI was added,
which is indicated by red fluorescence, and incubated for 20 min in
a dark place. The proportion of Annexin
V+/PI− cells were visualized by flow
cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA)
and analyzed with CellQuest Pro (BD Biosciences).
In situ terminal deoxyribonucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
The In situ Cell Death Detection kit,
Fluorescein (Roche Diagnostics GmbH, Mannheim, Germany) was used to
quantify apoptosis at single cell level by labeling DNA strand
breaks. Paraffin-embedded USL tissue sections were dewaxed by
heating at 60°C and washing in xylene (Sinopharm Chemical Reagent
Co., Ltd., Shanghai, China) and rehydrated with a graded series of
ethanol. They were incubated with a protease K working solution for
20 min at room temperature, and then incubated with
permeabilisation solution for 8 min. Slides were rinsed twice with
PBS and incubated with the TUNEL reaction mixture for 60 min at
37°C in the dark. The slides were then rinsed twice with PBS and
five fields of each section was observed by fluorescence microscopy
(IX51).
Senescence-associated β-galactosidase
(SA-β-gal) staining
The present study used a previously described method
by Dimri et al (27) to
test the positive percentage of activated SA-β-gal. Cells exposed
to mechanical strain were fixed by 3% formaldehyde for 5 min. In
order to detect highly expressed SA-β-gal, hUSLFs were incubated
with 1 mg/ml of 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside
(X-Gal; Wuhan Goodbio Technology Co., Ltd.) at 37°C and pH 6.0 for
12 h. Positively stained cells were blue when observed under a
light microscope (CKX31, Olympus Corporation) and the proportion of
positive cells was calculated.
Statistical analysis
All experimental data points are independent and
data are presented as the mean ± standard deviation. For normally
distributed data, one-way analysis of variance with Tukey's
post-hoc test used for multiple comparisons and Dunnett's test for
comparing each group with the control group. The Mann-Whitney U
test was used to compare two groups. Statistical analysis was
performed with statistical software SPSS 17.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Mechanical strain induces apoptosis and
senescence and the reduces the production of collagen in
hUSLFs
hUSLFs from passage 3–6 were plated on rat tail
collagen type I-coated dishes until they reached confluency. A
mechanical strain of 5333 µε at 0.3 Hz was applied for 4 h.
In our previous experiment (24),
mechanical strains of 0, 1333 µε, 2666 µε and 5333
µε were applied to hUSLFs for 4 h and Annexin V/PI double
staining was used to detect cellular apoptosis. Investigation
indicated that mechanical strain induced apoptosis in hUSLFs and
the apoptotic percentage (Annexin V+/PI−) in
cells exposed to 5333 µε was markedly higher compared with
other groups (0, 1333 µε and 2666 µε) (23). Apoptotic cells were notably higher
in the group exposed to mechanical loading (Fig. 1A). Senescent cells, which are
SA-β-gal positive, were also markedly increased in the mechanical
loading group (Fig. 1B). It has
thus been hypothesized that mechanical strain induces apoptosis and
senescence in hUSLFs. Collagen is the predominant component of ECM
in USL and it is produced by fibroblasts. The results of the
present study indicated that protein expression levels of COL1A1
were significantly reduced in the mechanical loading fibroblasts
(P<0.05; Fig. 1C and D), which
indicates that mechanical strain disrupts collagen metabolism in
hUSLFs.
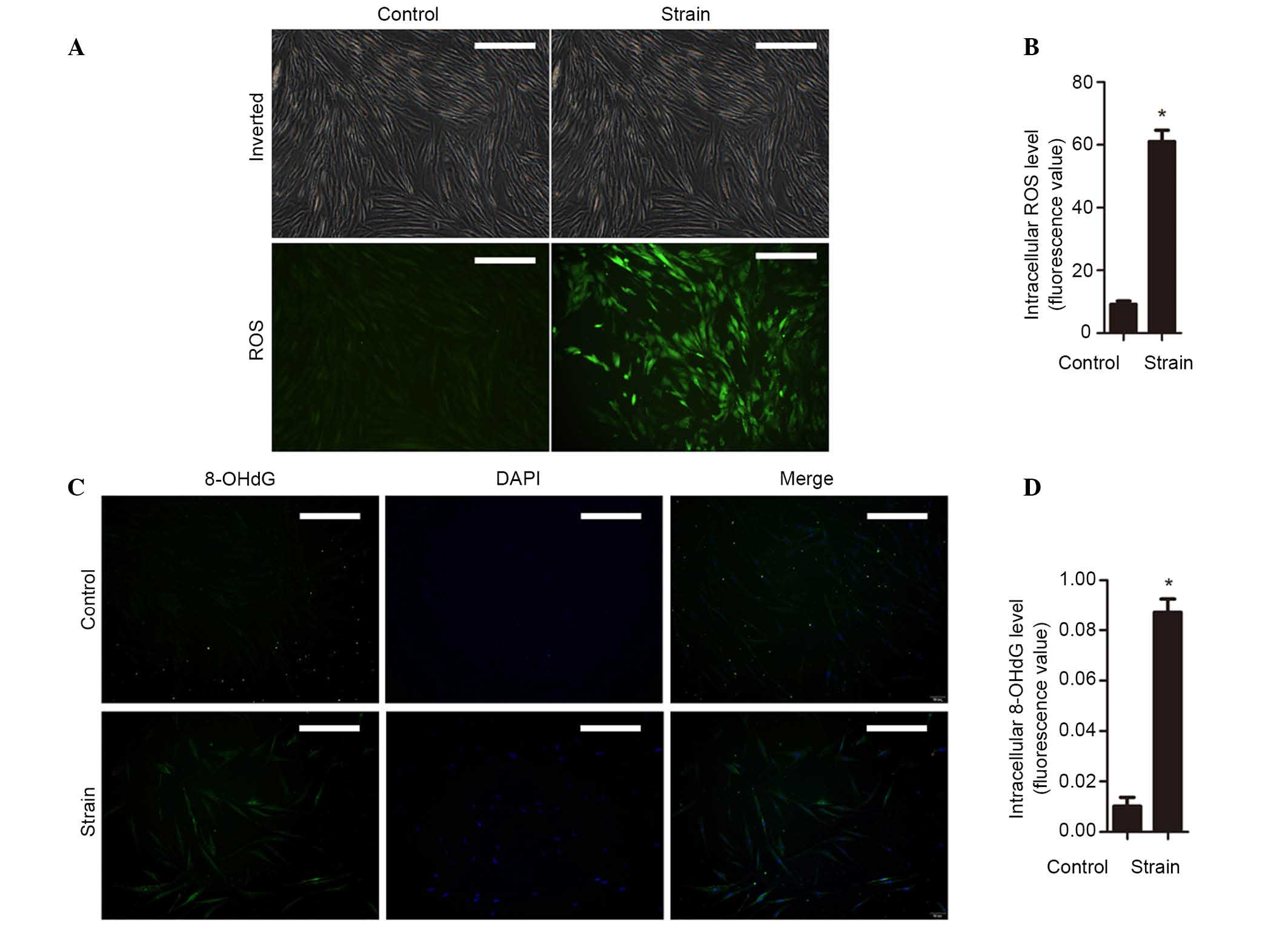
Mechanical strain increases intracellular
ROS and OS
Increased intracellular ROS levels promote cellular
apoptosis and senescence, and may affect cell metabolism (28,29).
In order to ascertain the effects of mechanical strain on
intracellular ROS levels, the level of ROS was detected by
fluorescent probe H2DCFDA. The results suggested that
the level of intracellular ROS were significantly increased by
mechanical stress of 5333 µε for 4 h (P<0.05; Fig. 2A and B). The levels of 8-OHdG in
hUSLFs were then determined. Fibroblasts loaded with strain were
observed to have significantly increased levels of 8-OHdG compared
with unstimulated cells (P<0.05; Fig. 2C and D). These results indicates
that mechanical strain results in excessive accumulation of
intracellular ROS and leads to OS in hUSLFs.
The PI3K/Akt signaling pathway is
activated by mechanical strain in hUSLFs
Mechanical strain activates the PI3K/Akt signaling
pathway and extracellular mechanical cues are transduced into
intracellular signaling in order to regulate a range of effects,
including apoptosis and senescence (20,21,23).
The transcription factor FOXO1 is phosphorylated by activated Akt
and regulates expression of numerous genes, including antioxidase
genes that regulate OS (20). In
order to ascertain whether mechanical strain activates the PI3K/Akt
signaling pathway in hUSLFs, mechanical strain of 5333 µε at
0.3 Hz was applied to fibroblasts (Fig. 3). Akt was activated within 10 min
and remained so for 1 h, and p-Akt levels returned to basal levels
in 3 h (P<0.01 at 10–120 min; Fig.
3A and B). FOXO1 was phosphorylated for 30 min following
exposure to mechanical strain, and returned to basal levels within
4 h (P<0.001 at 30–180 min; Fig. 3A
and C). In order to investigate the association between the
phosphorylation of FOXO1 and mechanical strain-activated Akt,
hUSLFs were incubated with the PI3K/Akt signaling inhibitor
LY294002 (20 µM) for 30 min prior to exposure to mechanical
strain. It was observed that Akt phosphorylation was blocked and
FOXO1 phosphorylation was significantly inhibited (P<0.05;
Fig. 3D and F). These results
indicate that mechanical strain activated the PI3K/Akt signaling
pathway, which in turn phosphorylates downstream FOXO1, thus
resulting in its inactivation.
Mechanical strain affects collagen
anabolism via activated PI3K/Akt signaling-induced OS
The present study also examined the effect of
mechanical strain on hUSLF via the PI3K/Akt/FOXO1 signaling
pathway. FOXO1 regulates OS through associated gene expression. The
present study demonstrated that the mRNA (P<0.01; Fig. 4A) and protein (P<0.01; Fig. 4B and C) expression levels of the
antioxidases GPX1 and Mn-SOD in fibroblasts were significantly
decreased following loading with 5333 µε strain for 4 h,
this also resulted in an increased intracellular ROS level
(P<0.001; Fig. 4D and E). To
further investigate the role of the PI3K/Akt/FOXO1 signaling
pathway in regulating OS, hUSLFs were incubated with PI3K/Akt
inhibitor LY294002 prior to exposure to mechanical strain. It was
observed that the expression levels of GPX1 and Mn-SOD were
significantly increased as Akt activation was blocked following
loading with mechanical strain (P<0.01; Fig. 4A–C). The levels of intracellular
ROS dropped evidently (P<0.001; Fig. 4D and E). These results demonstrate
that mechanical strain changes cellular OS via the PI3K/Akt/FOXO1
signaling pathway, which regulates the expression levels of GPX1
and Mn-SOD. The role of Akt in cellular apoptosis, senescence and
metabolism was also investigated. It was observed that inhibition
of PI3K/Akt signaling significantly suppressed apoptosis
(P<0.001; Fig. 5A and B) and
senescence (P<0.001; Fig. 5C and
D) and promoted the mRNA (P<0.01) and protein expression
levels of COL1A1 (P<0.05; Fig.
5E–G). These results indicate that mechanical strain induces
apoptosis and senescence, and disrupts the production of collagen
type I via activating PI3K/Akt/FOXO1-mediated OS.
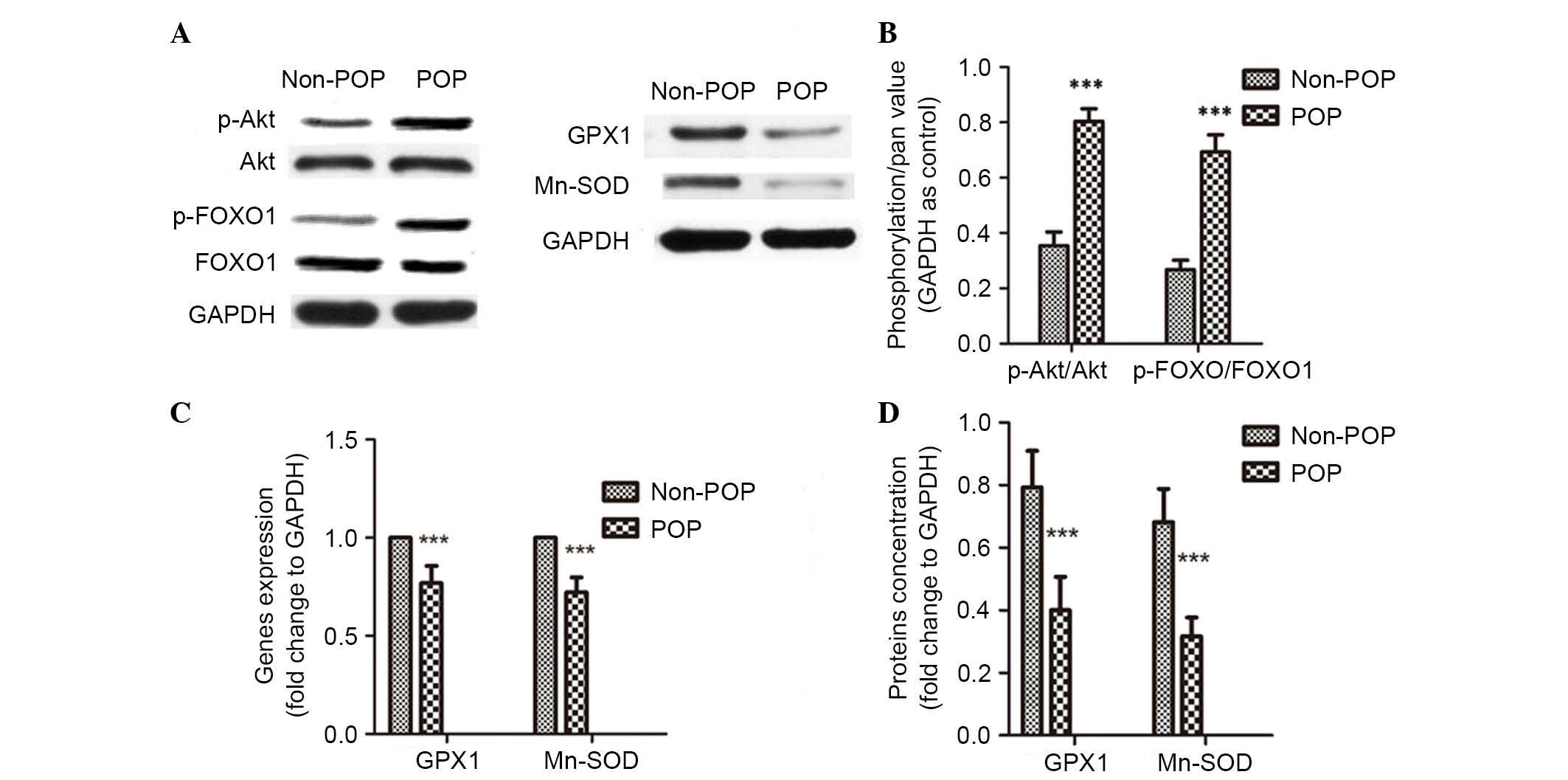
PI3K/Akt signaling pathway is activated
in USLs of POP patients
USLs were extracted from 20 patients with POP and 20
without POP in the course of hysterectomy. Age, body mass index and
menopausal status exhibited no significant difference between two
groups. Parity in the POP group was significantly increased
compared with the control group (P<0.05; Table I). Akt activation was detected in
USL tissue samples from the two groups. P-Akt levels were shown to
be significantly increased in USL tissue samples from the POP group
compared with the control group (P<0.001; Fig. 6A and B). The data was also analyzed
individually, in the POP group 12/20 USL tissue explants exhibited
marked expression of p-Akt, and in three explants Akt
phosphorylation was not notable. However, in control group, four
explants exhibited moderate or strong p-Akt expression (data not
shown). These results demonstrate Zthat the PI3K/Akt signaling
pathway is activated in the USLs of POP patients.
 | Table IDemographis of the POP group and
non-POP group. |
Table I
Demographis of the POP group and
non-POP group.
| Characteristic | POP group
(n=20) | Non-POP group
(n=20) | P-value |
|---|
| Age (years), median
(range) | 53.5 (45–76) | 52 (45–70) | NSa |
| Body mass index
(kg/m2), mean ± SD | 26.615±3.478 | 26.44±2.822 | NSa |
| Menopause status
(years), median (range) | 10 (1–36) | 8.5 (1–30) | NSa |
| Parity, mean ±
SD | 2.85±1.387 | 2±1.026 | P<0.05 |
Antioxidation decreases and the
production of collagen type I is reduced in USLs of POP
patients
Following identification of PI3K/Akt/FOXO1 signaling
pathway involvement in POP, its downstream gene targets were also
investigated (Fig. 6). It was
observed that mRNA (P<0.001; Fig.
6C) and protein expression levels (P<0.001; Fig. 6A and D) of GPX1 and Mn-SOD were
significantly decreased in the POP group, which indicates a reduced
defense against OS. Apoptosis in USLs was then detected by TUNEL
assay, and it was observed that the percentage of TUNEL-positive
cells was significantly higher in the POP group than in the control
group (P<0.001; Fig. 7A and B).
The present study investigated the metabolism of collagen in pelvic
supports and observed that mRNA (P<0.001) and protein
(P<0.001) expression levels of COL1A1 were significantly reduced
in the POP group (Fig. 7C–E).
PI3K/Akt signaling increases OS, which is involved in increased
levels of cell apoptosis and reduced collagen production in pelvic
supports of POP patients.
Discussion
USL, an important component of the pelvic support
system, is constantly altered by exposure to the mechanical
environment. Long-term high intra-abdominal pressure may result in
relaxation of pelvic supports leading to POP. Fibroblasts are the
predominant cell type in USLs. They transduce mechanical cues into
biochemical signals to regulate expression of specific genes. In
addition, hUSLF secretes ECM in response to physiological
conditions. Fibroblasts are key in the maintenance of tissue
homeostasis, repair and remodeling.
First, the present study investigated the mechanism
of mechanical force-induced apoptosis, which is involved in the
pathogenesis of POP. Clinical studies have identified that the risk
factors for POP are associated with mechanical stress and that
pelvic support relaxation may lead to POP. It was observed in the
present study that in USLs of POP patients, cell apoptosis was
increased and collagen anabolism was disrupted. In order to
investigate the mechanism of POP pathogenesis in vitro, a
model of hUSLFs subjected to mechanical strain was produced. A
four-point bending device was used to apply mechanical stress to
hUSLFs, it was observed that the stretched cells were randomly
distributed and a proportion did not remain on the plates (data not
shown). This mechanical strain model allowed investigation into the
pathogenesis of POP. First, the effects of mechanical strain on
hUSLFs were investigated and it was observed that the levels of
apoptosis and senescence increased and the production of collagen
type I decreased in stretch fibroblasts compared with unstretched
fibroblasts. This indicated that micropathological changes in
pelvic supports of POP had been imitated to a certain degree.
Excessive accumulation of intracellular ROS and OS
results in cell apoptosis, senescence and abnormal metabolism
(30). The present study
investigated whether ROS accumulation was present in stretched
cells and USLs of POP patients. The involvement of OS in the
pathogenesis of POP has been previously reported (19), and the results of the present study
are consistent. Mechanical strain was applied to fibroblasts and a
marked increase in the level of intracellular ROS was observed,
which resulted in increased OS in hUSLFs. The OS changes in the
mechanically loaded hUSLF model were consistent with those in the
USLs of POP patients, which verifies the validity of the model to
mimic POP at micropathological levels. The PI3K/Akt pathway is one
of the most important signaling pathways involved in OS. Activation
of PI3K/Akt is not unique to OS regulation as it also regulates
normal growth and metabolism (30). However, the present study
demonstrated that OS is important in apoptosis of stretched hUSLFs,
and that this may be mediated by the PI3K/Akt signaling
pathway.
Mechanical strain activates a number of signaling
pathways, including PI3K/Akt to transduce extracellular stimulation
into intracellular signals, and regulate various cellular
responses. The transcription factor FOXO1, a downstream target of
the PI3K/Akt signaling pathway, controls numerous genes, including
antioxidase genes that protect cells against oxidative injury
(22). The expression of PI3K/Akt
signaling regulates cell survival and proliferation partly by
phosphorylating FOXO proteins to promote their export from the
nucleus and degradation via the ubiquitin proteasome
pathway-dependent pathway (20).
In order to elucidate the effects of mechanical strain on the
PI3K/Akt signaling pathway, 5333 µε mechanical strain at 0.3
Hz was applied to hUSLFs. Akt was activated rapidly and remained
activated, prior to gradual inactivation. In addition, rapid Akt
activation was followed by FOXO1 phosphorylation resulting in FOXO1
nuclear exclusion and a reduced ability to regulate target genes.
It has been reported that transcription factor FOXO family bind to
target genes in the nucleus and regulate their expression,
including genes associated with OS, DNA injury, the cell cycle,
apoptosis, metabolism (20,22,23).
In the present study, hUSLFs were incubated with a specific
PI3K/Akt inhibitor LY294002 prior to exposure to mechanical strain.
It was observed that LY294002 blocked the activation of Akt and
FOXO1 phosphorylation was markedly reduced. This may be due to
another upstream signaling pathway activated by mechanical stress
that also regulates FOXO1. It has been reported that activation of
the extracellular signal-regulated kinase 1/2 or c-Jun
amino-terminal kinase signaling pathways phosphorylates FOXO1
(31–33). This suggests that mechanical
strain-induced FOXO1 phosphorylation is predominantly regulated by
PI3K/Akt signaling activation in hUSLFs.
The results of the present study demonstrate that
mechanical strain suppresses the expression of GPX1 and Mn-SOD. It
also decreases the ability to scavenge ROS, which results in
excessive ROS accumulation in hUSLFs. High levels of ROS in cells
disrupts the normal redox balance and cells enter a state of OS.
When OS is severe and cell defense is weak, cells may undergo
apoptosis, and a sustained increase in ROS may function as a common
trigger for activating senescence (30). Increased ROS in cells may also
affect metabolism, suppress cell viability and damage gene
expression or signaling transduction, which may block collagen
anabolism. In order to investigate whether mechanical
strain-induced OS promoted apoptosis and senescence. and interferes
with collagen production, hUSLFs were incubated with LY294002 prior
to the exposure to mechanical strain. It was observed that the
expression levels of GPX1 and Mn-SOD were increased and the levels
of intracellular ROS were decreased compared with cells exposed to
mechanical strain but not the inhibitor. In addition, the degree of
the apoptosis and senescence was reduced and COL1A1 expression was
increased following the inhibition of Akt. However, the levels did
to return to the basal standard, which indicates there may be
effects due to a small quantity of unphosphorylated FOXO1 that was
not blocked by LY294002, or may be regulated by other signaling
pathways. Although it is possible that the PI3KAkt/FOXO1 signaling
pathway is not the only pathway involved in the effects of
mechanical strain on hUSLFs, the results demonstrated that is the
predominant signaling pathway.
In conclusion, the in vitro experiments in
the present study demonstrated that mechanical strain induces
apoptosis and senescence, and reduces collagen type I production
via activated PI3K/Akt signaling pathway-mediated OS. Mechanical
strain activates Akt and, thus, downstream FOXO1 is phosphorylated
and the expression of GPX1 and Mn-SOD is suppressed, which results
in a decreased ability to scavenge ROS. In addition, the current
study observed that the PI3K/Akt signaling pathway is activated and
antioxidation declined in USLs of POP patients. The present study
indicates that mechanical strain results in PI3K/Akt-mediated OS,
which is key in the pathogenesis of POP and may have potential as a
therapeutic strategy for POP.
Acknowledgments
The authors would like to thank their colleagues in
the Department of Obstetrics and Gynecology, Renmin Hospital of
Wuhan University for helping extract uterosacral ligament explants
in the course of hysterectomy surgeries. The present study was
funded by the National Natural Science Foundation of China (grant
no. 81270684 and 81471442).
References
|
1
|
Jelovsek JE, Maher C and Barber MD: Pelvic
organ prolapse. Lancet. 369:1027–1038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Virtanen HS and Mäkinen JI: Retrospective
analysis of 711 patients operated on for pelvic relaxation in
1983–1989. Int J Gynaecol Obstet. 42:109–115. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gyhagen M, Bullarbo M, Nielsen TF and
Milsom I: Prevalence and risk factors for pelvic organ prolapse 20
years after childbirth: A national cohort study in singleton
primiparae after vaginal or caesarean delivery. BJOG. 120:152–160.
2013. View Article : Google Scholar
|
|
4
|
Chow D and Rodríguez LV: Epidemiology and
prevalence of pelvic organ prolapse. Curr Opin Urol. 23:293–298.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miedel A, Tegerstedt G, Mæhle-Schmidt M,
Nyrén O and Hammarström M: Nonobstetric risk factors for
symptomatic pelvic organ prolapse. Obstet Gynecol. 113:1089–1097.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paterno J, Vial IN, Wong VW, Rustad KC,
Sorkin M, Shi Y, Bhatt KA, Thangarajah H, Glotzbach JP and Gurtner
GC: Akt-mediated mechanotransduction in murine fibroblasts during
hypertrophic scar formation. Wound Repair Regen. 19:49–58. 2011.
View Article : Google Scholar
|
|
7
|
Zhou Y, Ling O and Bo L: Expression and
significance of lysyl oxidase-like 1 and fibulin-5 in the cardinal
ligament tissue of patients with pelvic floor dysfunction. J Biomed
Res. 27:23–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Budatha M, Silva S, Montoya TI, Suzuki A,
Shah-Simpson S, Wieslander CK, Yanagisawa M, Word RA and Yanagisawa
H: Dysregulation of protease and protease inhibitors in a mouse
model of human pelvic organ prolapse. PloS One. 8:e563762013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li BS, Hong L, Min J, Wu DB, Hu M and Guo
WJ: The expression of glutathione peroxidase-1 and the anabolism of
collagen regulation pathway transforming growth
factor-beta1-connective tissue growth factor in women with uterine
prolapse and the clinic significance. Clin Exp Obstet Gynecol.
40:586–590. 2013.PubMed/NCBI
|
|
10
|
Konstantonis D, Papadopoulou A, Makou M,
Eliades T, Basdra E and Kletsas D: The role of cellular senescence
on the cyclic stretching-mediated activation of MAPK and ALP
expression and activity in human periodontal ligament fibroblasts.
Exp Gerontol. 57:175–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue Y, Lypowy J, Hedhli N and Abdellatif
M: Ras GTPase-activating protein binds to Akt and is required for
its activation. J Biol Chem. 279:12883–12889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nho RS, Xia H, Kahm J, Kleidon J, Diebold
D and Henke CA: Role of integrin-linked kinase in regulating
phosphorylation of Akt and fibroblast survival in type I collagen
matrices through a beta1 integrin viability signaling pathway. J
Biol Chem. 280:26630–26639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson WR, Rubin CT and Rubin J:
Mechanical regulation of signaling pathways in bone. Gene.
503:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue Z, Zhang W, Desai LP, Gao H, Gunst SJ
and Tepper RS: Increased mechanical strain imposed on murine lungs
during ventilation in vivo depresses airway responsiveness and
activation of protein kinase Akt. J Appl Physiol (1985).
114:1506–1510. 2013. View Article : Google Scholar
|
|
15
|
Boccafoschi F, Bosetti M, Sandra PM,
Leigheb M and Cannas M: Effects of mechanical stress on cell
adhesion: A possible mechanism for morphological changes. Cell Adh
Migr. 4:19–25. 2010. View Article : Google Scholar :
|
|
16
|
Premaraj S, Souza I and Premaraj T:
Mechanical loading activates β-catenin signaling in periodontal
ligament cells. Angle Orthod. 81:592–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong SS, Ding WJ, Wu DB, Min J, Hong L, et
al: Effects of oxidative damage in human parametrial ligament
fibroblasts induced by mechanical stress. Chin J Clinicians
(Electronic Edition). 7:10775–10779. 2013.
|
|
18
|
Usta A, Guzin K, Kanter M, Ozgül M and
Usta CS: Expression of matrix metalloproteinase-1 in round ligament
and uterosacral ligament tissue from women with pelvic organ
prolapse. J Mol Histol. 45:275–281. 2014. View Article : Google Scholar
|
|
19
|
Kim EJ, Chung N, Park SH, Lee KH, Kim SW,
Kim JY, Bai SW and Jeon MJ: Involvement of oxidative stress and
mitochondrial apoptosis in the pathogenesis of pelvic organ
prolapse. J Urol. 189:588–594. 2013. View Article : Google Scholar
|
|
20
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu Z and Tindall DJ: FOXOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Downing JR: A new FOXO pathway required
for leukemogenesis. Cell. 146:669–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tzivion G and Hay N: PI3K-AKT-FoxO axis in
cancer and aging. Biochim Biophys Acta. 1813:19252011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong S, Li H, Wu D, Li B, Liu C, Guo W,
Min J, Hu M, Zhao Y and Yang Q: Oxidative damage to human
parametrial ligament fibroblasts induced by mechanical stress. Mol
Med Rep. 12:5342–5348. 2015.PubMed/NCBI
|
|
25
|
Ding WJ, Hong SS, Min J, Fang G, Zhang X,
Hu M, Yang Q and Hong L: Preliminary study on primary culture of
human parametrial ligament fibroblasts. Medical Journal of Wuhan
University. 35:16–19. 2014.
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Franklin JL: Redox regulation of the
intrinsic pathway in neuronal apoptosis. Antioxid Redox Signal.
14:1437–1448. 2011. View Article : Google Scholar :
|
|
29
|
Labunskyy VM and Gladyshev VN: Role of
reactive oxygen species-mediated signaling in aging. Antioxid Redox
Signal. 19:1362–1372. 2013. View Article : Google Scholar :
|
|
30
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mori R, Tanaka K, de Kerckhove M, Okamoto
M, Kashiyama K, Tanaka K, Kim S, Kawata T, Komatsu T, Park S, et
al: Reduced FOXO1 expression accelerates skin wound healing and
attenuates scarring. Am J Pathol. 184:2465–2479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Danciu TE, Gagari E, Adam RM, Damoulis PD
and Freeman MR: Mechanical strain delivers anti-apoptotic and
proliferative signals to gingival fibroblasts. J Dent Res.
83:596–601. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chae HD and Broxmeyer HE: SIRT1 deficiency
downregulates PTEN/JNK/FOXO1 pathway to block reactive oxygen
species-induced apoptosis in mouse embryonic stem cells. Stem Cells
Dev. 20:1277–1285. 2011. View Article : Google Scholar :
|